Abstract
Background
The electronic transitions between two fine levels depend on the transition probability. The transition probability depends on spectral line strength and oscillator strength. The oscillator strength depends on the number of oscillators and their energies. In this research, we will find the oscillator strengths of hyperfine multiplets of the Tantalum atom. The oscillator strength of hyperfine multiplet investigation aims to enhance our understanding of Tantalum's spectral characteristics. This work provides valuable information in the spectroscopy of material, atomic/molecular, and astrophysics.
Result
Fourier transform spectra from ultraviolet to far infrared regions have been obtained from TUGRAZ. Fourier transform spectra give the most reliable position of the wavelength of hyperfine multiplets. The Fourier transform spectra of Tantalum contain thousands of Tantalum I and II spectral lines. Each spectral line can be characterized by its upper and lower levels and corresponding angular momenta and hyperfine constants. These properties of the spectral lines were collected from the literature. Hyperfine multiplets for each fine structure were calculated, and they revealed their spectroscopic behavior with high precision.
Conclusion
In this study, Tantalum's hyperfine multiplet oscillator strength was calculated using advanced computational techniques to address its atomic structure. The fine structure “gf” values were obtained from literature, and intensities of the multiplets were determined. They combined with the gf values to calculate the oscillator strengths of the hyperfine multiplets.
Similar content being viewed by others
1 Background
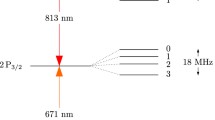
Tantalum, element 73 in the periodic table, is a rare, thick, blue-gray metal recognized for its high corrosion resistance. Named after the Greek legendary character Tantalus, it has a high melting point of 3017 °C and remarkable conductivity, making it important in a variety of sectors [1]. Tantalum atoms' hyperfine structure displays magnificent aspects in their atomic spectral lines as a result of interactions between the magnetic moments of their nuclei and electrons. This phenomenon produces extra splitting of spectral lines, resulting in several closely spaced lines rather than single ones [2]. Tantalum's hyperfine structure is important for understanding atomic behavior and is especially useful in spectroscopy and atomic physics studies. Scientists can learn about Tantalum atoms' intrinsic dynamics and structure by examining hyperfine splitting.
Knowledge of Tantalum's hyperfine structure has benefits in a variety of industries, including quantum computers and atomic clocks. Hyperfine structure comprehension enables precise measurements of atomic transitions, which is critical for the development of accurate timekee** devices and sophisticated computer technologies [3, 4]. In spectroscopy, oscillator strength is a metric that quantifies the chance of a transition between two energy levels within a molecule or atom while interacting with electromagnetic radiation, such as light. It represents the transition's capacity to absorb or emit light, with larger values suggesting greater probability. Oscillator strength, represented by a dimensionless number ranging from 0 to 1, aids understanding of the intensity of absorption or emission lines in spectra. It is determined by parameters such as the energy difference between states and the transition probability, which provide information on atomic and molecular characteristics [5].
In 1933, two groups, McMillan et al. and Gisolf et al., started the investigation of the hyperfine structure of the Ta atom [6, 7]. In the same year, Kiess investigated Ta's spectrum in detail. They classified 1890 lines out of 2629 lines of Ta [8]. Schmidt determined the quadrupole moment of the atomic nucleus of 181Ta [9]; Klinkenberg et al. investigated the Zeeman splitting of Ta I lines in the region 2500-8500A and gave many classifications of Ta I lines [10]. Berg et al. continued their investigation of the Ta I spectrum. They also found few ground-state levels and many high-odd levels [11]. Kamei studied the hyperfine structure of the spectrum of Ta I employing a hollow cathode discharge tube and a Fabry–Perot etalon and determined the quadrupole moments of Ta181 [12]. Bouazza considered previously available data to interpret even, odd, and multi-configurational fine structure and eigenvector percentage of levels the first time. The single electron has parameters determined for 181 Ta II for the least configuration by comparing ab initio calculations [13]. Windholz et al. determined energy values of 216 even and 290 odd parities of Ta I with uncertainties below 0.010 per centimeter and evaluated hfs constants of all found levels by wave number calibrated Fourier transform spectrum, extending from the UV-region (211 nm) to the infrared region (4.6 µm), and the wave numbers of altogether 3249 spectral lines were calculated with a global fit procedure [14]. Stephanie et al. detected the diatomic molecule Tantalum hydride (TaH) and its isotopologue Tantalum deuteride (TaD) for the first time by laser excitation spectroscopy. Two red-degraded bands, one arising from TaH at 636 nm and from TaD at 635 nm, have been recorded by intermodulated fluorescence spectroscopy, and rotational analysis shows that both bands are Ω = 2 ← 2 in character, with well-resolved Ω-doubling in the upper state of TaH [15]. Arcimowicz et al. presented the complex parametric studies of the fine- and hyperfine structure of Ta I up to second-order perturbation theory, considering the closed shell–open shell effects on excitations for the system of 47 even configurations [16]. Uddin et al. discovered 14 new energy levels of Ta II in a high-resolution Fourier transform spectrum from 72,000 to 81,000 cm−1; even levels were found between 0 and 44,000 cm−1. Through these data, nearly 100 spectral lines of Ta II are classified [17]. Windholz investigated the spectra for improved energy levels in Ta II, Pr II, and La II with large values of nuclear magnetic dipole moment and quadrupole moment to determine the center of gravity of spectral lines from resolved hfs [18]. Stachowska et al. presented a parametric study of the (fs) and (hfs) for even parity configurations of ionized (Ta II) using improved experimental data for 88 levels. A multi-configuration fitting procedure was performed for 47 configurations considering second-order perturbation theory, including the effects of closed-to-open shell excitations. The fs and hfs parameters, gJ-Landé factors, magnetic dipole, and electric quadrupole hyperfine structure constants A and B were calculated [19]. Bouchard et al. first analyzed the molecular beam study of TaN. They provided essential measurement support for P- and T-violation, dipole moment, branching fractions, transition lifetimes, and spin–orbit splitting of some states using dispersed laser-induced fluorescence technique [20]. Khan et al. investigated the emission spectra of Tantalum under the environment of different gases at various pressures using a LIBS spectrometer. The different parameters, such as emission intensity, excitation temperature, and electron number density of Ta plasma, have been evaluated as a function of pressure for various gases [21]. Althiyabi et al. computed energies, wavelengths, transition probabilities, weighted oscillator strengths of electric/magnetic dipole, electric/magnetic quadrupole, and lifetimes of the lowest 178 levels belonging to the (n = 3–6, l = 0–5) configurations in Be-like hafnium and Tantalum ions using the GRASP2018 code through the multi-configuration Dirac–Hartree–Fock (MCDHF) method with the Breit interaction and quantum electrodynamics (QED) corrections [22]. Guthöhrlein et al. determined magnetic hyperfine interaction constants A and electric quadrupole interaction constants B of 14 levels with even parity and 23 levels with odd parity using saturated optogalvanic laser spectroscopy [23]. Mocnik et al. determined the magnetic hyperfine interaction constants A and the electric quadrupole interaction constants B of 25 even parity levels and 32 odd parity levels by investigated the hyperfine structure of 41 Ta I lines. Respectively, they also found that certain Ta I levels indicated in Moore, Ch., Atomic energy levels, Vol. III, National Building Stand. (U.S.) Circ. No. 467, Washington, D.C.: U.S. GPO 1949 is nonexistent [24]. Brage et al. calculated oscillator strengths and hyperfine structure splitting for a set of transitions in Ta I and Ta II and found less than 10% uncertainty for hyperfine structure constants, which is predicted for oscillator strengths ranges from about 5 to 20% [25]. In 2000, Messnarz et al. investigated 200 lines of the neutral Tantalum atom using Doppler limited laser spectroscopy and computed the magnetic hyperfine interaction constants A and the electric quadrupole interaction constants B of levels 8 with even parity and levels 81 with odd parity. Additionally, 11 new levels of odd parity and 24 new levels of even parity were discovered [26]. In 2002, Eriksson et al. utilized Ta spectra emitted from a hollow cathode with a Fourier transform spectrometer and determine the center-of-gravity wavelengths for 199 lines and the hyperfine constants for 38 even and 97 odd levels of Ta II by analyzing the observed hyperfine patterns [27]. Deelen experimentally measured the hyperfine constant for 95 levels in neutral scandium using nonlinear least square fitting. Of those, 52 are reported for the first time. Furthermore, oscillator strength has been calculated for 590 hyperfine transitions [28]. Fu et al. used data from the Fourier transform spectrometer at the US National Solar Observatory and determined the hyperfine constant A for 36 levels of Co-I and 61 levels of Co-II [29]. Kaleem et al. estimated the hyperfine structural constant (A) of 95 odd and 104 even levels of the configurations 3d2(4p + 5p + 4s + 5s + 4d), 3d4s(4p + 5p + 5s + 6s + 4d + 5d), 3d3, 3d4p2, and 4s2(4p + 4d) of Sc-I using Fourier transform spectra [30].
2 Method
This research aims to find the oscillator strength of the hyperfine multiplet of Ta I. Hyperfine structures (hf) act as a fingerprint for element identification. It arises due to the interaction of electrons and the nucleus. The two most important interactions are magnetic dipole and electric quadrupole interactions. The former split the fine level’s structure, and the latter shifted the split lines. Magnetic dipole interaction occurs due to the interaction between the magnetic field of electrons and nuclear spin. The quadrupole interaction occurs due to the electrons' electric field at the nucleus's site. The hyperfine splitting is of the order of 0.001 to 1 cm−1. The energy contributions due to magnetic dipole \({(E}_{\mu })\) and electric quadrupole \({(E}_{Q})\) interactions are given in Eqs. (1) and (2), respectively.
where \(C=F\left(F+1\right)-I\left(I+1\right)-J(J+1)\), I, J, A, and B are nuclear spin, the angular momentum of fine level, magnetic dipole hf constant, and electric quadrupole hf constant, respectively. The expression for magnetic potential energy in the form of hyperfine constant A is written as:
Here, F is the total angular momentum of the atom, obtained by coupling nuclear spin and total electronic angular momenta. The energy of the hyperfine level was estimated by combining Eqs. (2), (3) and (4) as:
The wave number \((\sigma )\) is determined by the energy difference between the upper and lower levels. The fine structure gf value is multiplied by the normalized relative intensity, and a hyperfine oscillator strength \(g{f}_{hf}\) value is obtained. Mathematically, this can be written as
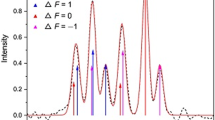
The term in the curly bracket gives the corresponding relative intensity of each hf component. One adds all relative intensities together and normalizes this so that the total intensity of all hfs components is 1, which is the Wigner 6j symbol.
3 Result
See Tables 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10.
3.1 Discussion
The oscillator strength of the hyperfine structure was calculated using magnetic dipole \({(E}_{\mu })\) and electric quadrupole \({(E}_{Q})\) constants. Oscillator strength (log gfs) was derived for 658 hyperfine transitions. Tables 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10 give details of the work. In each table, the first and third columns give the energies of the upper and lower levels. The second and fourth columns give the total quantum number “F” values of upper and lower levels, respectively. Each table's fifth, sixth, and seventh columns give the wavenumbers of the transition, intensities, and oscillator strengths of the hyperfine multiplets, respectively. Hyperfine constants were taken from the [3], and the intensity and position of hyperfine multiplets were calculated using Eqs. (3) and (4). Finally, using Eq. (5), the hyperfine multiplets were calculated.
4 Conclusion
In this study, we have calculated the oscillator strengths of hyperfine multiplets of the Tantalum atom. The hyperfine splitting of transition from 10 upper levels was examined. The levels are 30,664.684, 31,428.092, 31,500.99, 31,553.88, 34,001.2, 34,078.46, 34,094.69, 34,799.73, 35,876.55, and 37,561.29. There are 4, 9, 2, 2, 6, 4, 4, 8, 3, and 7 transition of these levels, respectively. Altogether, 658 hyperfine multiplets are calculated. The data not only provide crucial insights into the complex behavior of Tantalum atoms but also serve as valuable data for advancing studies on transition probabilities and lifetimes.
Availability of data and materials
The hyperfine constant data used in the manuscript can be downloaded from [3]. (Uddin, Zaheer. Hyperfine structure studies of Tantalum and Praseodymium . na, 2006).
Abbreviations
- Ta:
-
Tantalum
- TUGRAZ:
-
Graz University of Technology
- gf:
-
Fine structure value
- UV:
-
Ultraviolet
- hf:
-
Hyperfine
- log gfs:
-
Oscillator strength
- F:
-
Total quantum number
References
Ball P (2019) On the edge of the periodic table. Nature 565(7741):552–555
Ahmad N, Akram M, Gill KP, Asdaq SP, Akhtar RM, Saleem M, Baig MA (1997) Hyperfine structure studies of Tantalum. Zeitschrift für Physik D Atoms, Molecules and Clusters 41:159–163
Uddin Z (2006) Hyperfine structure studies of tantalum and praseodymium. na.
Wang C, Li X, Xu H, Li Z, Wang J, Yang Z et al (2022) Towards practical quantum computers: transmon qubit with a lifetime approaching 0.5 milliseconds. NPJ Quantum Inf 8(1):3
Stone PM (1962) Cesium oscillator strengths. Phys Rev 127(4):1151
McMillan E, Grace NS (1933) Hyperfine structure in the tantalum arc spectrum. Phys Rev 44(11):949
Gisolf JH, Zeeman P (1933) Nuclear moment of tantalum. Nature 132(3336):566–566
Kiess CC (1962) Description and analysis of the second spectrum of tantalum, Ta II. J Res Natl Bureau Stand Sect A Phys Chem 66A:111–161
Schmidt T (1943) About the quadrupole monent of the nucleus Ta-181 (78). Z Angew Phys 121(1–2):63–72
Klinkenberg PFA, Van den Berg GJ, Van den Bosch JC (1950) Structure and Zeeman-effect in the spectrum of the tantalum atom Ta i. Physica 16(11–12):861–889
Van den Berg GJ, Klinkenberg PFA, Van den Bosch JC (1952) Structure and Zeeman-effect in the spectrum of the tantalum atom Ta i, part ii. Physica 18(4):221–248
Kamei T (1955) Quadrupole Moments of Ta 181 and Lu 175. Phys Rev 99(3):789
Bouazza S (2012) Full hyperfine structure study of singly ionized Tantalum. Phys Scr 86(1):015302
Windholz L, Akhtar N, Uddin Z (2019) Revised energy levels and hyperfine structure constants of Ta I. J Quant Spectrosc Radiat Transf 224:512–536
Lee SY, Christopher CR, Manke KJ, Vervoort TR, Varberg TD (2014) The electronic spectrum of tantalum hydride and deuteride. Mol Phys 112(18):2424–2432
Arcimowicz B, Dembczyński J, Głowacki P, Ruczkowski J, Elantkowska M, Guthöhrlein GH, Windholz L (2013) Progress in the analysis of the even parity configurations of tantalum atom. Eur Phys J Spec Top 222(9):2085–2102
Uddin Z, Windholz L (2014) New levels of Ta II with energies higher than 72,000 cm− 1. J Quant Spectrosc Radiat Transfer 149:204–210
Windholz L (2017) The role of the hyperfine structure for the determination of improved level energies of Ta II. Pr II and La II Atoms 5(1):10
Stachowska E, Dembczyński J, Windholz L, Ruczkowski J, Elantkowska M (2017) Extended analysis of the system of even configurations of Ta II. At Data Nucl Data Tables 113:350–360
Bouchard JL, Steimle T, Kokkin DL, Sharfi DJ, Mawhorter RJ (2016) Branching ratios, radiative lifetimes, and transition dipole moments for tantalum nitride, TaN. J Mol Spectrosc 325:1–6
Khan S, Bashir S, Hayat A, Khaleeq-ur-Rahman M, Faizan-ul-Haq FUH (2013) Laser-induced breakdown spectroscopy of tantalum plasma. Phys Plasmas 20(7):073104
Althiyabi A, El-Sayed F (2022) Energies and transition data for Be-like hafnium and tantalum ions. At Data Nucl Data Tables 147:101528
Guthöhrlein GH, Windholz L (1993) Investigation of the hyperfine structure of TaI-lines. Zeitschrift für Physik D Atoms, Molecules and Clusters 27:343–347
Mocnik H, Arcimowicz B, Salmhofer W, Windholz L, Guthöhrlein GH (1996) Investigation of the hyperfine structure of Ta I-lines (III). Zeitschrift für Physik D Atoms, Mol Clust 36:129–136
Brage T, Proffitt CR, Leckrone DS (1999) Relativistic ab initio calculations of oscillator strengths and hyperfine structure constants in Tl II. J Phys B: At Mol Opt Phys 32(13):3183
Messnarz D, Guthöhrlein GH (2000) Investigation of the hyperfine structure of Ta I-lines (IV). Eur Phys J D Atomic Mol Opt Plasma Phys 12:269–282
Eriksson M, Litzén U, Wahlgren GM, Leckrone DS (2002) Spectral data for Ta II with application to the tantalum abundance in χ Lupi. Phys Scr 65(6):480
van Deelen F (2017) Hyperfine structure measurements in scandium for IR spectroscopy. Lund Observatory Examensarbeten.
Fu H, Xu Y, Fang D, Ma H, Liu M, Xu Q et al (2021) Hyperfine structure measurements of Co I and Co II with fourier transform spectroscopy. J Quant Spectrosc Radiat Transfer 266:107590
Kaleem M, Uddin Z, Xu Y, Dai Z (2023) Hyperfine structure constants of Sc I determined from Fourier transform spectra. Phys Scr 98(11):115409
Acknowledgements
Not applicable.
Funding
There is no funding for this research work.
Author information
Authors and Affiliations
Contributions
S. U. Rehman developed the Python program and confirmed the results, M. Zahid developed the Python program and wrote the methodology, A. A. Rajpoot developed the Python program and the lit survey, S. Shujat and A. Nasim prepared the manuscript and confirmed the conclusion, and Dr. M. Khan supervised in development of the model.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
There is no ethical issue in this research.
Consent for publication
Not applicable.
Competing interests
There is no conflict of interest among the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shujat, S.Q., Nasim, A., Ur Rehman, S. et al. Computation of hyperfine multiplet oscillator strengths in Tantalum atom. Beni-Suef Univ J Basic Appl Sci 13, 28 (2024). https://doi.org/10.1186/s43088-024-00489-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-024-00489-7