Abstract
Background
Heat stress (HS) has negative effects on poultry productivity, health and welfare resulting in economic losses. Broiler chickens are particularly susceptible to HS due to their high metabolic rate and rapid growth. The commensal intestinal bacterial populations have an important physiological role in the host and could ameliorate the negative effect of HS on the host. Thus, the aim of this study was to compare changes in the ileal (IL) microbiota in four different broiler lines during HS.
Results
Day-old broiler chicks from Giant Jungle Fowl (JF), Athens Canadian Random Bred (ACRB), 1995 Random Bred (L1995), and Modern Random Bred (L2015) lines were raised under thermoneutral (TN) conditions until day (d) 28. On d 29 birds were subjected to TN (24 °C) or chronic cyclic HS (8 h/d, 36 °C) condition till d 56. On d 56 two birds per pen were euthanized, and IL luminal content (IL-L) and mucosal scra**s (IL-M) were collected for bacterial DNA isolation. Libraries were constructed using V3–V4 16S rRNA primers and sequenced using MiSeq. DNA sequences were analyzed using QIIME2 platform and SILVA 132 database for alpha and beta diversity, and taxonomic composition, respectively. Functional property of microbiota was predicted using the PICRUSt 2 pipeline and illustrated with STAMP software. Shannon index was significantly elevated in IL-M under HS. β-diversity PCoA plots revealed separation of microbial community of L2015-TN from JF-TN, JF-HS, ACRB-TN, and ACRB-HS in the IL-M. PERMANOVA analysis showed a significant difference between microbial community of L1995-HS compared to ACRB-HS and JF-TN, L1995-TN compared to ACRB-HS and JF-TN, L2015-HS compared to ACRB-HS and ACRB-TN, L2015-HS compared to JF-TN, L2015-TN compared to ACRB-HS and JF-TN, and ACRB-HS compared to JF-TN in the IL-L. The impact of HS on microbial composition of IL-M was more prominent compared to IL-L with 12 and 2 taxa showing significantly different relative abundance, respectively. Furthermore, differences in microbiota due to the genetic line were more prominent in IL-M than IL-L with 18 and 8 taxa showing significantly different relative abundance, respectively. Unlike taxonomy, predicted function of microbiota was not affected by HS. Comparison of L2015 with JF or ACRB showed significant changes in predicted function of microbiota in both, IL-M and IL-L. Differences were most prominent between L2015 and JF; while there was no difference between L2015 and L1995.
Conclusions
These data indicate the genetic line × temperature effect on the diversity and composition of IL microbiota. Moreover, the data showcase the effect of host genetics on the composition of IL microbiota and their predicted function. These data are of critical importance for devising nutritional strategies to maintain GIT microbial balance and alleviate the negative effects of HS on broiler chickens’ performance and health.
Similar content being viewed by others
Background
Selection for high growth rate and feed efficiency over the past 80 years has made phenomenal progress in terms of breast yield, feed efficiency, and reduction of market age [1, 2] in meat-type chickens (broilers). However, modern broilers are more susceptible to environmental or bacterial challenges and cannot maintain superb performance under these challenging conditions and the negative effects of challenges on modern birds are more significant [2, 3].
Heat stress (HS) is an environmental challenge that threatens all animal and plant species [4]. Avian species, due to the unique physiology, such as higher body temperature and feathers, are more susceptible to the negative consequences of HS [5]. During the HS, core body temperature increases in broiler chickens [6], and HS negatively affects broilers performance, and GIT integrity and provokes immune responses [6, 7].
Gastrointestinal tract (GIT) microbiota has an undeniable role in performance, GIT health and development of host immune responses [8,9,10,11,12,13]. The impact of GIT microbiota on the host could be via direct host-microbe interaction or indirectly through microbial metabolites. Challenging conditions, such as HS, affects community composition and function of GIT microbiota [6, 14] and leads to the dysbiosis of GIT microbial community [8, 15]. Thus, dysbiosis of GIT microbiota during HS might contribute to poor performance, and disruption of GIT integrity.
The impact of HS on GIT microbial composition and function is not fully understood. In addition, there is no information regarding the consequences of genetic selection on broilers GIT microbiota. Therefore, we hypothesized that the susceptibility of modern broiler chickens to HS compared to their ancestors reported previously [3], might be related to their microbial community composition and function. Accordingly, the current experiment was conducted to evaluate the effect of HS and genetic selection on the ileal (IL) microbiota of broiler chickens.
Methods
Birds, diets, and management
The study was conducted in accordance with the recommendations in the guide for the care and use of laboratory animals of the National Institutes of Health and the protocols were approved by the University of Arkansas Animal Care and Use Committee under protocols 18,083 and 16,084. All methods were carried out in accordance with relevant guidelines and regulations. This study was performed and reported in accordance with ARRIVE guidelines (https://arriveguidelines.org/). Embryonated eggs from four lines, including Giant Jungle Fowl (JF), Athens Canadian Random Bred (ACRB), 1995 Arkansas Random Bred (L1995), and Modern Random Bred (L2015) were incubated together. Embryonated eggs were incubated in a Jamesway multi-stage incubator from embryonic d 0–18 at 37.6 °C dry bulb and 29.4 °C wet bulb. At embryonic d 18, eggs were assessed for fertility and fertile eggs were transferred by line to a single hatcher unit from embryonic d 18 to hatch (d 21) with temperature set at 36.7 °C dry bulb and 28.9 °C wet bulb. These conditions are close to ideal for the 1995 and 2015 bird, however the ACRB birds were typically incubated at a warmer temperature. Detailed characteristics of the lines were reported previously [3]. The study was conducted as a 4 × 2 factorial arrangement with 4 lines and 2 environmental conditions as main factors. Day-old broiler chicks from the four chicken lines were hatched at the University of Arkansas and vent-sexed. Males were individually wing-banded with a number and barcode, and housed in environmentally controlled chambers in the Poultry Environmental Research Laboratory (University of Arkansas). Chicks were separated by line and placed into twelve environmental chambers with each chamber consisting of two equally sized pens allowing for 24 pens total (6 pens per line). Twenty-five male chicks of the same line were randomly placed in each pen and kept at an approximate density of one bird per 0.5 m2 in all pens. The stocking density in this study was kept low enough for all lines at placement so that stocking density at older ages would have a minimal impact on the HS experience. All lines were allowed ample floor space, far below typical industry stocking densities at older ages. All birds had ad libitum access to commercially available standard corn-soybean meal diet and water. During the first week, birds were provided with a 23 h light/1 h dark lighting program and a 20 h light/4 h dark lighting program was used from day (d) 8–56. Rearing temperature gradually decreased from 32 °C (d 1–3) to 31 °C (d 4–6), 29 °C (d 7–10), 27 °C (d 11–14), and 24 °C from d 15 to 28. From d 29 to 42, half of the pens for each line (3 pens) were raised under TN condition; while the rest of the pens (3 per line) were subjected to chronic cyclic HS (8 h/d, 36 °C). For the chronic cyclic HS groups, from d 29 to 56, the temperature was increased to 36 °C at 9 AM and decreased to 24 °C at 5 PM.
16S rRNA gene sequencing and bioinformatics
On d 56, two birds per replicate were selected based on the average pen weight, euthanized by cervical dislocation, and luminal content (L) and mucosal scra**s (M) were collected from IL. Bacterial DNA was isolated using PowerSoil kit (Qiagen, Valencia, CA) and a QIAcube instrument (Qiagen) as per manufacturer’s protocol. DNA concentration was measured by NanoDrop (TermoFisher Scientific, Inc., Waltham, MA), and DNA quality was assessed by TapeStation System (Agilent Technologies, Santa Clara, CA). 16S rRNA gene amplicon libraries were generated following the 16S Metagenomic Sequencing Library Preparation workflow from Illumina (Illumina, Inc., San Diego, CA) using PCR primers targeting the variable V3–V4 region of the 16S rRNA gene. Concentration and quality of the amplicons were determined using QIAxcel DNA Hi Resolution cartridge, QIAxcel ScreenGel 1.6.0 software, and QIAxcel Advanced System (Qiagen) per manufacturing instructions. The pooled DNA library was diluted to a final concentration of 4 pM and mixed with PhiX (Illumina, Inc., 4 nmol) control (20% v/v) and pair-end 2 × 300 bp sequenced using the Illumina MiSeq platform and a MiSeq Reagent Kit v3 (Illumina, Inc). The 16S rRNA gene sequences determined in this study were deposited in the NCBI Sequence Read Archive database (SRA accession # PRJNA794190).
Quantitative Insight Into Microbial Ecology (QIIME) software package 2 (version 2021.8 or higher, http://qiime2.org) [16] was used to perform quality control and analysis of the sequence reads. Raw fastq files were demultiplexed using q2-demux and quality filtered and dereplicated with q2-dada2. Sequences with average Phred score lower than 19 were removed. Taxonomy was assigned to the amplicon sequence variants (ASVs) using the q2-feature classifier classify-sklearn naïve Bayes taxonomy classifier against the SILVA database version 132 (https://www.arb-silva.de/download/archive/qiime/). MAFFT were used for multiple ASVs sequence alignment via q2‐alignment and used to construct a phylogeny with fasttree2 via q2-phylogeny. Samples of IL-L and IL-M were rarefied to 5650 and 1335 sequences, respectively for alpha- and beta-diversity analysis. Alpha-diversity was measured using the Shannon (H), Simpson, and Chao 1 indices. Differences between alpha-diversity indices were tested using the Kruskal–Wallis test (QIIME 2). To estimate the similarity of microbial community structure between groups (beta-diversity), principal coordinate analysis (PCoA) based on Weighted UniFrac distance matrix were performed (QIIME2). To assess the association between microbial community and treatments, pairwise PERMANOVA analysis implemented in QIIME2 was performed on a Weighted UniFrac distance matrix of 48 samples. The significance of PERMANOVA was obtained by 999 permutation tests.
Relative abundance of taxa for each line and temperature were calculated at the phylum and genus levels. Relative abundance data were obtained by normalization of the phylum and genus composition to the total number of reads in each sample and were analyzed using ANOVA procedure (JMP Pro 2016) and significance between treatments were determined using LSD test (P < 0.05). In order to predict the function of IL-L and IL-M microbiota, data analysis was performed through the Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt)2 pipeline [17]. Then, PICRUST output for the level 3 of the Kyoto Encyclopedia of Genes and Genomes (KEGG) [18] were analyzed and illustrated with Statistical Analysis of Metagenomic Profiles (STAMP) software version 2.1.3 [19]. Within STAMP analysis, ANOVA followed by two group comparison was performed using Welsh t-test with Benjamini–Hochber false discovery rate (FDR) analysis [20].
Results
Ileal luminal content
Alpha-diversity indices are shown in Fig. 1, and Additional file 1: Table S1. Shannon and Chao 1 indices showed that there was no significant interaction between temperature and genetic line on richness or evenness of IL-L microbial community. There were no significant differences in bacterial diversity (Shannon and Chao 1 indices) among genetic lines (Fig. 1A, and Additional file 1: Table S1), while HS birds had higher Shannon and Chao 1 indices in comparison to TN birds (Fig. 1B, and Additional file 1: Table S1). Simpson index showed that neither was there a significant interaction between temperature and genetic line, nor was there a significant effect of the main factors on richness or evenness of IL-L microbial community (data not shown). β-diversity PCoA plots revealed no separation of microbial communities due to genetic line × temperature interaction (Fig. 2). PERMANOVA analysis showed a significant (P = 0.001) genetic line × temperature interaction (Additional file 1: Table S2). There was a significant difference in β-diversity between microbial community of L1995-HS compared to ACRB-HS and JF-TN, L1995-TN compared to ACRB-HS and JF-TN, L2015-HS compared to ACRB-HS and ACRB-TN, L2015-HS compared to JF-TN, L2015-TN compared to ACRB-HS and JF-TN, and ACRB-HS compared to JF-TN.
Alpha diversity in the ileal content of Jungle Fowl (JF), Athens Canadian Random Bred (ACRB), 1995 Random Bred (L1995), and 2015 Modern Random Bred (L2015) chicken lines raised under thermoneutral (TN) or heat stress (HS) condition (8 h/d; 36 °C) on d 56. A Shanon’s diversity index for genetic lines. B Shanon’s diversity index for ambient temperature. Boxplots show the quartiles, median, and extremities of the values
Beta diversity in the ileal content of Jungle Fowl (JF), Athens Canadian Random Bred (ACRB), 1995 Random Bred (L1995), and 2015 Modern Random Bred (L2015) chicken lines raised under thermoneutral (TN) or heat stress (HS) condition (8 h/d; 36 °C) on d 56. 2D principal coordinate analysis (PCoA) plot is based on weighted UniFrac distance matrix. Each sphere represents a sample
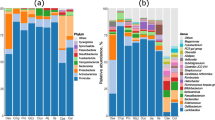
The relative abundance of taxa at the phylum level is shown in Fig. 3A with Firmicutes, Proteobacteria, and Actinobacteria as the top 3 phyla. There was no effect of genetic line × temperature interaction on relative abundance of taxa at the phylum level. However, relative abundance of Proteobacteria significantly increased due to the HS (Fig. 3B). Changes in relative abundance of the top 20 genera are shown in Fig. 4A with Lactobacillus, Enterococcus, and Candidatus Arthromitus as the top 3 genera. There was no effect of genetic line × temperature on the relative abundance of genera. However, the relative abundance was affected by genetic line and HS (Fig. 4B–K). Relative abundance of Blautia was significantly increased due to HS (Fig. 4B).
Changes in relative bacterial abundance (%) in the ileal content of Jungle Fowl (JF), Athens Canadian Random Bred (ACRB), 1995 Random Bred (L1995), and 2015 Modern Random Bred (L2015) chicken lines raised under thermoneutral (TN) or heat stress (HS) condition (8 h/d; 36 °C) on d 56 at phylum level. A Taxonomic profile of ileal content microbiota. B Effect of ambient temperature on the relative abundance of Proteobacteria. Different letters denote statistically significant (P < 0.05) differences
Changes in relative bacterial abundance (%) in the ileal content of Jungle Fowl (JF), Athens Canadian Random Bred (ACRB), 1995 Random Bred (L1995), and 2015 Modern Random Bred (L2015) chicken lines raised under thermoneutral (TN) or heat stress (HS) condition (8 h/d; 36 °C) on d 56 at genus level. A Taxonomic profile (top 20 genera) of ileal content microbiota. B Effect of ambient temperature on the relative abundance of Blautia. Effect of genetic line on relative abundance of C CHKCI001, D Lactococcus, E Staphylococcus, F Weissella, G Brachybacterium, H Candidatus Arthromitus, I Streptococcus, J [Eubacterium] hallii group, and K Rothia. Different letters denote statistically significant (P < 0.05) differences
Relative abundance of Lactococcus (Fig. 4D), Staphylococcus (Fig. 4E), Weissella (Fig. 4F), Brachybacterium (Fig. 4G), Candidatus Arthromitus (Fig. 4H), and Rothia (Fig. 4K) were significantly higher in JF compared to other genetic lines. Relative abundance of CHKCI001 (Fig. 4C), and [Eubacterium] hallii group (Fig. 4J) were significantly higher in ACRB birds compared to other groups. Finally, relative abundance of Streptococcus was significantly higher in L2015 compared to JF and ACRB (Fig. 4I).
The predicted function of the microbiota is shown in Figs. 5 and 6. There was no difference in predicted function of microbiota among TN and HS (data not shown). Furthermore, there was no difference in predicted function of microbiota among L2015 and L1995 (data not shown). There were 20 pathways at KEGG level 3 with distinctive enrichment between L2015 and JF (Fig. 5). There were 18 pathways at KEGG level 3 with distinctive enrichment between L2015 and ACRB (Fig. 6). Among these, the L2015 microbiota had higher numbers of functional genes involved in carbohydrate metabolism including amino sugar and nucleotide sugar metabolism, and inositol phosphate metabolism compared to JF. Compared to ACRB, genes involved in carbohydrate metabolism including amino sugar and nucleotide sugar metabolism, and fructose and mannose metabolism were enriched in L2015. Functional genes involved in xenobiotics biodegradation and metabolism were enriched in L2015 compared to both JF and ACRB. This included higher benzoate degradation, and xylene degradation in L2015 compared to JF, and higher aminobenzoate degradation, limonene and pinene degradation, benzoate degradation, ethylbenzene degradation, and naphthalene degradation compared to ACRB. Interestingly, compared to L2015, functional genes involved in metabolism of cofactors and vitamins were enriched in both JF and ACRB. In comparison with L2015, JF had more genes involved in ubiquinone and other terpenoid-quinone biosynthesis, and retinol metabolism; while, ACRB had higher genes involved in retinol metabolism and thiamine metabolism.
Ileal mucosal scra**s
Alpha-diversity indices are shown in Fig. 7, and Additional file 1: Table S3. Shannon and Simpson indices showed that there was no significant interaction between temperature and genetic line on richness or evenness of IL-L microbial community. There were no significant differences in bacterial diversity (Shannon and Simpson indices) among genetic lines (Fig. 7A, and Additional file 1: Table S3), while HS birds had higher Shannon and Simpson indices in comparison to TN birds (Fig. 7B, and Additional file 1: Table S3). Chao 1 index showed that neither was there a significant interaction between temperature and genetic line, nor was there a significant effect of the main factors on richness or evenness of IL-L microbial community (data not shown). β-diversity PCoA plots revealed separation of microbial communities due to genetic line × temperature interaction (Fig. 8). Microbial community of L2015-TN was separated from JF-TN, JF-HS, ACRB-TN, and ACRB-HS (Fig. 8). PERMANOVA analysis showed a significant (P = 0.041) genetic line × temperature interaction (Additional file 1: Table S4). Diversity of microbial community was significantly different between JF-HS compared to ACRB-TN.
Alpha diversity in the ileal mucosa of Jungle Fowl (JF), Athens Canadian Random Bred (ACRB), 1995 Random Bred (1995), and 2015 Modern Random Bred (2015) chicken lines raised under thermoneutral (TN) or heat stress (HS) condition on d 56. Shanon’s diversity index for genetic lines (A). Shanon’s diversity index for ambient temperature (B). Boxplots show the quartiles, median, and extremities of the values
Beta diversity in the ileal mucosa of Jungle Fowl (JF), Athens Canadian Random Bred (ACRB), 1995 Random Bred (1995), and 2015 Modern Random Bred (2015) chicken lines raised under thermoneutral (TN) or heat stress (HS) condition on d 56. 2D principal coordinate analysis (PCoA) plot is based on weighted UniFrac distance matrix. Each sphere represents a sample
The relative abundance of taxa at the phylum level is shown in Fig. 9A with Firmicutes, Bacteroidetes, and Proteobacteria as the top 3 phyla. There was no effect of genetic line × temperature interaction on relative abundance of taxa at the phylum level. However, relative abundance of Proteobacteria significantly increased due to the HS (Fig. 9B). In addition, L2015 had significantly higher relative abundance of Firmicutes (Fig. 9C) and lower relative abundance of Bacteroidetes (Fig. 9E) compared to both JF and ACRB. Finally, relative abundance of Proteobacteria was significantly higher in ACRB compared to other genetic lines (Fig. 9D). Changes in relative abundance of the top 20 genera are shown in Fig. 10A with Lactobacillus, Bacteroides, and Candidatus Arthromitus as the top 3 genera. The genetic line × temperature interaction effect was significant on relative abundance of Bacillus and Parasutterella (Fig. 10B, C). Under TN conditions, relative abundance of Bacillus was significantly lower in L1995 and L2015 compared to ACRB, while there was no difference among genetic lines under HS (Fig. 10B). Relative abundance of Parasutterella was significantly higher in ACRB-TN compared to JF-TN and L2015-TN, while under HS, relative abundance of Parasutterella was significantly higher in L2015 compared to other genetic lines (Fig. 10C). Relative abundance of 11 genera including Blautia (Fig. 10D), [Ruminococcus] torques group (Fig. 10E), Ruminiclostridium 5 (Fig. 10F), [Eubacterium] hallii group (Fig. 10G), Tyzzerella (Fig. 10H), Christensenellaceae R-7 group (Fig. 10I), Ruminococcaceae UCG-014 (Fig. 10J), Negativibacillus (Fig. 10K), GCA-900066575 (Fig. 10L), CHKCI001 (Fig. 10M), and Bilophila (Fig. 10N) were increased due to HS. There were 15 genera with differential relative abundance among the genetic lines (Fig. 10O–AC). Relative abundance of Lactobacillus was significantly higher in L1995 and L2015 compared to JF and ACRB (Fig. 10P). Besides, relative abundance of Faecalibacterium (Fig. 10O) and Mailhella (Fig. 10AA) were significantly lower in L1995 and L2015 compared to JF and ACRB. Furthermore, relative abundance of Campylobacter (Fig. 10Q), Streptococcus (Fig. 10Y), and Tyzerella 3 (Fig. 10AC) were significantly higher in L2015 compared to other lines. JF had significantly higher relative abundance of Negativibacillus compared to other genetic lines (Fig. 10W). In addition, relative abundance of Ruminococcoceae UCG-014 (Fig. 10U), and Bacteroides (Fig. 10X) were significantly higher in JF compared to L1995 and L2015. Finally, relative abundance of Butyriciococcus was significantly higher in ACRB compared to other genetic lines (Fig. 10Z).
Changes in relative bacterial abundance (%) in the ileal mucosal scra**s of Jungle Fowl (JF), Athens Canadian Random Bred (ACRB), 1995 Random Bred (L1995), and 2015 Modern Random Bred (L2015) chicken lines raised under thermoneutral (TN) or heat stress (HS) condition (8 h/d; 36 °C) on d 56 at phylum level. A Taxonomic profile of ileal content microbiota. B Effect of ambient temperature on the relative abundance of Proteobacteria. Effect of genetic line on relative abundance of C Firmicutes, D Proteobacteria, and E Bacteroidetes. Different letters denote statistically significant (P < 0.05) differences
Changes in relative bacterial abundance (%) in the ileal mucosal scra**s of Jungle Fowl (JF), Athens Canadian Random Bred (ACRB), 1995 Random Bred (L1995), and 2015 Modern Random Bred (L2015) chicken lines raised under thermoneutral (TN) or heat stress (HS) condition (8 h/d; 36 °C) on d 56 at genus level. A Taxonomic profile (top 20 genera) of ileal mucosal scra**s microbiota. Genetic line × temperature interaction effect on B Bacillus, and C Parasutterella. Effect of ambient temperature on the relative abundance of D Blautia, E [Ruminococcus] torques group, F Ruminiclostridium 5, G [Eubacterium] hallii group, H Tyzzerella, I Christensenellaceae R-7 group, J Ruminococcaceae UCG-014, K Negativibacillus, L GCA-900066575, M CHKCI001, and N Bilophila. Effect of genetic line on relative abundance of O Faecalibacterium, P Lactobacillus, Q Campylobacter, R Subdoligranulum, S [Eubacterium] hallii group, T Christensenellaceae R-7 group, U Ruminococcaceae UCG-014, V Sellimonas, W Negativibacillus, X Bacteroides, Y Streptococcus, Z Butyriciococcus, AA Mailhella, AB Helicobacter, and AC Tyzzerella 3. Different letters denote statistically significant (P < 0.05) differences
Predicted Function of the microbiota is shown in Figs. 11 and 12. There was no difference in predicted function of microbiota among TN and HS (data not shown). Furthermore, there was no difference in predicted function of microbiota among L2015 and L1995 (data not shown). There were 76 pathways at KEGG level 3 with distinctive enrichment between L2015 and JF (Fig. 11 and Additional file 1: Fig. S1). There were 58 pathways at KEGG level 3 with distinctive enrichment between L2015 and ACRB (Fig. 12 and Additional file 1: Fig. S2). Among these, the L2015 microbiota had higher numbers of functional genes involved in carbohydrate metabolism including glycolysis/gluconeogenesis, pyruvate metabolism, and propanoate metabolism compared to JF. Compared to ACRB, the L2015 microbiota had higher numbers of functional genes involved in carbohydrate metabolism including glycolysis/gluconeogenesis, pyruvate metabolism, fructose and mannose metabolism, and amino sugar and nucleotide sugar metabolism. Furthermore, functional genes involved in lipid metabolism including synthesis and degradation of ketone bodies, and glycerolipid metabolism were enriched in L2015 compared to both JF and ACRB. Finally, genes involved in xenobiotics biodegradation and metabolism were enriched in L2015 compared to both JF and ACRB.
Compared to L2015, functional genes involved in immune system including NOD-like receptor signaling pathway, and antigen processing and presentation were enriched in both JF and ACRB. Furthermore, functional genes involved in the endocrine system including adipocytokine signaling pathway, and PPAR signaling pathway were enriched in both JF and ACRB in comparison to L2015. Finally, compared to L2015, functional genes involved in amino acid metabolism and metabolism of cofactors and vitamins including arginine and proline metabolism, valine, leucine and isoleucine biosynthesis, pantothenate and CoA biosynthesis, vitamin B6 metabolism, and biotin metabolism were enriched in both JF and ACRB.
Discussion
Understanding the interplay between host and its microbiota is of great importance especially regarding their dynamic in various environmental conditions. To our knowledge, this is the first experiment comparing the genetic line × temperature interaction effect on GIT microbiota in poultry. The effect of high ambient temperature on the alpha-diversity of GIT microbiota depends on the duration and intensity of heat exposure, and GIT segment [21]. In our study, alpha diversity was significantly increased in IL-L and IL-M due to HS, indicating a shift in the GIT microbial community which might be due to the favorable environment for the overgrowth of opportunistic bacteria and partially justify the negative effects of HS on performance and health in L1995 and L2015 as previously reported by our group [3]. Previously, Wang et al. [22] reported higher alpha diversity indices in the ileal content of chickens raised under high ambient temperature compared to TN condition [22]. In this experiment, we indicated separation of microbial community of L2015-TN from JF-TN, JF-HS, ACRB-TN, and ACRB-HS in the IL-M. Similarity of microbial community of L2015-HS with JF and ACRB might be due to reduction in feed intake due to HS and its impact on the diversity of microbial community. Genetic lines such as JF and ACRB have lower feed intake compared to modern commercial strains [3] and feed intake significantly decrease during HS in L2015 and L1995, but not in JF and ACRB [3].
Composition and function of microbial population in various segments of the GIT can influence feed efficiency, although the extent of interplay between the host and microbiome is unclear [23]. Reduction in relative abundance of Bacillus under HS in the IL-M of ACRB, but not other genetic lines could be indicative of higher reliance of this genetic line on Bacillus species for proper physiological function. Previous reports show the alleviating effects of Bacillus-based probiotics during HS [24], thus the effect of supplementation of Bacillus-based probiotics on the performance of ACRB under HS needs further investigation. Relative abundance of Parasutterella was significantly higher in ACRB-TN compared to JF-TN and L2015-TN, while under HS relative abundance of Parasutterella was significantly higher in L2015 compared to other genetic lines. Parasutterella is a strict anaerobe belonging to the phylum Proteobacteria [25] and positive correlation between relative abundance of Parasutterella and performance was previously reported in chickens [26], which is not the case in our experiment. We observed an increase in the relative abundance of Proteobacteria in IL-L and ILM due to the HS. Performance data from this trial were previously published [3] indicating an association between an increase in abundance of Proteobacteria and reduction in feed intake. Furthermore, we indicated higher relative abundance of Blautia in IL-L and IL-M, and higher relative abundance of Ruminiclostridium 5 in IL-M of HS compared to TN group. Ruminiclostridium 5 and Blautia are correlated with higher abdominal fat percentage in chickens [ HS affects community composition and function of ileal microbiota. The effect of HS on microbial composition of the ileum is site-specific and is more prominent on the ileal mucosa than ileal luminal content. Furthermore, taxonomic, and functional analysis showed a signature like microbiota for each genetic line. Shift in community function was largest in L2015 compared to JF, with no difference between L2015 and L1995. This highlights the effect of temperature and genetic composition on the diversity of ileal microbiota and function.Conclusion
Availability of data and materials
The 16S rRNA gene sequences determined in this study were deposited in the NCBI Sequence Read Archive (SRA) database (http://nih.gov/bioproject/browse; SRA accession # PRJNA794190).
Abbreviations
- ACRB:
-
Athens-Canadian Random Bred
- ASV:
-
Amplicon sequence variant
- d:
-
Day
- FDR:
-
False discovery rate
- GIT:
-
Gastrointestinal tract
- HS:
-
Heat stress
- IL:
-
Ileum
- IL-L:
-
Ileal luminal bacterial population
- IL-M:
-
Ileal mucosal bacterial population
- JF:
-
Giant Jungle Fowl
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- L:
-
Luminal
- LAR:
-
Low abundance reads
- L1995:
-
1995 Random bred
- L2015:
-
Modern random bred
- M:
-
Mucosal
- PCoA:
-
Principal coordinate analysis
- PERMANOVA:
-
Non-parametric multivariate ANOVA
- PICRUSt:
-
Phylogenetic Investigation of Communities by Reconstruction of Unobserved States
- QIIME:
-
Quantitative Insight Into Microbial Ecology
- SRA:
-
NCBI sequence read archive
- STAMP:
-
Statystical analysis of metagenomic profiles
- TN:
-
Thermoneutral
References
Zerjal T, Härtle S, Gourichon D, Guillory V, Bruneau N, Laloë D, et al. Assessment of trade-offs between feed efficiency, growth-related traits, and immune activity in experimental lines of layer chickens. Genet Sel Evol. 2021;53(1):44. https://doi.org/10.1186/s12711-021-00636-z.
Rauw WM, Kanis E, Noordhuizen-Stassen EN, Grommers FJ. Undesirable side effects of selection for high production efficiency in farm animals: a review. Livest Prod Sci. 1998;56(1):15–33. https://doi.org/10.1016/S0301-6226(98)00147-X.
Tabler TW, Greene ES, Orlowski SK, Hiltz JZ, Anthony NB, Dridi S. Intestinal barrier integrity in heat-stressed modern broilers and their ancestor wild jungle fowl. Front Vet Sci. 2020. https://doi.org/10.3389/fvets.2020.00249.
Warren R, Price J, Graham E, Forstenhaeusler N, VanDerWal J. The projected effect on insects, vertebrates, and plants of limiting global warming to 1.5°C rather than 2°C. Science. 2018;360(6390):791–5. https://doi.org/10.1126/science.aar3646.
Emami NK, Jung U, Voy B, Dridi S. Radical response: effects of heat stress-induced oxidative stress on lipid metabolism in the avian liver. Antioxidants. 2020. https://doi.org/10.3390/antiox10010035.
Emami NK, Greene ES, Kogut MH, Dridi S. Heat stress and feed restriction distinctly affect performance, carcass and meat yield, intestinal integrity, and inflammatory (chemo)cytokines in broiler chickens. Front Physiol. 2021. https://doi.org/10.3389/fphys.2021.707757.
Greene ES, Emami NK, Dridi S. Research Note: Phytobiotics modulate the expression profile of circulating inflammasome and cyto(chemo)kine in whole blood of broilers exposed to cyclic heat stress. Poult Sci. 2021;100(3):100801. https://doi.org/10.1016/j.psj.2020.10.055.
Sohail MU, Hume ME, Byrd JA, Nisbet DJ, Shabbir MZ, Ijaz A, et al. Molecular analysis of the caecal and tracheal microbiome of heat-stressed broilers supplemented with prebiotic and probiotic. Avian Pathol. 2015;44(2):67–74. https://doi.org/10.1080/03079457.2015.1004622.
Emami NK, Daneshmand A, Naeini SZ, Graystone EN, Broom LJ. Effects of commercial organic acid blends on male broilers challenged with E. coli K88: performance, microbiology, intestinal morphology, and immune response. Poult Sci. 2017;96(9):3254–63. https://doi.org/10.3382/ps/pex106.
Emami NK, Calik A, White MB, Kimminau EA, Dalloul RA. Effect of probiotics and multi-component feed additives on microbiota, gut barrier and immune responses in broiler chickens during subclinical necrotic enteritis. Front Vet Sci. 2020;7(972):572142. https://doi.org/10.3389/fvets.2020.572142.
Emami NK, White MB, Calik A, Kimminau EA, Dalloul RA. Managing broilers gut health with antibiotic-free diets during subclinical necrotic enteritis. Poult Sci. 2021;100(5):101055. https://doi.org/10.1016/j.psj.2021.101055.
Iqbal Y, Cottrell JJ, Suleria HAR, Dunshea FR. Gut microbiota-polyphenol interactions in chicken: a review. Animals (Basel). 2020;10(8):1391. https://doi.org/10.3390/ani10081391.
Yan W, Sun C, Yuan J, Yang N. Gut metagenomic analysis reveals prominent roles of Lactobacillus and cecal microbiota in chicken feed efficiency. Sci Rep. 2017;7(1):45308. https://doi.org/10.1038/srep45308.
Sepulveda J, Moeller AH. The effects of temperature on animal gut microbiomes. Front Microbiol. 2020;11:384. https://doi.org/10.3389/fmicb.2020.00384.
He J, He Y, Pan D, Cao J, Sun Y, Zeng X. Associations of gut microbiota with heat stress-induced changes of growth, fat deposition, intestinal morphology, and antioxidant capacity in ducks. Front Microbiol. 2019;10:903. https://doi.org/10.3389/fmicb.2019.00903.
Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37(8):852–7. https://doi.org/10.1038/s41587-019-0209-9.
Weber N, Liou D, Dommer J, MacMenamin P, Quiñones M, Misner I, et al. Nephele: a cloud platform for simplified, standardized and reproducible microbiome data analysis. Bioinformatics. 2018;34(8):1411–3. https://doi.org/10.1093/bioinformatics/btx617.
Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2015;44(D1):D457–62. https://doi.org/10.1093/nar/gkv1070.
Parks DH, Tyson GW, Hugenholtz P, Beiko RG. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics. 2014;30(21):3123–4. https://doi.org/10.1093/bioinformatics/btu494.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Stat Methodol. 1995;57(1):289–300.
Patra AK, Kar I. Heat stress on microbiota composition, barrier integrity, and nutrient transport in gut, production performance, and its amelioration in farm animals. J Anim Sci Technol. 2021;63(2):211–47. https://doi.org/10.5187/jast.2021.e48.
Wang XJ, Feng JH, Zhang MH, Li XM, Ma DD, Chang SS. Effects of high ambient temperature on the community structure and composition of ileal microbiome of broilers. Poult Sci. 2018;97(6):2153–8. https://doi.org/10.3382/ps/pey032.
Shah TM, Patel JG, Gohil TP, Blake DP, Joshi CG. Host transcriptome and microbiome interaction modulates physiology of full-sibs broilers with divergent feed conversion ratio. NPJ Biofilms Microbiomes. 2019;5(1):24. https://doi.org/10.1038/s41522-019-0096-3.
Wang WC, Yan FF, Hu JY, Amen OA, Cheng HW. Supplementation of Bacillus subtilis-based probiotic reduces heat stress-related behaviors and inflammatory response in broiler chickens. J Anim Sci. 2018;96(5):1654–66. https://doi.org/10.1093/jas/sky092.
Rychlik I. Composition and function of chicken gut microbiota. Animals (Basel). 2020;10(1):103. https://doi.org/10.3390/ani10010103.
Han H, Zhou Y, Liu Q, Wang G, Feng J, Zhang M. Effects of ammonia on gut microbiota and growth performance of broiler chickens. Animals (Basel). 2021;11(6):1716. https://doi.org/10.3390/ani11061716.
**ang H, Gan J, Zeng D, Li J, Yu H, Zhao H, et al. Specific microbial taxa and functional capacity contribute to chicken abdominal fat deposition. Front Microbiol. 2021. https://doi.org/10.3389/fmicb.2021.643025.
Liu J, Stewart SN, Robinson K, Yang Q, Lyu W, Whitmore MA, et al. Linkage between the intestinal microbiota and residual feed intake in broiler chickens. J Anim Sci Biotechnol. 2021;12(1):22. https://doi.org/10.1186/s40104-020-00542-2.
Ravindran V, Abdollahi MR. Nutrition and digestive physiology of the broiler chick: State of the art and outlook. Animals (Basel). 2021. https://doi.org/10.3390/ani11102795.
Stanley D, Hughes RJ, Geier MS, Moore RJ. Bacteria within the gastrointestinal tract microbiota correlated with improved growth and feed conversion: challenges presented for the identification of performance enhancing probiotic bacteria. Front Microbiol. 2016. https://doi.org/10.3389/fmicb.2016.00187.
Lv H, Huang Y, Wang T, Zhai S, Hou Z, Chen S. Microbial composition in the duodenum and ileum of Yellow broilers with high and low feed efficiency. Front Microbiol. 2021. https://doi.org/10.3389/fmicb.2021.689653.
Wei S, Morrison M, Yu Z. Bacterial census of poultry intestinal microbiome. Poult Sci. 2013;92(3):671–83. https://doi.org/10.3382/ps.2012-02822.
Servin AL. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev. 2004;28(4):405–40. https://doi.org/10.1016/j.femsre.2004.01.003.
Belenguer A, Duncan SH, Holtrop G, Anderson SE, Lobley GE, Flint HJ. Impact of pH on lactate formation and utilization by human fecal microbial communities. Appl Environ Microbiol. 2007;73(20):6526–33. https://doi.org/10.1128/aem.00508-07.
Pan D, Yu Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes. 2014;5(1):108–19. https://doi.org/10.4161/gmic.26945.
Servin AL, Coconnier MH. Adhesion of probiotic strains to the intestinal mucosa and interaction with pathogens. Best Pract Res Clin Gastroenterol. 2003;17(5):741–54. https://doi.org/10.1016/s1521-6918(03)00052-0.
Collado MC, Gueimonde M, Hernández M, Sanz Y, Salminen S. Adhesion of selected Bifidobacterium strains to human intestinal mucus and the role of adhesion in enteropathogen exclusion. J Food Prot. 2005;68(12):2672–8. https://doi.org/10.4315/0362-028x-68.12.2672.
Nii T, Jirapat J, Isobe N, Yoshimura Y. Effects of oral administration of Lactobacillus reuteri on mucosal barrier function in the digestive tract of broiler chicks. J Poult Sci. 2020;57(1):67–76. https://doi.org/10.2141/jpsa.0190035.
Duncan SH, Louis P, Flint HJ. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl Environ Microbiol. 2004;70(10):5810–7. https://doi.org/10.1128/AEM.70.10.5810-5817.2004.
Konsak BM, Stanley D, Haring VR, Geier MS, Hughes RJ, Howarth GS, et al. Identification of differential duodenal gene expression levels and microbiota abundance correlated with differences in energy utilisation in chickens. Anim Prod Sci. 2013;53(12):1269–75. https://doi.org/10.1071/AN12426.
Huang P, Zhang Y, **ao K, Jiang F, Wang H, Tang D, et al. The chicken gut metagenome and the modulatory effects of plant-derived benzylisoquinoline alkaloids. Microbiome. 2018;6(1):211. https://doi.org/10.1186/s40168-018-0590-5.
Kogut MH, Genovese KJ, Swaggerty CL, He H, Broom L. Inflammatory phenotypes in the intestine of poultry: not all inflammation is created equal. Poult Sci. 2018;97(7):2339–46. https://doi.org/10.3382/ps/pey087.
Kogut MH. The gut microbiota and host innate immunity: regulators of host metabolism and metabolic diseases in poultry. J Appl Poult Res. 2013;22(3):637–46. https://doi.org/10.3382/japr.2013-00741.
te Pas MFW, Borg R, Buddiger NJH, Wood BJ, Rebel JMJ, van Krimpen MM, et al. Regulating appetite in broilers for improving body and muscle development—a review. J Anim Physiol Anim Nutr. 2020;104(6):1819–34. https://doi.org/10.1111/jpn.13407.
Cherry JA, Siegel PB. Selection for body weight at eight weeks of age: 15. Feed passage and intestinal size of normal and dwarf chicks. Poult Sci. 1978;57(2):336–40. https://doi.org/10.3382/ps.0570336.
Svihus B, Itani K. Intestinal passage and its relation to digestive processes. J Appl Poult Res. 2019;28(3):546–55. https://doi.org/10.3382/japr/pfy027.
Zhang J-M, Sun Y-S, Zhao L-Q, Chen T-T, Fan M-N, Jiao H-C, et al. SCFAs-induced GLP-1 secretion links the regulation of gut microbiome on hepatic lipogenesis in chickens. Front Microbiol. 2019. https://doi.org/10.3389/fmicb.2019.02176.
Acknowledgements
Not applicable.
Funding
This work was funded by a grant from Arkansas Experimentation Station Animal Health (FY2019) to SD and SO, and by in house USDA-ARS CRIS # 8042-31000-108-00D to MP-W.
Author information
Authors and Affiliations
Contributions
SD designed the experiment and purchased the reagents. EG, SO, TT, NA and SD conducted the trial and processed the animals at the end of trial. NE, LLS, and MPW performed the microbiota analyses. NE wrote the first draft of the manuscript. MPW and SD edited and reviewed the manuscript with input from all the co-authors. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the recommendations in the guide for the care and use of laboratory animals of the National Institutes of Health and the protocols were approved by the University of Arkansas Animal Care and Use Committee under protocols 18083 and 16084. All methods were carried out in accordance with relevant guidelines and regulations. This study was performed and reported in accordance with ARRIVE guidelines (https://arriveguidelines.org/).
Consent for publication
Not applicable.
Competing interests
Authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mention of trade name, proprietary product, or specific equipment does not constitute guarantee or warranty by USDA and does not imply its approval to the exclusion of other suitable products.
Supplementary Information
Additional file 1: Table S1.
Comparison of microbial community richness (Chao 1) in the luminal population. Table S2. Beta-diversity in luminal population. Table S3. Comparison of microbial community diversity in mucosal population (Simpson’s diversity). Table S4. Beta-diversity in mucosal population. Figure S1. Comparison in predicted microbiota function mucosal population between modern random bred (L2015) and jungle fowl (JF). Figure S2. Comparison in predicted microbiota function in mucosal population between modern random bred (L2015) and Athens-Canadian Random bred (ACRB).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Emami, N.K., Schreier, L.L., Greene, E. et al. Ileal microbial composition in genetically distinct chicken lines reared under normal or high ambient temperatures. anim microbiome 4, 28 (2022). https://doi.org/10.1186/s42523-022-00183-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42523-022-00183-y