Abstract
Background
Many analgesic methods have been used to control post-mastectomy pain. Both thoracic paravertebral and serratus anterior blocks are recent regional techniques with promising results. The aim of this study was to compare safety and analgesic efficacy of both techniques in controlling post-mastectomy pain.
Methods
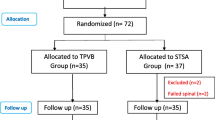
The study was conducted from January 1, 2019, till January 10, 2019, on 60 female patients ASA class ≤ 2 undergoing modified radical mastectomy. After induction of balanced general anesthesia patients received either continuous thoracic paravertebral block (group P) or continuous serratus anterior block (group S). Twenty milliliters of levobupivacaine 0.25% were injected in each technique under ultrasound guidance followed by continuous infusion of 5 ml/h levobupivacaine 0.125% through a 22-gage catheter. IV morphine was given postoperatively by patient-controlled analgesia. In both groups, we measured time to first dose morphine, total 24 and 48 h morphine consumption, vital signs, visual analog scale, and side effects of each technique.
Results
The demographic data (age, body mass index, and duration of surgery) were comparable in both groups. The time for first dose of morphine was significantly longer in group P (368 ± 36 min) than group S (270 ± 37.65 min) with P value < 0.001. Total morphine consumption in milligram at both 24 and 48 h were significantly less in group P (8.1 ± 0.8, and 11.5 ± 1 respectively) than in group S (10.1 ± 1.3 and 14.2 ± 1.4), with limited side effects in both groups.
Conclusion
Both continuous paravertebral and serratus anterior plane blocks are safe good alternatives to control post-mastectomy pain. However continuous paravertebral block provides better analgesic profile.
Similar content being viewed by others
Introduction
Breast cancer is one of the most common types of cancer in women, the standard surgery for which is modified radical mastectomy surgery with axillary dissection (MRM) (Cancer Research, 2010; Office for National Statistics, 2010; Sharma et al., 2010; American Cancer Society, 2019). MRM causes acute post-operative pain that progresses to chronic persistent pain in 25–60% of cases, hence, the importance of proper control of post-mastectomy pain (Andersen & Kehlet, 2011). Regional analgesic methods aiming at blockade of the lateral cutaneous branches of the thoracic intercostal nerves (T2–T12) provide analgesia to the anterolateral chest wall in these patients (Mayes et al., 2016a). This blockade can be achieved by several methods including thoracic epidural and intercostal nerve blocks (Marret et al., 2006). These techniques carry many risks including pneumothorax and hemodynamic instability (Marret et al., 2006). Thoracic paravertebral block (PVB) is a relatively recent good alternative technique to block spinal nerves at the site of their emergence from the intervertebral foramina (Karmakar, 2001; Santonastaso et al., 2019). A relatively recent study suggested that ultrasound-guided serratus anterior plane block (SPB) is another good analgesic alternative (Blanco et al., 2013). Another recent study compared both SPB and PVB as a single-injection technique (Gupta et al., 2017), however, the continuous technique of both blocks needs further study. Our hypothesis was that PVB has a better analgesic profile with less needed morphine consumption than SPB.
Aim of work
The aim of this study was to compare the safety and efficacy of ultrasound (US)-guided continuous serratus anterior plane block (SPB) to continuous thoracic paravertebral block (PVB) as a method of pain relief following MRM with axillary dissection.
Patients and methods
Institutional ethical committee approval number IRB0004025 was obtained, and then the paper obtained trial registry number PACTR201812842421823 from Pan African clinical trial registry. A written informed consent was obtained from each participating patient. The study was conducted from January 1, 2019, till January 10, 2019, on 60 female patients undergoing MRM in the National Cancer Institute, Cairo. Inclusion criteria included female patients (18‑60 years) who planned for a modified radical mastectomy of the American Society of Anesthesia (ASA) class ≤ II. Exclusion criteria included patients’ refusal, patients with coagulopathy (INR ≥ 2), local infection, and patients with renal insufficiency (creatinine ≥ 1.5 mg/d ≥ dL).
Primary outcome parameters were total of 24 and 48 h post-operative morphine consumption by patient-controlled analgesia (PCA). Secondary outcome parameters were time to first dose morphine, VAS, mean arterial blood pressure (MBP), heart rate (HR) post-operative for 48 h, and complications as postoperative nausea, vomiting, and respiratory depression.
Randomization was done by permuted block technique and each participating patient was given a number kept in a sealed envelope. After induction of balanced general anesthesia, the envelope was opened and either PVB or SPB was performed by one of the first two authors (both are well trained in the two blocks). Both the patient and the attending anesthesia resident in the operating room were blinded for the block performed.
A preoperative visit was performed to each patient during whom the patient was learned to use the PCA, measure her VAS, and press bolus dose if VAS reached 4. In each patient, we induced a balanced general anesthesia (midazolam 2 mg, fentanyl 2 μg/kg, propofol 2 mg/kg, and atracurium 0.5 mg/kg), patient was ventilated for 2 min using sevoflurane 3% on O2 then patients were intubated. Anesthesia was maintained using sevoflurane inhalational anesthesia 1-2 MAC guided by vital signs on O2/Air with FiO2 0.5. After intubation and before the start of surgery, the anesthesia resident indorsed the patient and left the operating room till one of the two blocks was performed. Patients were randomly allocated into 2 equal groups:
Group S (serratus group) (30 patients)
In which patients were placed in the lateral position with the diseased side up, a linear ultrasound probe (10–12 MHz) of the ultrasound machine (M-Turbo, SonoSite Inc., USA) was placed over the mid-clavicular region in a sagittal plane. The probe was moved downwards and laterally till the fifth rib in the mid-axillary line. The following muscles were identified overlying the fifth rib: latissimus dorsi (superficial and posterior), teres major (superior), and serratus muscles (deep and inferior). The thoracodorsal artery was used to identify the plane superficial to the serratus muscle. Skin infiltration with local anesthetic then the needle (20-gage Touhy needle; B Braun, Germany) was introduced in plane under direct visualization to the fascial plane superficial to the serratus anterior muscle between it and the latissimus dorsi muscle (Blanco et al., 2013). After negative aspiration, 20 mL of levobupivacaine 0.25% (Chirocaine®) was injected in 5 mL increments. Afterward, a 22 gage peripheral nerve catheter was threaded into the space. The needle was removed and the catheter secured with adhesive. Five milliliters per hour of levobupivacaine (0.125%) was administered through the catheter.
Group P (Paravertebral group) (30 patients)
In which patients were placed in the lateral position with the diseased side up. The paravertebral space was located using US guidance. The paravertebral space between the third and fourth thoracic vertebrae was identified in a parasagittal view approximately 3 cm lateral to midline on the side of surgery. A local anesthetic was injected caudal to the ultrasound transducer. A 20-gage Tuohy needle was inserted in plane beneath the ultrasound transducer and directed to the paravertebral space. Normal saline (5 mL) was injected via the needle to identify the paravertebral space and observe the anterior displacement of the pleura. Twenty milliliters of levobupivacaine 0.25% was injected after negative aspiration in 5 ml increments. Then a 22-gage peripheral nerve catheter was threaded into the space. The needle was removed and the catheter was secured with adhesive. Five milliliters per hour of levobupivacaine (0.125%) was administered through the catheter. In both groups, morphine was given by patient-controlled analgesia (PCA) (Fresenius pilot C. Master PCA, Wendelstein, Germany) device connected to the IV line immediately postoperative in the recovery room. The pump settings were morphine 1 mg/ml; bolus dose 1 mg, lockout interval 20 min, and maximum dose 4 mg/h. In both groups, we measured time to first needed morphine dose, total 24 and 48 h morphine consumption, VAS and vital signs at 0, 4, 8, 24, and 48 h postoperative, in addition to side effects (postoperative nausea, vomiting, and respiratory depression) in each group. All-time intervals were measured from the end of surgery and patient transfer to the recovery room so that the zero time starts once the patient is in the recovery room.
Basis of sample size calculation
The sample size was calculated based on a recent study (Gupta et al., 2017) that shows the analgesic efficacy of ultrasound-guided paravertebral block versus serratus block for modified radical mastectomy pain and the results for PCA morphine consumption postoperatively after 24 and 48 h (Gupta et al., 2017). We used these parameters for calculating the minimum sample size needed per group under the following circumstances: Significance level or probability of type I error = 0.01, power of the test statistics to be 90%, expected within-group standard deviation of 2.4 and a critical difference of 3.2 (difference in morphine consumption), a two-tailed testing and a ratio of sample size group 2/sample size group 1 = 1, a minimum of 17 patients per group with a total of 34 patients were enough to see such effect. Many biological variables do not follow a normal distribution as the VAS score, so we increased the sample size to involve 30 patients per group (total of 60 patients) to assume approximately normal distribution.
Statistical analysis
Data was analyzed using SPSS version 25.0 Comparison of post-operative morphine consumption (mg) measured after 24 and 48 h was done using t test after testing for normality, otherwise, non-parametric tests. The test of normality used was Shapiro-Wilk test, the non-parametric test name is Mann-Whitney U test.
Results
Sixty patients completed the study. The patients of both study groups (30 patients each) were comparable regarding demographic data (age, body mass index (BMI)), and duration of surgery as shown in Table 1 with no significant difference.
The time for the first dose morphine was significantly longer in paravertebral group as shown in Table 2. Both total 24 and 48 h morphine consumption were significantly less in paravertebral group.
There was no significant difference in VAS between both groups at all time intervals of the study till 48 h as shown in Fig. 1.
Only few cases of nausea and vomiting were reported in patients of the study groups with no significant difference between both groups (Table 3). No cases of respiratory depression were recorded in our study patients.
Regarding vital signs, both groups were comparable in the mean blood pressure (MBP) and the heart rate (HR) at all time intervals of the study without any significant difference as shown in Figs. 2 and 3.
Discussion
This randomized study compared US-guided continuous PVB with continuous SPB for analgesia after MRM. The results of our study were in favor of PVB regarding the longer duration of pain control (98 min longer) and less morphine consumption (by > 20%) than SPB. The incidence of side effects in both groups was low with no major complications. This superiority of PVB can be explained by the mechanism of action of SPB which blocks only the lateral cutaneous branches of the intercostal nerves (T2–T4) (Mayes et al., 2016b) with spare of their anterior cutaneous branches and supraclavicular nerves. Furthermore, SPB may not achieve adequate somatic and sympathetic blockade in the axillary region, unlike PVB that achieves ipsilateral anesthesia of thoracic dermatomes, affecting both somatic and sympathetic innervation (Tighe & Karmakar, 2013; Krediet et al., 2015). The thoracic paravertebral space (TPVS) is a wedge-shaped space containing spinal nerves, the sympathetic chain, white and gray rami communicantes, vessels, and connective tissue. The TPVS also communicates with both the epidural and intercostal spaces (Karmakar, 2001; Tighe & Karmakar, 2013; Krediet et al., 2015). Our results coincide with that of a recent study that found that PVB is superior to SPB in post-mastectomy pain, but they compare single injection and for 24 h only (Hetta & Rezk, 2016). Post-mastectomy pain syndrome is a type of chronic neuropathic pain that may follow MRM due to central sensitization, so proper postoperative analgesia prevents central sensitization and chronic neuropathic pain (Caviggioli et al., 2011).
A recent study demonstrated that SPB with a bolus of 30 mL of 0.25% levobupivacaine provided a good analgesic effect up to 12 h postoperatively. In our study, the time for the first required dose morphine was less. This difference may be related to our use of a lesser volume of 0.25% levobupivacaine (only 20 mL) in addition to their use of dexmedetomidine as an adjunctive analgesic (Abdallah et al., 2019). A recent study of continuous SPB has good results but they studied its effect after different surgeries (hybrid transthoracic esophagectomy) (Barbera et al., 2017). Insertion of a catheter may prolong the action of SPB (Broseta et al., 2015; Fujiwara et al., 2015). In another study, after injection of 15–20 ml of levobupivacaine 0.25% at T4 level for PVB, the duration of analgesia was shorter (137.5 min) than in our study (368 min). This difference highlights the efficacy of inserting a catheter for continuous infusion rather than a single injection (Wahba & Kamal, 2014).
A recent randomized controlled study found that SPB gives comparable results with thoracic epidural analgesia (TEA) for controlling acute thoracotomy pain and morphine consumption, but SPB group patients were hemodynamically more stable than TEA (Khalil et al., 2017).
Continuous PVB was associated with better analgesia in the postoperative period; the time for first dose morphine was significantly longer in paravertebral group. Both total 24 and 48 h of morphine consumption were significantly less in paravertebral group. However, SPB still has the advantages of being a safe and relatively easy technique.
The strength points of our study include using the same volume and concentration of local anesthetic in both groups that provide a fair comparison, also continuous analgesia using a catheter. The limitations of our study include that we could not assess the exact onset time of analgesia as patients were under general anesthesia, in addition to the limited follow-up time of the study to 48 h that did not allow us to assess the effect on chronicity of pain. We suggest further studies to assess the efficacy of adding adjuncts to the local anesthetic and to follow up patients for longer periods.
Conclusion
We concluded that both continuous paravertebral and serratus anterior plane blocks are safe and effective methods of pain control after modified radical mastectomy; however, paravertebral block has a better analgesic profile with less needed morphine.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abdallah NM, Bakeer AH, Youssef RB, Zaki HV, Abbas DN (2019) Ultrasound-guided continuous serratus anterior plane block: dexmedetomidine as an adjunctive analgesic with levobupivacaine for post-thoracotomy pain. A prospective randomized controlled study. J Pain Res 12:1425–1431
American Cancer Society. Cancer facts and figures 2019. Atlanta, Ga. American Cancer Society, 2019. Last accessed 5 Feb 2019
Andersen KG, Kehlet H (2011) Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain 12:725–746
Barbera C, Milito P, Punturieri M, Asti E, Bonavina L (2017) Serratus anterior plane block for hybrid transthoracic esophagectomy: a pilot study. J Pain Res 10:73–77
Blanco R, Parras T, Mc Donnell JG, Prats-Galino A (2013) Serratus plane block: a novel ultrasound-guided thoracic wall nerve block. Anaesthesia 68:1107–1113
Broseta AM, Errando C, De Andrés J, Díaz-Cambronero O, Ortega-Monzó J (2015) Serratus plane block: the regional analgesia technique for thoracoscopy? Anaesthesia 70:1329–1330
Cancer Research UK: Breast cancer incidence statistics, 2010. http://www.cancerresearchuk.org (accessed 01/03/2013).
Caviggioli F, Maione L, Forcellini D, Klinger F, Klinger M (2011) Autologous fat graft in postmastectomy pain syndrome. Plast Reconstr Surg 128:349
Fujiwara S, Komasawa N, Minami T (2015) Pectral nerve blocks and serratus-intercostal plane block for intractable postthoracotomy syndrome. J Clin Anesth 27:275–276
Gupta K, Srikanth K, Girdhar KK, Chan V (2017) Analgesic efficacy of ultrasound-guided paravertebral block versus serratus plane block for modified radical mastectomy: a randomized, controlled trial. Indian J Anaeth. 16:381–386
Hetta DF, Rezk KM (2016) Pectoralis-serratus interfascial plane block vs. thoracic paravertebral block for unilateral radical mastectomy with axillary evacuation. J Clin Anesth 34:91–97
Karmakar MK (2001) Thoracic paravertebral block. Anesthesiology 95:771–780
Khalil AE, Abdallah NM, Bashandy GM, Kaddah TA (2017) Ultrasound-guided serratus anterior plane block versus thoracic epidural analgesia for thoracotomy pain. J Cardiothorac Vasc Anesth 3
Krediet AC, Moayeri N, van Geffen G-J, Bruhn J, Renes S, Bigeleisen PE et al (2015) Different approaches to ultrasound-guided thoracic paravertebral block: an illustrated review. Anesthesiology. 123(2):459–474
Marret E, Vigneau A, Salengro A, Noirot A, Bonnet F (2006) Effectiveness of analgesic techniques after breast surgery: a meta-analysis. Ann Fr Anesth Reanim 25:947–954
Mayes J, Davison E, Panahi P, Patten D, Eljelani F, Womack J, Varma M (2016a) An anatomical evaluation of the serratus anterior plane block. Anaesthesia. 71(9):1064–1069
Mayes J, Davison E, Panahi P, Patten D, Eljelani F, Womack J et al (2016b) An anatomical evaluation of the serratus anterior plane block. Anaesthesia 71:1064–1069
Office for National Statistics. Breast cancer: incidence, mortality and survival, 2010. http://www.ons.gov.uk (accessed 01/03/2013).
Santonastaso DP, de Chiara A, Russo E, Musetti G, Lucchi L, Sibilio A et al (2019) Single shot ultrasound-guided thoracic paravertebral block for opioid-free radical mastectomy: a prospective observational study. J Pain Res. 12:2701–2708
Sharma GN, Dave R, Sanadya J, Sharma P, Sharma KK (2010) Various types and management of breast cancer: an overview. J Adv Pharm Technol Res. 1(2):109–126
Tighe SQ, Karmakar MK (2013) Serratus plane block: do we need to learn another technique for thoracic wall blockade? Anaesthesia 68:1103–1106
Wahba SS, Kamal SM (2014) Thoracic paravertebral block versus pectoral nerve block for analgesia after breast surgery. Egypt J Anaesth 30:129–135
Acknowledgements
Not applicable.
Funding
By the authors themselves without receiving any external funding.
Author information
Authors and Affiliations
Contributions
EM and SA have done the nerve blocks in patients while NA has done the sample size calculation and statistical analysis. All authors have a major contribution in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Institutional ethical committee approval number IRB0004025 was obtained from Institutional Review Board of National Cancer Institute, Cairo University (IRB-NCI) on December 11, 2018, Organization no: IORG0003381. Then the paper obtained trial registry number PACTR201812842421823 from Pan African clinical trial registry. A written informed consent was obtained from each participating patient.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mahran, E., Adlan, S. & Alieldeen, N. Comparative randomized study of continuous serratus anterior plane block versus continuous paravertebral block in post-mastectomy pain. Ain-Shams J Anesthesiol 12, 45 (2020). https://doi.org/10.1186/s42077-020-00091-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42077-020-00091-w