Abstract
Background
Early detection of biliary atresia (BA) is a great challenge providing the main useful way to improve its clinical consequence. Promising metabolomics provides an effective method for determining innovative biomarkers and biochemical ways for improving early diagnosis. This study aimed to determine the benefit of serum and urinary potential bile acid metabolites in the differentiation of BA from non-biliary atresia (non-BA) cases using tandem mass spectrometry (MS/MS). Fourteen bile acids metabolites were measured quantitively by MS/MS in serum and urine samples from 102 cholestatic infants and 102 control infants, in addition to the assay of the total serum bile acid enzymatically.
Results
After the diagnostic clinical and laboratory workflow, cholestatic infants were divided into BA (37 infants) and non-BA (65 infants) subgroups. Remarkably on analysis of serum individual bile acid concentrations, there were significant differences between cholestatic BA and non-BA regarding serum (glycocenodeoxycholic acid (GCDCA), taurochenodeoxycholic acid (TCDCA), taurocholic acid (TCA), and GCDCA/chenodeoxycholic acid (CDCA) ratio) (p < 0.001, for all), while there was no significant difference between the two groups regarding serum level of (cholic acid (CA), glycocholic (GCA), or TCDCA/CDCA ratio). There were no significant differences in either the urinary individual bile acids or urinary primary bile acids (conjugated or unconjugated) between BA and non-BA. Further principal component analysis (PCA) analysis was done and receiver operating characteristic (ROC) analysis was performed using score plots of the positive factors in the first two principal components PC1 (CA, GCA, GCDCA, TCA, TCDCA) and PC2 (CA, CDCA, lithocholic (LCA), ursodeoxycholic acid (UDCA)) for establishing the differences between the two diseased groups and revealed that the area under the curve (AUC) for PC1 was (0.770) higher than AUC for PC2 (0.583) indicating that the positive components of PC1 may be potential biomarkers for differentiation between the two cholestatic groups.
Conclusions
Metabolomics of serum bile acid levels using tandem mass spectrometry might change the paradigm differentiating BA from non-BA saving patients from unnecessary invasive procedures.
Similar content being viewed by others
Introduction
Neonatal cholestasis (NC) is a common neonatal hepatobiliary disorder occurring within the first three months of life [1]. Biliary atresia (BA) is considered the major reason of NC occurring in (35–41%) of the cases followed by progressive familial intrahepatic cholestasis (PFIC) (10%), preterm neonates (10%), metabolic and endocrinological conditions (9–17%), Alagille syndrome (AGS) (2–6%), infectious diseases (1–9%), mitochondriopathy (2%), biliary sludge (2%), and, lastly, idiopathic causes (13–30%) [2].
BA is a life-threatening disease that is characterized by a rapidly develo** fibro-obliteration of the bile ducts resulting in liver failure [3]. It is considered the commonest cause of end-stage liver disease in infancy [4] and the main indication for liver transplantation during childhood [5]. It is a great challenge to differentiate it from other causes of cholestasis and obstructive cholangiopathy due to shared clinical, biochemical, imaging, and histopathological features [6].
Despite being the golden standard of the diagnosis of BA, intraoperative cholangiogram (IOC) is a procedure that can clearly visualize the biliary tree, confirming the diagnosis of BA, but it is an invasive and undesirable procedure and could considerably increase morbidity. Furthermore, the diagnosis should be done as early as possible to facilitate timely Kasai portoenterostomy (KPE) before 3 months of life to improve native liver survival and decrease the need for liver transplantation during childhood [7]. So, we are in urgent need to identify a less invasive and non-operative technique to rule out BA without delay and avoid an unnecessary operation or liver biopsy [8].
Bile acids are the end-products of cholesterol catabolism in the liver [9, 10]. Conjugated primary bile acids are synthesized and secreted into the bile where deconjugation and conversion into secondary bile acids occur by intestinal bacteria [11], then they undergo enterohepatic circulation as most bile acids are reabsorbed with a trace amount lost in feces or spilled out into the systemic circulation and urine [12].
The importance of bile acids involved in physiological functions, their role in pathological processes [13], and their potential pharmacological uses have sparked an increased interest in deciphering their patterns in various biological matrices [14]. Changes in bile acid profiles can be exploited as disease biomarkers [15, 16], hence complete bile acid profiling, particularly those detected at relatively low concentrations in pathological settings, necessitates precise, and sensitive approaches [17]. With advances such as mass spectrometer-dependent metabolomics, it is possible to identify and quantify in vivo metabolites with molecular mass <1.5 KDa and so develop a profile of biomarkers for certain diseases or diseases [18]. Compared with traditional diagnostic methods, even small changes in metabolites can help to detect early pathologic changes more sensitively [19].
The metabolic profiling of bile acids varies in humans of different ages and different disease states [20], both gastrointestinal and non-gastrointestinal diseases [21, 22]. As bile acids are the major constituents of bile, their excretion is disrupted in BA with a decrease in luminal bile acid concentration and less generation of deconjugated and secondary bile acids that results in alterations in the enterohepatic circulation and disruption in total bile acid pool [8].
Thus, the aim of this study was to evaluate the potential value of the intermediates involved in bile acid metabolism (bile acid profiles) in the differentiation between BA and non-BA cholestasis in infants.
Methods
The institutional research board of the National Liver Institute, Menoufia University, approved the study (00134/2018 INTM), and written informed consent was obtained from participants’ parents or guardians. A case-control study was conducted on (204) infants, distributed as (102) cholestatic infants, aged from 1 to 365 days, and (102) matched control infants aged from 5 to 365 days. The cholestatic infants were selected from the inpatient wards, pediatric hepatology, gastroenterology, and nutrition department, National Liver Institute Hospital, Menoufia University, in the period from March 2019 to March 2020, using a nonprobability convenience sampling method. The control infants were recruited from Menoufia University Hospitals, complaining of other diseases such as chest infection or gastroenteritis. Cholestasis in children was defined as a conjugated bilirubin level of more than 1 mg/dL with a total bilirubin level of less than 5 mg/dL or when the conjugated bilirubin is more than 20% of the total bilirubin that is more than 5 mg/dL [23].
First, history taking and physical examination of all patients were conducted carefully, and their data were collected retrospectively from electronic records including etiologic work-up of cholestasis hepatitis B surface antigen, hepatitis C virus antibody, TORCH screening (Toxoplasma, rubella, herpes simplex virus (HSV) type 1 HSV type 2), and cytomegalovirus (CMV) immunoglobulin M and immunoglobulin) G) and abdominal ultrasound in all diseased cases for differential diagnosis. Some cases underwent specifically selected investigations according to their provisional diagnoses, such as CMV polymerase chain reaction, succinylacetone in urine, galactose and galactose-1-phosphate in the blood, magnetic resonance cholangiopancreatography (MRCP), and hepatobiliary scintigraphy (HBS). Liver tissue biopsies were taken from suspected cases of cholestasis as part of the diagnostic work except for cases with infection, Down syndrome, cases with endocrinal causes, Arthrogryposis–renal dysfunction–cholestasis (ARC) syndrome, acute liver failure, and hepatic focal lesion. From all, 69 cases underwent liver biopsy. Biliary obstruction was confirmed in 39 cholestatic infants. Then, they underwent IOC and 37 cases were diagnosed as biliary atresia and a Kassi operation was done. Two cases of them had a patent biliary tract that exclude the diagnosis of biliary atresia. Exclusion criteria: (i) cholestatic children with other comorbidities like diabetes mellitus or viral hepatitis and (ii) cases that underwent treatment.
Sample collection
Blood and urine samples were collected from all subjects included in this study at the same time in the early morning after fasting for 3 h. Six milliliters of the venous blood was divided as follows: 3ml was added to the plain vacationer tube, was left 15 min for coagulation, and centrifuged at 3000 rpm for 5 min, and then, the sera were aliquoted into 2 vials; one was used for measurement of liver and kidney function tests using RX Daytona plus, clinical chemistry analyzer (Randox Laboratories Limited, UK), and total bile acids using auto chemistry analyzer CDT240 (Diasystem Scandinavia AB, Sweden). The other vial was stored at −80 until analysis of bile acid metabolites by MS/MS (ACQUITY UPLC/MS/MS. Waters Corporation, Milford MA, USA). Two milliliters was collected into an EDTA tube for a complete blood count using Coulter Counter T660 (Coulter Electronics, Hialeah, Fl, USA). The remaining 1 ml was aliquoted into a sodium citrate tube for measurement of prothrombin time. Urine samples were collected from patients in special sterile plastic tubes and then evacuated in a special container without the addition of any preservatives and stored immediately at −80°C until analysis.
Chemicals and reagents
Bile acid standards
Cholic acid (CA), chenodeoxycholic acid (CDCA), deoxycholic acid (DCA), lithocholic acid (LCA), ursodeoxycholic acid (UDCA), glycocholic acid (GCA), glycochenodeoxycholic acid (GCDCA), glycodeoxycholic acid (GDCA), glycoursodeoxycholic acid (GUDCA), taurocholic acid (TCA), taurochenodeoxycholic acid (TCDCA), taurodeoxycholic acid (TDCA), tauroursodeoxycholic acid (TUDCA), and taurolithocholic acid (TLCA) were purchased from Sigma Chemical Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Methanol, acetonitrile, and formic acid were of HPLC grade and purchased from Fisher Scientific (Loughborough, UK). HPLC grade water was obtained from Millipore pure water purification system (Bransteasd Diamond RO model D12671) (USA).
Sample preparation and assay method
Both blood and urine samples were prepared according to Sugita et al. [24] method with some modifications. First, 100 μL of serum/urine was added to 400-μL ice-cold methanol to precipitate proteins and vortexed, then centrifuged at 13500 rpm for 15 min. The resulting supernatant was separated in clean Eppendorf and centrifuged again at 13,500 rpm for another 15 min. Finally, 50 μL of the supernatant was mixed with 100 μL water/formic acid solution and injected into (ACQUITY UPLC H-Class. Waters corporation, Milford MA, USA) using ACQUITY UPLC BEH C18, 1.7 μm, 2.1×50mm, column (Waters), at 50°C and flow rate of 0.28 mL/min. Mobile phase A was water/formic acid (1000: 1, v/v), and mobile phase B was 100% acetonitrile. The samples were eluted with 80% mobile phase A and 20% mobile phase B for an initial 2.1 min after injection, then with a linear gradient of mobile phase B of 20 to 30% over 5.2 min, followed by mobile phase B at 80% over 8 min, which was held for 0.5 min. The column was equilibrated with 80% mobile phase A for 2 min before the injection of the next sample. The injection volume of the sample was 5 μL, and the mass spectrometer was operated on electrospray negative mode using the multiple reaction monitoring (MRM). Data analysis was performed using Target Lynx applications manager software ver. 4.1 (waters) to obtain the calibration equations and the quantitative concentration of each bile acid in the samples. The validation range was from 0 to 40 μmol/L.
Pathological assessment
The liver specimens were fixed in 10% buffered formaldehyde paraffin-embedded and stained with the routine hematoxylin and eosin stain. Ancillary special stains, Masson’s trichrome stain, and Perls’ stains were done to assess fibrosis and iron deposition, respectively. Liver biopsies were assessed by an independent hepatopathologist for adequacy of biopsy. Each liver tissue was assessed according to Russo et al. [25] scoring system for the presence of the followings: visible bile plugs, portal tract edema, portal tract inflammation, bile duct proliferation, and periductal neutrophilic infiltration. Lobular necroinflammation, giant cell transformation, hepatocyte swelling, extramedullary hematopoiesis, canalicular cholestasis, and fatty infiltration were also assessed.
Statistical analysis
Data analysis was performed using SPSS software for Windows version 20.0 (Armonk, NY: IBM Corp). Differences between patients and controls were evaluated using Student’s unpaired t test (for continuous variables) and the chi-square (χ2) test (for categorical variables). The Mann–Whitney U test is applied for statistical comparison between two sets of data if one or both have a skewed distribution. Comparison between more than 2 patient groups for non-parametric data is done using the Kruskall-Wallis test. Fisher’s exact and Monte Carlo are correct for chi-square when more than 20% of the cells have an expected count of less than 5. PCA was used as a multivariate technique for analysis in metabolomics to detect the metabolites that augment the potential of the metabolic biomarkers analyzed in the biological fluids. ROC curves were constructed based on the data of PCA for differentiation between BA and non-BA infants.
Results
The patient’s data were retrospectively evaluated for their ability to distinguish BA from other causes of cholestasis and the laboratory and diagnostic workflow were performed (Table 1), which revealed that there was no significant difference between the cholestatic BA infants and the cholestatic non-BA, regarding the serum total bile acids and total bilirubin while, there was a highly significant difference regarding GGT (p <0.001). On ultrasound examination, 36 cases in the BA group showed small, contracted, or nonvisualized gallbladder versus 32 cases of cholestatic non-BA (p <0.001). Bile duct and canalicular plugs, periductal neutrophilic infiltrate, ductular proliferation, and mononuclear infiltrate in the duct were significantly observed in BA cases compared to non-BA atresia cases (p <0.001, for all). The presence of significant portal tract edema, marked fibrosis, and inflammation was significantly found in BA cases compared to non-BA atresia cases (p < 0.001, p < 0.001, and p = 0.004, respectively). On the other hand, hepatocyte swelling, prominent pseudo-rosette formation, hepatocyte multinucleated giant cells, and extramedullary hematopoiesis were significantly observed in non-BA compared to BA cases (p = 0.009, p < 0.001, p 0.002, and p = 0.018, respectively) (Table 2) (Fig. 1).
Histopathological characteristics of biliary atresia versus non-biliary atresia cholestatic diseases. a A case of biliary atresia showing marked portal tract expansion by edema (H&E 100×) and fibrosis (incite, Masson trichrome 40×). b A case of biliary atresia showing pathognomic intraluminal bile plugs (arrows) (H&E 200×). c A case of biliary atresia showing bile ductular proliferation (arrows) (H&E 200×). d A case of giant cell hepatitis showing numerous giant cell transformations (arrows) (H&E 200×). e A case of Alagille syndrome showing lacks of the main bile duct branch, not accompanying the artery (X mark) (H&E 200×). f A case of progressive familial intrahepatic cholestasis showing prominent hepatic rosetting with cholestatic rosettes formation (arrows) (H&E 200×)
Remarkably, two non-BA infants underwent intraoperative cholangiogram despite their preoperative workup. Together, these data highlighted the challenge of differentiating BA from non-BA without operative intervention.
Bile acid profiling in cholestatic BA, cholestatic non-BA infants, and healthy control
The serum and urinary primary conjugated bile acids were significantly elevated (p < 0.001) in both BA and non-BA groups compared with control samples (182.25 ± 103.4 and 127.93 ± 109.95 versus 3.30 ± 2.36 μmol/L and 51.44 ± 49.69 and 29.27 ± 29.92 versus1.20 ± 1.54 μmol/L). There was a significant difference between the cholestatic biliary atresia subgroup and control regarding serum primary unconjugated bile acids (p < 0.036). Furthermore, there were significant differences between the cholestatic BA subgroup and the cholestatic non-BA subgroup regarding serum and urinary secondary conjugated bile acids (p < 0.001 for both) with no difference in serum or urinary levels of primary (unconjugated and conjugated) and secondary unconjugated bile acids (Table 3).
On analysis of individual bile acid concentrations, there were significant differences between cholestatic BA and non-BA regarding serum levels of (GCDCA, TCDCA, TCA, and GCDCA/CDCA ratio), while there was no significant difference regarding serum level of CA, CDCA, GCA, CDCA TCDCA/CDCA ratio or urinary CA, CDCA, GCDCA, TCDCA, TCA (Tables 4 and 5). Moreover, there was no statistically significant difference regarding serum or urinary levels of both conjugated and unconjugated secondary individual bile acids between the two patients’ groups (Tables 6 and 7).
Principle component analysis (PCA)
PCA of the metabolic profiling of serum individual bile acids of the two cholestatic groups (BA and non-BA) showed that out of all the extracted PCs, the first two PCs (PC1, PC2) had eigenvalues larger than one (eigenvalue >1) with a value 2.803 and 1.652, respectively (Table 8). The serum bile acids possessing positive loading factors in the first two extracted PCs with contribution percent to the total variance (20.02% and 11.81%, respectively) were CA, GCA, GCDCA, TCA, and TCDCA for PC1 and CA, CDCA, LCA, and UDCA for PC2.
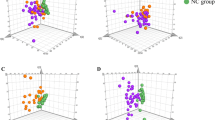
- Then, the ROC analysis was performed using score plots of the first two principal components for visualization of the data and establishing the differences between the two diseased groups; the area under the curve (AUC) for PC1 was 0.770 and for PC2 was .583 (Figs. 2 and 3).
The non-BA cholestatic infants were followed up by the pediatrician and were diagnosed furthermore as following infection (n=20), PFIC (n=16), inspissated bile syndrome (n=9), patients with metabolic causes (n=7), patients with Down syndrome (n=3), with endocrinal causes (n=2), AGS syndrome (n=3), one patient for each of ARC syndrome, giant cell hepatitis, acute liver failure, hepatic focal lesion, and choledochal cyst.
Discussion
Since the Kasai hepatoportoenterostomy had evolved over time, a growing number of studies have shown that the younger the age at Kasai, the better the native liver survival and overall BA survival [19, 26]. However, distinguishing BA from other causes of cholestatic jaundice remains difficult due to the limitations of traditional methods, and numerous noninvasive tests that have been developed to aid in the diagnosis. Growing evidence implies that metabolite profiling may be beneficial in identifying diagnostic biomarkers in BA [27]. The changes in the serum and urinary bile acid levels in the cholestatic patients compared to the control suggest different disturbances in metabolism [28].
This study assessed the serum total bile acid (enzymatically) and the metabolic profile of 14 bile acids in cholestasis infants for differential diagnosis of the BA group from the non-BA group. There was no statistically significant difference regarding serum total bile acid in BA and non-BA patients (p = 0.549 and 0.158), respectively as it was reported by Golden et al. [29] and Zhao et al. [8].
Using the breakthrough in the technology, particularly tandem mass spectrometry, Zhou et al. [30] and Mushtaq et al. [31] hypothesized that the individual bile acids were higher in BA when compared to other types of cholestatic hepatobiliary disorders or neonatal jaundice [30]. In this regard, the present study showed that there were significant differences regarding the conjugated bile acids (TCA, TCDCA, GCDCA, and GCDCA/CDCA) while no significant differences were reported regarding secondary bile acids between cholestatic BA and cholestatic non-BA. Similarly, Golden et al. [29] reported that there was a significant difference in serum primary BAs (TCDCA and GCDCA) between BA and cholestatic non-BA in infants. These results revealed that biliary obstruction causes a drastic buildup of primary individual bile acids [16]. Such increases were formerly reported in other cholestatic situations, such as intrahepatic cholestasis of pregnancy [32], supporting the possible role of primary bile acids as a biomarker for altered biliary circulation [16].
The enterohepatic circulation was significantly blocked in BA, and only a minimal amount of bile acids reached the colon, causing a great reduction of CA and CDCA in plasma. As the block of enterohepatic circulation, conjugated BAs (TCDCA, GCDCA, TCA, and GCA) in the liver were considerably elevated and eliminated via alternative export systems at the hepatic sinusoidal membrane which cause increased conjugated bile acid levels in plasma [33].
Furthermore, Zhao et al. [8] reported that levels of CA and CDCA were significantly lower in cholestatic infants than in control. While CDCA level was significantly lower in the BA group than the non-BA group because of the more severe fibrotic or cirrhotic changes. CDCA is hydrophilic bile acid and supposed to provide a hepatoprotective function which impaired due to cirrhosis [8].
Accordingly, Zhou et al. [34] studied plasma bile acid profiles in BA and neonatal hepatitis syndrome and reported that unconjugated bile acids (CA and CDCA) were significantly decreased in BA and neonatal hepatitis than in control.
In contrast to primary bile acids, secondary species are not at comparable levels in serum samples, and their relative abundance was insignificant between the two cholestatic subgroups. These findings were due to the absence of the interaction of the intestinal bacteria with primary bile acids in BA, which decrease the levels of deconjugated and secondary BAs [29].
Fortunately, the mean level of primary conjugated bile acids was higher than the primary unconjugated, as conjugation results in less toxic and more water-soluble bile acids, thus protecting against cellular damage from such toxic compounds that initiate oxidative stress and cell death signaling [35].
TCDCA and GCDCA are generated by taurine and glycine conjugation of CDCA in the liver. As the higher (TCDCA and GCDCA levels) and lower CDCA levels in BA, the ratio of TCDCA: CDCA and GCDCA: CDCA could be used to compare BA with other cholestatic diseases [8, 30]. In this regard, the present study reported that the ratio of GCDCA: CDCA was significantly higher in BA than in non-BA. However, there was no significant difference between them regarding TCDCA: CDCA ratio. Similarly, Zhao et al. [8] reported that GCDCA: CDCA ratio was significantly higher in BA infants than in non-BA infants. Accordingly, Zhou et al. [34] reported that TCDCA: CDCA ratio was significantly higher in BA infants than in neonatal hepatitis.
In addition to inhibition of CYP7A1, biliary obstruction also down-regulates CYP8B1 expression in the liver [36]. This gene encodes sterol 12α-hydroxylase, a main pathway in the bile biosynthesis favoring synthesis of other primary bile acids instead of CDCA synthesis, and thus determines the TCDCA to CDCA and GCDCA to CDCA ratios [16].
Regarding urinary individual bile acids, the present study revealed that the most abundant bile acid species in cholestatic patients compared to control were TCA, TCDCA, GCA, and GCDCA but at a lower level than serum with no significant difference between BA and non-BA subgroups. These results were in line with Golden et al. [29] who reported that bile acids in the urine were predominantly conjugated with no significant difference between cholestatic non-BA and BA.
Suzuki et al. [37] had previously investigated urinary bile acid metabolites as a possible screening tool without any conclusive results; however, in a study performed by Nanashima et al. [38], they reported that urinary sulfated bile acids increased in patients with cholestasis. Another study done by Trottier et al. [39] had revealed that biliary obstruction changes differentially the circulating and/or urinary levels of the various bile acids.
To display and explore analysis purposes for variables of BAs in the metabolome, modern chemometric tools were used such as principal component analysis (PCA), as simultaneous comparisons of a large number of complex objects were facilitated by reducing the dimensionality of the data set. The resulting data were displayed as score plots which are generally reflected by the first two principal components, representing the distribution of samples in multivariate space [40]. Accordingly, the present study revealed that the identified serum BAs possessing positive loading factors in the first two extracted PCs were CA, GCA, GCDCA, TCA, and TCDCA for PC1 and CA, CDCA, LCA, and UDCA for PC2.
The AUC is a direct indication of the efficiency and clinical applicability of the diagnostic method [41]. According to the previous data, the current study showed that the ROC curves had varied diagnostic powers for the significant metabolites in the first two components between the two cholestatic groups as the AUC of PC1 was 0.770 while it was for PC2 0.583. These results indicated that the positive components of PC1 had the highest score and deemed as “potential marker metabolites” for differentiation between the two cholestatic groups
This study had some limitations such as the variable pathologies of the cholestatic non-BA patients, which may clarify the wide variability in the concentrations of urine, and serum bile acids in this study in addition to, the difficult subgroups analysis due to the small sample size, although they may provide valuable information that can be potentially a useful diagnostic tool for understanding the mechanism of the disease.
Conclusion
Assessing the serum BA levels of CA, GCA, GCDCA, TCA, and TCDCA collectively using tandem mass spectrometry may be helpful in differentiating the cholestatic biliary atresia from cholestatic non-biliary atresia infants with avoidance of unnecessary invasive procedures. This might provide new insights into the biochemical changes in cholestasis.
Availability of data and materials
All data generated or analyzed during this study are included in this article.
Abbreviations
- ARC:
-
Arthogryposis—renal dysfunction cholestasis
- AGS:
-
Alagille syndrome
- AUC:
-
Area under the curve
- BA:
-
Biliary atresia
- CA:
-
Cholic acids
- CDCA:
-
Chenodeoxycholic acid
- CMV:
-
Cytomegalovirus
- DCA:
-
Deoxycholic acid
- GCA:
-
Glycocholic acid
- GCDCA:
-
Glycochenodeoxycholic acid
- GUDCA:
-
Glycoursodeoxycholic acid
- HSV:
-
Herpes simplex virus
- IgG:
-
Immunoglobulin G
- IgM:
-
Immunoglobulin M
- IOC:
-
Intraoperative cholangiogram
- KPE:
-
Kasi portoenterostomy
- LC-MS:
-
Liquid chromatography-mass spectroscopy
- LCA:
-
Lithocholic acid
- MS/MS:
-
Tandem mass spectrometry
- NC:
-
Neonatal cholestasis
- Non-BA:
-
Non-biliary atresia
- PCA:
-
Principal component analysis
- PFIC:
-
Progressive familial intrahepatic cholestasis
- ROC curve:
-
Receiver operating characteristic
- TCA:
-
Taurocholic acid
- TCDCA:
-
Taurochenodeoxycholic acid
- TDCA:
-
Taurodeoxycholic acid
- TLCA:
-
Taurolithocholic acid
- UDCA:
-
Ursodeoxycholic acid
- GGT:
-
Gamma glutamyl transaminase
References
Fernando M, Ra**drajith S (2020) Neonatal and infantile cholestasis: an overlooked health burden with unmet needs. Indian J Gastroenterol 39(6):531–538
Götze T, Blessing H, Grillhösl C, Gerner P, Hoerning A (2015) Neonatal cholestasis–differential diagnoses, current diagnostic procedures, and treatment. Front Pediatr 3:43
Kaur J, Wadhwa N (2019) Neonatal cholestasis revisited! Curr Med Res Pract 9(1):18–24
Vij M, Rela M (2020) Biliary atresia: pathology, etiology and pathogenesis. Fut Sci OA 6(5):FSO466
Ghazy RM, Adawy NM, Khedr MA, Tahoun MM (2018) Biliary atresia recent insight. Egyptian Pediatric Association. Gazette. 66(1):1–8.6
César MAJ, Misael PP, Amaya S, Alejandro M, Orozco B, Marina E et al (2020) A systematic review of etiology, pathogenesis, diagnosis and treatment. 1(3):43–49
Khayat A, Alamri AM, Saadah OI (2021) Outcomes of late Kasai portoenterostomy in biliary atresia: a single-center experience. J Int Med Res 49(5):1–13
Zhao D, Zhou K, Chen Y, **e W, Zhang Y (2020) Development and validation of bile acid profile-based scoring system for identification of biliary atresia: a prospective study. BMC Pediatr 20(1):1–9
Li T, Chiang JY (2015) Bile acids as metabolic regulators. Curr Opin Gastroenterol 2:159
Ferrell JM, Chiang JY (2021) Bile acid receptors and signaling crosstalk in the liver, gut and brain. Liver Res 5(3):105–118
Chiang JYL (2017) Bile acid metabolism and signaling in liver disease and therapy. Liver Res 1(1):3–9
Vaz FM, Ferdinandusse S (2017) Bile acid analysis in human disorders of bile acid biosynthesis. Mole Aspects Med 56:10–24
Ticho AL, Malhotra P, Dudeja PK, Gill RK, Alrefai WA (2019) Intestinal absorption of bile acids in health and disease. Comprehens Physiol 10(1):21
Li Y, Lu LG (2018) Therapeutic roles of bile acid signaling in chronic liver diseases Journal of Clinical and Translational. Hepatology 6(4):425–430
Legido-Quigley C, McDermott L, Vilca-Melendez H, Murphy GM, Heaton N, Lindon JC et al (2011) Bile UPLC-MS fingerprinting and bile acid fluxes during human liver transplantation. Electrophoresis 32(15):2063–2070
Zhao H, Wang C, Zhao N, Li W, Yang Z, Liu X, Le W, Zhang X (2018) Potential biomarkers of Parkinson’s disease revealed by plasma metabolic profiling. J Chromatography B. 1081:101–108
Li T, Apte U (2015) Bile acid metabolism and signaling in cholestasis, inflammation, and cancer. Adv Pharmacol 74:263–302
Abdelsattar S, Kasemy ZA, Elsayed M, Elrahem TA, Zewain SK (2021) Targeted metabolomics as a tool for the diagnosis of kidney disease in type II diabetes mellitus. Bri J Biomed Sci:1–7
Zhang A, Sun H, Yan G, Wang P, Wang X (2015) Metabolomics for biomarker discovery: moving to the clinic. Biomed Res Int 2015
Amplatz B, Zöhrer E, Haas C, Schäffer M, Stojakovic T, Jahnel J et al (2017) Bile acid preparation and comprehensive analysis by high performance liquid chromatography–high-resolution mass spectrometry. Clin Chim Acta 464:85–92
Yang C, Chen C, Chou Y, Chiu H, Tsai W, Shiesh S (2017) Bile acid profiles in neonatal intrahepatic cholestasis caused by citrin deficiency. Clin Chim Acta 475:28–35
Fu X, **ao Y, Golden J, Niu S, Gayer CP (2020) Serum bile acids profiling by liquid chromatography-tandem mass spectrometry (LC-MS/MS) and its application on pediatric liver and intestinal diseases. Clin Chem Lab Med (CCLM). 58(5):787–797
Feldman AG, Sokol RJ (2019) Neonatal cholestasis: emerging molecular diagnostics and potential novel therapeutics. Nat Rev Gastroenterol Hepatol 16(6):346–360
Sugita T, Amano K, Nakano M, Masubuchi N, Sugihara M, Matsuura T (2015) Analysis of the serum bile acid composition for differential diagnosis in patients with liver disease. Gastroenterol Res Pract 2015
Russo P, Magee JC, Boitnott J, Bove KE, Raghunathan T, Finegold M et al (2011) Design and validation of the biliary atresia research consortium histologic assessment system for cholestasis in infancy. Clin Gastroenterol Hepatol 9(4):357–362
Jimenez-Rivera C, Jolin-Dahel KS, Fortinsky KJ, Gozdyra P, Benchimol EI (2013) International incidence and outcomes of biliary atresia. J Pediatr Gastroenterol Nutr 56(4):344–354
Zhang A, Sun H, Wang X (2012) Serum metabolomics as a novel diagnostic approach for disease: a systematic review. Anal Bioanal Chem 404(4):1239–1245
Kriegermeier A, Green R (2020) Pediatric cholestatic liver disease: review of bile acid metabolism and discussion of current and emerging therapies. Front Med 7:149–149
Golden J, Zagory JA, Fenlon M, Goodhue CJ, **ao Y, Fu X et al (2018) Liquid chromatography–mass spectroscopy in the diagnosis of biliary atresia in children with hyperbilirubinemia. J Surg Res 228:228–237
Zhou K, Lin N, **ao Y, Wang Y, Wen J, Zou G (2012) Elevated bile acids in newborns with Biliary Atresia (BA). PLoS One 7(11):e49270
Mushtaq I, Logan S, Morris M, Johnson AW, Wade AM, Kelly D et al (1999) Screening of newborn infants for cholestatic hepatobiliary disease with tandem mass spectrometry commentary: what is tandem mass spectrometry? Bmj 319(7208):471–477
Tribe RM, Dann AT, Kenyon AP, Seed P, Shennan AH, Mallet A (2010) Longitudinal profiles of 15 serum bile acids in patients with intrahepatic cholestasis of pregnancy. Offic J Am College Gastroenterol| ACG 105(3):585–595
Arab JP, Cabrera D, Arrese M (2018) Bile acids in cholestasis and its treatment. Ann Hepatol 16(1):53–57
Zhou K, Wang J, **e G, Zhou Y, Yan W, Pan W et al (2015) Distinct plasma bile acid profiles of biliary atresia and neonatal hepatitis syndrome. J Proteome Res 14(11):4844–4850
Luo L, Aubrecht J, Li D, Warner RL, Johnson KJ, Kenny J et al (2018) Assessment of serum bile acid profiles as biomarkers of liver injury and liver disease in humans. PLoS One 13(3):e0193824
Schaap FG et al (2009) High expression of the bile salt-homeostatic hormone fibroblast growth factor 19 in the liver of patients with extrahepatic cholestasis. Hepatology 49(4):1228–1235
Suzuki M, Muraji T, Obatake M, Nio M, Ito K, Suzuki K et al (2011) Urinary sulfated bile acid analysis for the early detection of biliary atresia in infants. Pediatr Int 53(4):497–500
Nanashima A, Obatake M, Sumida Y, Abo T, Yamane Y, Nomura M et al (2009) Clinical significance of measuring urinary sulfated bile acids in adult patients with hepatobiliary diseases. Hepato Gastroenterol 56(90):299–302
Trottier J, Białek A, Caron P, Straka RJ, Milkiewicz P (2011) Profiling circulating and urinary bile acids in patients with biliary obstruction before and after biliary stenting. PloS One 6(7):e22094
** B, Gu H, Baniasadi H (2014) Raftery D: Statistical analysis and modeling of mass spectrometry-based metabolomics data. In: mass spectrometry in metabolomics. Springer, pp 333–353
**a J, Broadhurst DI, Wilson M, Wishart DS (2013) Translational biomarker discovery in clinical metabolomics: an introductory tutorial. Metabolomics 9(2):280–299
Acknowledgements
The authors would like to express their sincere appreciation to all the chemists of the Clinical Biochemistry and Molecular Diagnostics Department, National Liver Institute, for their help to the laboratory tests and to the guardians of the participant patients for their contribution to this work.
Funding
This research was performed using the instruments supplied by a grant from the Science and Technology Development Fund (STDF) (N2338).
Author information
Authors and Affiliations
Contributions
SAE, SA, and DS were the major contributor in performing the practical section of this work and the interpretation of the laboratory data. They also contributed to the writing of the manuscript. SAA and SAE performed full clinical examination (general and hepatological), complete history taking regarding nutrition and growth charts with stress on birth date, sex, gestational age, antenatal, prenatal history, consanguinity, pedigree construction, recording of previous neonatal deaths, and similar affected cases within the family. DS had performed the pathological section of the study. SAA contributed to the interpretation of the clinical data and supervision of the final diagnosis of the cases. HE and AE contributed to the conception, design, and revision of the work. The authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approval was obtained from the Ethics Committee of the National Liver Institute, Menoufia University (00134/2018 INTM). Informed written consent was obtained from the guardians of participants before enrollment in the study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eshiaty, S.A., Abdelsattar, S., Sweed, D. et al. The value of blood and urine metabolomics in differential diagnosis of cholestasis in infants. Egypt Liver Journal 13, 6 (2023). https://doi.org/10.1186/s43066-023-00244-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43066-023-00244-0