Abstract
Objective
Osteoprotegerin is a member of the tumor necrosis factor receptor superfamily. The study aimed to assess serum osteoprotegerin and endothelial dysfunction relationship in obese non-diabetic subjects. Elucidating the association linking serum osteoprotegerin level to obesity and insulin resistance.
Materials and methods
Sixty obese non-diabetic subjects attending the outpatient endocrine clinic were compared with 20 controls. All subjects went through history taking, clinical examination, and anthropometric measuring. Laboratory tests included fasting blood glucose, HbA1c, HOMA-IR, lipid profile, and serum osteoprotegerin level—assessment of endothelial dysfunction measured by carotid intimal thickening and flow meter dilatation.
Results
Obese subjects had significantly higher rates of insulin resistance and dyslipidemia compared with controls (P = 0.017 and 0.002, respectively). Serum osteoprotegerin levels were significantly lower in obese subjects compared with controls (P = 0.011). In obese subjects, there was a significant positive correlation between osteoprotegerin and HOMA-IR (r = 0.272; P = 0.035), whereas there was no significant correlation between OPG and endothelial dysfunction.
Conclusion
The study showed that osteoprotegerin significantly decreased in obese non-diabetic individuals in comparison with control subjects. A significant positive correlation between osteoprotegerin and HOMA-IR has been reported in obese non-diabetic subjects. OPG can be considered a serum biomarker to predict insulin resistance in obese individuals.
Similar content being viewed by others
Introduction
One of the most notable health issues is obesity, considered a risk factor for plenty of chronic illnesses. It was recently added to the World Health Organization’s objective list of non-communicable diseases around the globe [1].
According to the 2019 Healthy 100 Million survey, Egypt has one of the highest obesity rates in the world. Of the 49.7 million Egyptian adults (≥ 18 years) surveyed, 39.8% were obese (BMI ≥ 30 kg/m2) [2]. Diabetes mellitus type 2 has a high frequency in adult Egyptians, which might be attributed to obesity. Additionally, obesity has increased diabetes complications; sleep apnea, fatty livers, hypertension, ischemic heart disease, and death. Obesity propensity is mostly influenced by genetic factors, and genotype expression is influenced by the environment.
Osteoprotegerin (OPG) is a receptor activator of nuclear factor kappa B ligand, a TNF-related apoptosis-inducing ligand, and belongs to the (TRAIL) family. TRAIL is a member of the TNF superfamily [3]. Recently, OPG was recognized as a novel secreted protein related to the tumor necrosis factor receptor that shares in bone density regulation. Its expression is increased in osteoclastogenesis, bone resorption, and inflammatory bone disease. OPG has been demonstrated to contribute to vascular calcification, which is considered a cardiovascular disease risk factor [4].
Plasma OPG levels are highly associated with endothelial dysfunction, and serum OPG level provides an independent prognostic benefit for X-syndrome as a risk criterion for cardiovascular disease [5]. The RANK/RANKL/OPG may play a crucial role in vascular calcification according to mounting studies [4]. Inflammatory cell chemotaxis is substantially enhanced by OPG. OPG stimulates two processes commonly occurring in atherosclerosis: matrix metalloproteinase release and apoptosis in vascular smooth muscle cells and endothelium [6]. It was shown that OPG has an atherosclerotic effect by stimulating the production of intercellular adhesion molecules on endothelial cells (ECs). such as vascular adhesion molecule-1 (VCAM-1) and his E-selectin. This, in turn, increases leukocyte adherence, which is a prelude to EC dysfunction [7].
The study aimed to assess the relationship between serum (OPG) level and endothelial dysfunction in obese non-diabetic subjects.
Materials and methods
Population of the study
The study included 60 obese non-diabetic subjects visiting the Endocrine and Obesity Clinic in Kasr Alainy throughout the period from April to October 2022 in comparison to a lean group consisting of 20 subjects as control.
Inclusion criteria
All participants who were obese with BMI ≥ 35, neither diabetics nor pre-diabetics.
Exclusion criteria
-
Hypertension
-
History of stroke
-
History of myocardial infarction
-
History of intake of lipid-lowering drugs
-
History of renal disease
Methods
All subjects involved in the study went through the following:
-
Full history taking
-
Thorough clinical examination
-
Anthropometric measures:
-
Height, weight, waist circumference, and hip circumference.
-
Anthropometric measures were calculated by the following formulae:
-
BMI: (kg/m.2)—waist/hip ratio: waist circumference/hip circumference (cm/cm) − WHt R ratio: waist/height (cm/cm) − Body adiposity index (BAI) = (Hip circumference/height (cm/m) × 1.5) − 18
-
Laboratory tests: including fasting blood sugar, HBA1C, HOMA-IR, lipid profile (Total cholesterol, TG, LDL, HDL), and serum osteoprotegerin.
Sampling
All subjects were requested to fast for 6–8 h before sampling. A venipuncture, in a totally aseptic environment, was followed to aspirate 6 ml of blood distributed into three tubes; one was a plain tube (4 ml), the other was a violet top EDTA vacutainer tube (2 ml) and the third was grey top fluoride vacutainer tube (2 ml).
Assay
All subjects included were assayed for the following:
-
I.
Routine investigations.
As for the serum tube; the blood was left to clot for fifteen minutes. Afterward, it was rapidly separated within one hour from withdrawal by centrifugation.
Blood was centrifuged at 3000 rpm (1000 × g) for 10 min.
Serum was collected after centrifugation and was kept in 2 aliquots (2 ml micro-centrifuge tube each) at − 20 °C till assay for the following: one for lipid profile including cholesterol (total, HDL, and LDL), triglycerides, and insulin.
The fluoride tube was used to assay fasting blood glucose.
The EDTA tube was used to measure HBA1C.
Diagnoses of IR were based on the homeostasis model assessment (HOMA-IR) equation taking 2.9 as a cut-off for insulin resistance.
-
II.
Specific investigations.
The other serum aliquot was used to assay serum osteoprotegerin levels by commercially available ELISA technique.
Doppler ultrasonography
Done with the same operator to measure:
-
1.
Carotid intima-media thickness
-
2.
Flow-mediated dilatation (FMD) of the brachial artery: FMD is determined by the percentage of change in brachial artery diameter after stimulus concerning the baseline diameter. The brachial artery diameter is initially measured at rest after placing the participant in a constant temperature and quiet room with his/her hand standing in a comfortable position. After determination of the baseline diameter, ischemia is performed by inflating the cuff 50 mmHg over the systolic pressure. After 3 min the tourniquet is released and as a result, an increase in blood flow occurs resulting in vasodilatation of the brachial artery, and the maximum diameter is measured.
Statistical analysis
Data analysis packages were calculated using the software SPSS version 21.
Qualitative data was expressed by percentage and number. Quantitative data was expressed by median, interquartile range, mean, and standard deviation.
Student’s t-test and ANOVA test were performed for parametric data. Chi-square, and Fisher exact tests, however, were done for non-parametric data.
The level of significance was set at p equal to or below 0.05.
Osteoprotegerin validity was assessed using ROC curve analysis.
Results
In the current study, 60 obese non-diabetic subjects (BMI ≥ 35) attending an outpatient endocrine clinic in Kasr Al-Ainy were included and were compared with 20 healthy controls.
Comparison between obese subjects and control groups regarding demographic data, anthropometric data, and classification of WHR and WHtR were summarized in Table 1.
Obese subjects were significantly older when compared with controls (mean age 38.87 ± 11.82 vs 27.00 ± 2.96; P < 0.001). Obese subjects had significantly increased weight, BMI, waist circumference, hip circumference, body adiposity index, waist-to-hip ratio, and waist-to-height ratio when compared with controls (P = < 0.001, < 0.001, < 0.001, < 0.001, < 0.001, 0.003, and < 0.001 respectively).
Comparisons between Obese subjects and control groups regarding laboratory investigations were summarized in Table 2. Obese subjects had significantly higher serum levels of fasting blood sugar, glycosylated hemoglobin, LDL, triglycerides, T.cholesterol, and HOMA-IR when compared with control subjects (P = 0.002, < 0.001, 0.0128, 0.023, 0.001 and 0.024 respectively). Serum levels of HDL were significantly decreased in obese subjects when compared to control subjects (P = 0.005).
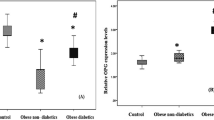
Comparison between obese subjects and control groups regarding serum Osteoprotegerin level was illustrated in Fig. 1. Serum osteoprotegerin level was significantly decreased among obese subjects when compared with controls (1.39 ± 1.59 vs 5.05 ± 4.29 pg/mL; P = 0.011).
A comparison between obese subjects and control groups regarding endothelial dysfunction parameters was demonstrated in Table 3. The table shows no significant difference between both studied groups regarding endothelial dysfunction parameters including carotid intimal thickening or flow meter dilatation measured by duplex study.
Correlations of serum osteoprotegerin were calculated with different parameters in obese subjects and summarized in Table 4.
The table shows a significant positive correlation between osteoprotegerin and HOMA-IR among obese subjects (r = 0.272; P = 0.035). No significant positive correlation between osteoprotegerin and other variables was noted.
The receiver operating characteristic (ROC) curve for the detection of obese subjects using serum osteoprotegerin was calculated and then drawn. The curve is illustrated in Fig. 1. It shows that the cut-off value of 2.26 pg/mL of serum osteoprotegerin had significant discriminative ability for the detection of obese subjects from controls (P = 0.044), with an area under curve 0.690, 95% confidence interval ranging between 0.505 and 0.874. With 93.3% sensitivity and 65% specificity.
Discussion
Osteoprotegerin was first identified in 1997 as a decoy receptor for nuclear factor kappa B ligand (RANKL), thereby inhibiting osteoclast differentiation and thus bone resorption. OPG is associated with numerous physiological mechanisms [8]. Many studies have attempted to clarify the role of OPG in insulin resistance, vascular calcification, and obesity. Still, the results were inconsistent. Studies using transgenic mice have shown that OPG protects the vasculature by preventing both plaque calcification and progression. In DM-type patients, OPG has been associated with morbidity like CAD, subclinical atherosclerosis, and moreover, mortality [9].
Research has indicated that in normoglycemic adults, the incidence of stroke, CAD, and other cardiovascular events increases in concurrent with the levels of circulating OPG. Moreover, elevated serum OPG is an independent risk factor for the progression of atherosclerosis and vascular mortality [10]. However, obesity itself is not a determinant of increased OPG levels. However, IR appears to play an important role in OPG upregulation in relation to obesity [11].
Serum OPG level was statistically decreased among obese subjects when compared with controls (1.39 ± 1.59 vs 5.05 ± 4.29 pg/ml; P = 0.011).
Similarly, Rashad et al. [12] in a study that included 150 normal-weight healthy women as a control group and 150 women with obesity subcategorized into two subgroups; non-diabetic (n = 70) and with DM type 2 (n = 80), reported that non-diabetic control group had higher OPG serum levels than obese women (p < 0.001) and obese non-diabetic obese had lower serum levels of OPG in compared to obese diabetics to p < 0.001.
These findings should be discriminated from their finding that levels of OPG were higher in obese women than controls (p < 0.001) regarding mRNA expression.
Kotanidou et al. [11] categorized obese participants into two subgroups according to levels of fasting insulin, using 15.00 μUI/ml as a cut-off value, and found that OPG levels were again significantly higher in the hyper-insulinemic state (p < 0.001).
A study conducted by O’Sullivan et al. [13] on 115 obese male adults proved that OPG is elevated in obese adult men with glucose intolerance.
The idea of the correlation of OPG levels to impaired glucose and insulin metabolism could be a possible explanation for this variation.
Furthermore, the variations in the patient populations throughout these studies may contribute to the disparities in the results of OPG levels in obesity. The selection criteria varied, despite the fact that age, androgen status, and estrogen status are important factors that influence OPG serum levels [14].
In the current study, a significant positive correlation was reported between OPG and HOMA-IR among obese subjects (r = 0.272; P = 0.035).
Another positive association between serum OPG and HOMA-IR was found in a study conducted by Akinci et al. [15] on 128 women affected previously with gestational diabetes mellitus and 67 controls. In Gannagé-Yared et al. [16] a study suggested that insulin resistance was positively correlated to OPG levels among Caucasian obese individuals.
In contrast, a significant negative correlation between OPG levels and insulin resistance, as measured by the HOMA-IR equation, was identified in obese sub-Saharan African women in the Ayina et al. [17] study, signifying that elevated OPG levels may suggest high insulin sensitivity. Also, Ashley et al. [18] reported a negative association between OPG and HOMA-IR in 100 healthy obese subjects.
Meanwhile, in the Erol et al. [19] study, no significant correlations were found between OPG and HOMA-IR. Also, O’Sullivan et al. [13] ended with the same result.
Based on the contradictory results of previous studies, we suggest that serum OPG levels may be a marker of IR in obese individuals. It remains controversial whether high serum OPG levels in this particular population reflect insulin sensitivity or resistance. Differences in age, gender, ethnicity, metabolic status, and insulin sensitivity in the study population using HOMA-IR may partially explain this discrepancy. In the current study, no discernible difference was found between the two studied groups. regarding endothelial dysfunction parameters including carotid intimal thickening or flow meter dilatation measured by Duplex study.
In agreement with our study, Gunes et al. [20] reported no association between OPG levels and carotid intimal thickening or flow meter dilatation in DM type 2 patients.
In contrast to our study, de Ciriza et al. [21] conducted a study on 238 asymptomatic patients with metabolic syndrome, they stated that serum level of OPG positively correlated with intima-media thickness (r = 0.2, p = 0.005). Moreover, the study also reported that serum OPG was correlated with a high risk of abnormal intimal media thickening.
On the other hand, the results of Morony et al.’s [22] experimental study suggested that OPG has vaso-protective properties by vascular calcification reduction.
No significant correlation between OPG and demographic, anthropometric, or laboratory data including lipid profile.
In contrast, Erol et al. [19] reported a negative correlation between age and OPG level. While Ayina et al. [17] reported that age was positively correlated with OPG levels (r = 0.831, p = 0.003).
As regards the correlation between anthropometric measurements and serum OPG concentration levels, results showed significant positive correlations between WHR serum level and OPG; however, no correlation was determined with WC or BMI. WHR, rather than WC or BMI, is a predictor for obesity-related risk stratification. OPG may be the pathogenic link between obesity-related risk stratification and WHR.
Conclusion
The present study demonstrated that OPG was significantly reduced in obese, non-diabetic individuals compared to healthy non-obese controls. Furthermore, a significant positive correlation between OPG and HOMA-IR has been reported in obese non-diabetic subjects. However, no significant association between OPG and endothelial dysfunction has been reported
Recommendations
OPG could be used as a biomarker in predicting insulin resistance in obese non-diabetic patients.
More prospective studies on larger scales are needed to assess the relation between serum osteoprotegerin (OPG) level and endothelial dysfunction in obese non-diabetic patients. However, further studies are needed to identify new pathways for vascular affection in obesity.
Availability of data and materials
Not applicable.
Abbreviations
- HOMA-IR:
-
Homeostatic Model Assessment-Insulin Resistance
- OPG:
-
Osteoprotegerin
- BMI:
-
Body mass index
- RANKL:
-
Receptor activator of nuclear factor kappa-Β ligand
- TNF:
-
Tumor necrotic factor
- TRAIL:
-
TNFRelated apoptosis-inducing ligand
- TNFSF:
-
Tumor necrosis factor superfamily
- TNFRSF:
-
Tumor necrosis factor receptor superfamily
- RANK/RANKL/OPG:
-
Receptor activator of nuclear factor kappa-B/receptor activator of nuclear factor kappa-B ligand/osteoprotegerin
- VACM-1:
-
Vascular cell adhesion protein 1
- EC:
-
Endothelial cell
- WHR:
-
Waist Hip Ratio
- BAI:
-
Body adiposity index
- TG:
-
Triglycerides
- LDL:
-
Low-density lipoprotein
- HDL:
-
High-density lipoprotein
- EDTA:
-
Ethylene-diamine-tetra-acetic acid
- FMD:
-
Flow-mediated dilatation
- ANOVA:
-
Analysis of variance
- ROC:
-
Receiver operating characteristic
- mRNA:
-
Messenger ribonucleic acid
References
Kontis V, Mathers CD, Rehm J et al (2014) Contribution of six risk factors to achieving the 25×25 non-communicable disease mortality reduction target: a modeling study. The Lancet 384(9941):427–437. https://doi.org/10.1016/s0140-6736(14)60616-4
Malik VS, Willet WC, Hu FB (2020) Nearly a decade on — trends, risk factors and policy implications in global obesity. Nat Rev Endocrinol 16(11):615–616. https://doi.org/10.1038/s41574-020-00411-y
Walsh MC, Choi Y. Biology of the RANKL-RANK-OPG System in Immunity, Bone, and Beyond. Frontiers in Immunology. 2014;5:1–11. https://doi.org/10.3389/fimmu.2014.00511
Rochette L, Meloux A, Rigal E, Zeller M, Cottin Y, Vergely C (2019) The role of osteoprotegerin and its ligands in vascular function. Int J Mol Sci 20(3):705. https://doi.org/10.3390/ijms20030705
Barbu CG, Arsene AL, Florea S et al (2017) Cardiovascular risk assessment in osteoporotic patients using osteoprotegerin as a reliable predictive biochemical marker. Mol Med Rep 16(5):6059–6067. https://doi.org/10.3892/mmr.2017.7376
Quercioli A, Mach F, Bertolotto M et al (2012) The receptor activator of NF-κB ligand (RANKL) increases the release of neutrophil products associated with coronary vulnerability. Thromb Haemost 107(01):124–139. https://doi.org/10.1160/th11-05-0324
Zauli G, Corallini F, Bossi F et al (2007) Osteoprotegerin increases leukocyte adhesion to endothelial cells both in vitro and in vivo. Blood 110(2):536–543. https://doi.org/10.1182/blood-2007-01-068395
Baud’huin M, Duplomb L, Teletchea S et al (2013) Osteoprotegerin: multiple partners for multiple functions. Cytokine Growth Factor Rev 24(5):401–409. https://doi.org/10.1016/j.cytogfr.2013.06.001
Guzel S, Seven A, Kocaoglu A et al (2012) Osteoprotegerin, leptin, and IL-6: Association with silent myocardial ischemia in type 2 diabetes mellitus. Diab Vasc Dis Res 10(1):25–31. https://doi.org/10.1177/1479164112440815
Reinhard H, Lajer M, Gall MA et al (2010) Osteoprotegerin and Mortality in Type 2 Diabetic Patients. Diabetes Care 33(12):2561–2566. https://doi.org/10.2337/dc10-0858
Kotanidou EP, Kotanidis CP, Giza S et al (2019) Osteoprotegerin increases parallel to insulin resistance in obese adolescents. Endocr Res 44(1–2):9–15. https://doi.org/10.1080/07435800.2018.1480630
Rashad NM, El-Shal AS, Shalaby SM, Abdel-Nour HM, Sarhan WM (2021) Osteoprotegerin expression and serum values in obese women with type 2 diabetes mellitus. Mol Biol Rep 48(11):7095–7104. https://doi.org/10.1007/s11033-021-06699-x
Sullivan Eoin O’, Ashley DT, Davenport C et al (2013) A comparison of osteoprotegerin with adiponectin and high-sensitivity C-reactive protein (hsCRP) as a marker for insulin resistance. Metabolism 62(1):34–38. https://doi.org/10.1016/j.metabol.2012.06.005
Szulc P, Hofbauer LC, Heufelder AE, Roth S, Delmas PD (2001) Osteoprotegerin serum levels in men: correlation with age, estrogen, and testosterone status. J Clin Endocrinol Metab 86(7):3162–3165. https://doi.org/10.1210/jcem.86.7.7657
Akinci B, Celtik A, Yuksel F et al (2011) Increased osteoprotegerin levels in women with previous gestational diabetes develo** metabolic syndrome. Diabetes Res Clin Pract 91(1):26–31. https://doi.org/10.1016/j.diabres.2010.09.028
Gannagé-Yared MH, Yaghi C, Habre B et al (2008) Osteoprotegerin concerning body weight, lipid parameters insulin sensitivity, adipocytokines, and C-reactive protein in obese and non-obese young individuals: results from both cross-sectional and interventional study. Eur J Endocrinol 158(3):353–359. https://doi.org/10.1530/eje-07-0797
Ayina Ayina CN, Sobngwi E, Essouma M, et al. Osteoprotegerin concerning insulin resistance and blood lipids in sub-Saharan African women with and without abdominal obesity. Diabetology & Metabolic Syndrome.2015;7(1):14. https://doi.org/10.1186/s13098-015-0042-3
Ashley DT, Sullivan Eoin O, C Davenport et al (2011) Similar to adiponectin, serum levels of osteoprotegerin are associated with obesity in healthy subjects. Metabolism 60(7):994–1000. https://doi.org/10.1016/j.metabol.2010.10.001
Erol M, Özlem Bostan Gayret, Hikmet Tekin Nacaroğlu, et al. Association of osteoprotegerin with obesity, insulin resistance and non-alcoholic fatty liver disease in children. Iranian Red Crescent Medical Journal. 2016;18(11). https://doi.org/10.5812/ircmj.41873
Mutlu Gunes, Sule Temizkan, Tugce Apaydin, Can Ilgin, Goncagül Haklar, Dilek Gogas Yavuz. Serum osteoprotegerin levels, endothelial function and carotid intima-media thickness in type 2 diabetic patients. Journal of Diabetes and Its Complications. 2021;35(12):108073–108073. doi:https://doi.org/10.1016/j.jdiacomp.2021.108073
Pérez de Ciriza C, Moreno M, Restituto P et al (2014) Circulating osteoprotegerin is increased in the metabolic syndrome and is associated with subclinical atherosclerosis and coronary arterial calcification. Clin Biochem 47(18):272–278. https://doi.org/10.1016/j.clinbiochem.2014.09.004
Morony S, Yin, Zhang Z et al (200AD) Osteoprotegerin inhibits vascular calcification without affecting atherosclerosis in ldlr (−/−) mice. Circulation 117(3):411–420. https://doi.org/10.1161/circulationaha.107.707380
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Concept: S.R., R.S.; design: S.R., R.S; supervision: A.S, H.W.Z.H, R.S., S.A; materials: A.S, H.W.Z.H, R.S., S.R, S.A; data collection and/or processing: S.R; analysis and/or interpretation: A.S, H.W.Z.H, R.S., S.R, S.A; literature review: S.R., R.S.; writing: H.W.Z.H, S.R, R.S.; critical review: A.S, H.W.Z.H, R.S., S.R, S.A. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was conformed confidentially to the Declaration of Helsinki. The Research Ethics Committee (REC) of the Faculty of Medicine, Cairo University, reviewed and approved the study protocol (Approval serial #ms-62–2022). The purpose of the study was explained to each participant and they were reassured of confidentiality. An informed written consent was taken from them.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shennawy, A.M., Hanna, H.W.Z., Raed, S. et al. Value of serum osteoprotegerin in the assessment of endothelial dysfunction in obesity. Egypt J Intern Med 36, 39 (2024). https://doi.org/10.1186/s43162-024-00297-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43162-024-00297-6