Abstract
Background
Automated [89Zr]Zr-radiolabeling processes have the potential to streamline the production of [89Zr]Zr-labelled PET imaging agents. Most radiolabeling protocols use [89Zr][Zr(ox)4]4− as the starting material and oxalate is removed after radiolabeling. In some instances, radiolabeling with [89Zr]ZrCl4 as starting material gives better radiochemical yields at lower reaction temperatures. In this work, a fully-automated process for production of [89Zr]ZrCl4 is reported and its use for the synthesis of [89Zr]ZrDFOSq-bisPhPSMA and [89Zr]ZrDFOSq-TATE.
Results
A simple automated process for the isolation of [89Zr]ZrCl4 by trap** [89Zr][Zr(ox)4]4− on a bicarbonate-activated strong anion exchange cartridge followed by elution with 0.1 M HCl in 1 M NaCl was developed. [89Zr]ZrCl4 was routinely recovered from [89Zr][Zr(ox)4]4− in > 95% yield in mildly acidic solution of 0.1 M HCl in 1 M NaCl using a fully-automated process. The [89Zr]ZrCl4 was neutralized with sodium acetate buffer (0.25 M) removing the requirement for cumbersome manual neutralization with strong base. The mixture of [89Zr]ZrCl4 was used for direct automated radiolabeling reactions to produce [89Zr]Zr-DFOSquaramide-bisPhPSMA and [89Zr]ZrDFOSquaramide-TATE in 80–90% over all RCY in > 95% RCP.
Conclusions
This method for the production of [89Zr]ZrCl4 does not require removal of HCl by evaporation making this process relatively fast and efficient. The fully automated procedures for the production of [89Zr]ZrCl4 and its use in radiolabeling are well suited to support the centralized and standardized manufacture of multiple dose preparations of zirconium-89 based radiopharmaceuticals.
Similar content being viewed by others
Background
The radioactive half-life of zirconium-89 and relatively low translation energy of the positron emission from zirconium-89 (t1/2 = 78.4 h, β+ = 22.3%, Eave(β+) = 396.9 keV) have led to the isotope becoming the radionuclide of choice for radiolabelling of monoclonal antibodies to make antibody-based tracers for Positron Emission Tomography (PET) (Verel et al. 2003; Feo et al. 2022; Yoon et al. 2020; Heskamp et al. 2017; Dongen et al. 2021; McInnes et al. 2017; Jauw et al. 2019). The high molecular weight and interactions mediated by the Fc portion of antibodies (Fc = Fraction crystallisable) lead to radiolabelled antibodies taking days to clear from the blood pool and localise in the target tissue. PET tracers that rely on peptides or small molecules to achieve selectivity accumulate in target tissue more rapidly and are consequently often radiolabelled with radionuclides with shorter radioactive half-lives such as gallium-68 (t1/2 = 68 min) or fluorine-18 (t1/2 = 109.8 min). PET tracers that are radiolabelled with gallium-68 are currently playing a major role in diagnosis by PET imaging, but the short radioactive half-life of gallium-68 often requires the radiopharmaceutical to be synthesised on-site. There is increased interest in the potential to use zirconium-89 for peptide-based tracers where the longer radioactive half-life of zirconium-89 would make centralized manufacture of PET tracers to a certified good manufacturing process (GMP) standard feasible. In principle, the distribution of GMP PET tracers from a centralized manufacturer to hospitals would greatly increase the number of clinical sites that could perform PET scans although the additional radiation dose associated with zirconium-89 needs to be considered. The longer radioactive half-life also offers the possibility of imaging at later time points where reduced background signal can lead to the identification of lesions that are not present on scans using gallium-68 labelled tracers (Felix et al. 2022; Rosar et al. 2023).
A common route for the preparation of zirconium-89 is proton bombardment of yttrium-89 in a 89Y(p,n)89Zr reaction. The zirconium-89 radionuclide is then purified from the 89Y starting material by resin that has been functionalised with hydroxamate functional groups that form strong interactions with [89Zr]ZrIV. Elution of the resin with oxalic acid (0.05–1 M) leads to excellent recovery of [89Zr]ZrIV where the oxalic acid deprotonates and serves as a dianionic bidentate ligand to form complexes best formulated as [[89Zr]Zr(ox)4]4−. The isolated [[89Zr]Zr(ox)4]4− solution is well suited for both transportation and complexation reactions presumably through transfer chelation to ligands such as desferrioxamine (H3DFO) and H3DFO-antibody conjugates. Although the solutions of [[89Zr]Zr(ox)4]4− in oxalic acid can be used for directing labelling of H3DFO antibody conjugates it is essential that oxalic acid/oxalate is removed before administration as it is highly toxic. The removal of oxalate/oxalic acid is often achieved by size-exclusion chromatography for antibody conjugates which adds an extra manipulation with radioactive materials. Separation of oxalate from [89Zr]ZrIV labelled peptide-based or peptidomimetic-based probes with lower molecular weight is more challenging. For radiolabelling both antibodies and small molecules/peptides with [89Zr]ZrIV it is possible that removing oxalate before addition of the biomolecule could be a better approach and certain radiolabelling reactions have been demonstrated to proceed better when [89Zr]ZrCl4 is used as starting material rather than [[89Zr]Zr(ox)4]4−. It is possible to isolate [89Zr]ZrCl4 by loading [[89Zr]Zr(ox)4]4− onto a strong anion exchange cartridge in the chloride form followed by elution with aqueous HCl (1 M) (Holland et al. 2009). The HCl is then removed by boiling the eluate at 110 °C under a continuous stream of argon and it is then reconstituted in 0.9% saline or 0.1 M HCl for radiolabelling. Removing HCl by evaporation increases the time required for synthetic preparations and can lead to loss of activity due to the formation of aerosols potentially increasing radiation exposure, and contamination. A promising alternative to the solid target 89Y(p,n)89Zr methods for the production of [89Zr]Zr is the proton bombardment of aqueous solutions of [89Y]YCl3 and [89Y](NO3)3 (Pandey et al. 2014). A potential advantage of this liquid target approach is that it avoids the solid target processing steps (do Carmo et al. 2020).
In this work, we describe a new processes for the synthesis of [89Zr]ZrCl4 from [89Zr][Zr(ox)4]4− using a bicarbonate-activated polystyrene-based strong anion exchange cartridge. This processes directly produces solutions of [89Zr]ZrCl4 in mixture of dilute HCl (0.1 M) in aqueous NaCl (1 M). When working with radioactivity it is important to keep the amount of radiation used as low as is possible (ALARA) and to minimize radiation exposure to those involved in manufacture whilst synthesizing pure, sterile products in a reproducible fashion to support centralized manufacture and distribution to multiple clinical sites. With these goals in mind we translated the chemistry for the production of [89Zr]ZrCl4 to an automated procedure using a commercially available iPHASE MultiSyn radiosynthesizer. We have then extended the approach to use [89Zr]ZrCl4 produced on the synthesizer to prepare a small molecule and peptide tracers, [89Zr]ZrDFOSq-bisPSMA (Fig. 1, a bivalent inhibitor of prostate specific membrane antigen) (Noor et al. 2020), [89Zr]ZrDFOSq-TATE (Fig. 1, a somatostatin subtype-2 receptor-targeting peptide) (Noor et al. 2021). This work builds on previous contributions for automated production of [89Zr]ZrIV-based radiopharmaceuticals that used [89Zr][Zr(ox)4]4− as a starting material (Dongen et al. 2019; Wichmann et al. 2023).
Results
Synthesis of [ 89 Zr]ZrCl 4
One goal of this study was to develop automated standardized, reproducible syntheses of [89Zr]ZrCl4, [89Zr]ZrDFOSq-bisPSMA and [89Zr]ZrDFOSq-TATE that could form the basis of procedures used to prepare tracers suitable for clinical studies. It was deemed preferable to remove oxalate/oxalic acid and produce [89Zr]ZrCl4 which was then used for the radiolabeling reactions. We first repeated published procedures for the preparation of [89Zr]ZrCl4 using a Quaternary Methyl Ammonium (QMA) strong anion exchange cartridge (Waters Sep-pak QMA, with a quaternary amine functional group in the chloride form) that was pre-conditioned with acetonitrile (Pandya et al. 2019). Consistent with literature procedure, maximum recovery of [89Zr]ZrCl4 required elution of the cartridge with 1 M HCl (~ 90%, Additional file 1: Figure S2, Table S1). Acetonitrile is considered a ‘Class 2’ solvent by both the European Medicines Agency and the U.S. Food and Drug Administraion where use “should be limited” (Q3C(R8) Impurities 2021; ICH guideline Q3C (R8) 2022; Constable et al. 2007; Grodowska and Parczewski 2010), so pre-conditioning the column with either ethanol or dimethylsulfoxide was investigated but both resulted in lower recovered yields of [89Zr]ZrCl4 (55%-76%, Fig. 2, Table S1) as did elution with 0.25 M HCl (~ 80%, Fig. 2, Additional file 1: Table S1).
An alternative anion exchange cartridge, a functionalized crosslinked polystyrene-divinylbenzene polymer in the bicarbonate form (PS-HCO3 SAX cartridge, Huayi Isotopes) was also investigated. This column is marketed for separation of fluorine-18 where the manufacturer’s instructions state that this column does not require pre-conditioning with organic solvents prior to use as it is pre-treated with ethanol (Class 3 solvent) and plugged tightly at both ends to prevent contamination and manufactured according to Good Manufacturing (GMP) requirements. Following application of [[89Zr][Zr(ox)4]4− to the cartridge (in either 1 M or 0.05 M oxalic acid mixtures) [89Zr]ZrCl4 was eluted with HCl (1 M) in > 90% recovered yield (Fig. 2, Table S1). Radiolabelling of DFO-derivatives requires neutralisation of 1 M hydrochloric acid with a strong base such as sodium carbonate. The efficiency of elution with mixtures of aqueous sodium chloride and dilute hydrochloric acid (0.1 M and 0.05 M) was investigated to investigate if eluting with an increased chloride ion concentration but with a lower concentration of acid was possible. The combination of hydrochloric acid (0.05 M) with sodium chloride solution (2.5 M NaCl) resulted in excellent recovery of ~ 92%. This is slighter lower recovery than when 1 M hydrochloric acid is used, but the recovery of [89Zr]ZrCl4 in a dilute (0.05 M) hydrochloric acid solution has the significant advantage that this eluent can be readily buffered with sodium acetate buffer and does not require the addition of a stronger base (sodium carbonate) (Fig. 2).
Synthesis of [ 89 Zr]ZrDFOSq-bisPhPSMA from [ 89 Zr]ZrCl 4
The [89Zr]ZrCl4 eluent (0.1 M HCl/1 M NaCl) was used to prepare [89Zr]ZrDFOSq-bisPhPSMA that we published previously (Noor et al. 2020, 2021), with the omission of the ‘neutralization’ step with sodium carbonate and C18 SPE purification. Addition of [89Zr]ZrCl4 eluent (100 MBq, 0.1 M HCl/1 M NaCl) to a mixture of the respective ligand (100 ng) in sodium acetate (0.25 M) buffer and heating the reaction mixtures to 75 °C for 15 min allowed isolation of each compound in > 95% radiochemical yield and purity that does not require any SPE purification. This new methodology allowed the use of a significantly lower peptide concentration (100 ng/MBq compared with 2.0 µg/MBq) than our initial method increasing the molar activity to 18.8 GBq/μmol at the end of synthesis. Another advantage of using [89Zr]ZrCl4 and dissolving the ligands in ethanol was that the final product does not contain either oxalate or dimethyl sulfoxide which were removed in our initial synthesis by a final purification through a C18 solid-phase extraction cartridge.
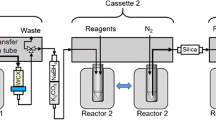
Automated production of [ 89 Zr]ZrCl 4 , [ 89 Zr]ZrDFOSq-bisPhPSMA and [ 89 Zr]ZrDFOSq-TATE
The new synthetic methodology for the preparation of [89Zr]ZrDFOSq-bisPhPSMA and [89Zr]ZrDFOSq-TATE directly from [89Zr]ZrCl4 was next translated to a fully automated procedure using a commercial iPHASE MultiSyn synthesis module using disposable cassettes (Wichmann et al. 2023, 2021). An iPhase zirconoium-89 radiolabelling disposable cassette (MSH-300) was modified and configured according to Fig. 3a then mounted on the Multisyn module (Fig. 3b). The iPHASE Multisyn programmed sequence was downloaded on the control software and prompts were followed to commence the synthesis. In the first step excellent trap** (> 95%) [89Zr]ZrIV was observed on the PS-HCO3 cartridge and high recoveries (90%) of [89Zr]ZrCl4 were achieved as observed on the built-in radiodetectors on manifold 3. The cartridge was eluted at a slower flow rate with a mixture of NaCl (1.0 M) and HCl (0.1 M) over 5 min to provide maximum recovery. The [89Zr]ZrCl4 (~ 150–1000 MBq) was directly eluted into the reaction vial containing H3DFOSq-bisPhPSMA dissolved in a mixture of aqueous sodium acetate buffer (0.25 M), ethanol (10%) and ascorbic acid (0.05%). The transfers of liquids were controlled through remote control software by opening and closing the gas pressure/vacuum valves or by using the syringe drivers. The reaction vessel was then heated at 75 °C for 15 min resulting in the synthesis of [89Zr]ZrDFOSq-bisPhPSMA in an overall > 80% radiochemical yield, based on [[89Zr]Zr(ox)4]4−, with a radiochemical purity of > 95% determined by radio-TLC (Figure S1) and radio-HPLC (Figure S2) with a final step that includes in-line sterile filtration (Table S2). The final sterile filtered product was collected in 25 mL evacuated vials, a total volume of 12–14 mL in 0.9% w/v of saline was obtained as a clear colorless solution with no visible particles and a pH of 5–6. No residual oxalate detected using QuantiQuik™ Oxalate Quick Test Strips.
Adapting the procedure developed for the synthesis of [89Zr]ZrDFOSq-bisPhPSMA to [89Zr]ZrDFOsq-TATE resulted in radiochemical yields of ~ 60% with ~ 30% of the radioactivity being retained in the reaction vial, tubing, syringe and the sterile filter. [89Zr]ZrDFOsq-TATE is less soluble than [89Zr]ZrDFOsq-bisPhPSMA in the aqueous sodium acetate (0.25 M), ethanol (10%) and ascorbic acid (0.05%) mixture. Increasing the ethanol concentration to 20% in the reaction vessel increased the yield to 67%. Adding an addition washing step with an ethanol vial (2 mL) to position #10 (Fig. 3c) to rinse the reaction vial and the cassette followed by an additional transfer of the product mixture in 0.9% w/v saline allowed isolation of [89Zr]ZrDFOsq-TATE in > 85 ± 3% radiochemical yields based on [89Zr][Zr(ox)4)]4− with > 95% radiochemical purity (Additional file 1: Figures S3 and S4, Table S3). The final product (12–14 mL) was filtered through a sterile filter into evacuated vials such that final formulation contained 0.9% saline containing ≤ 20% ethanol and 0.05% ascorbic acid. This material would be sufficient for multiple administrations and could be further diluted to ensure the ethanol concentration is ≤ 10% (Serdons et al. 2008).
The new automated procedure produced similar yields and purity when two different commercial sources of [[89Zr][Zr(ox)4]4− that are provided with different concentration of oxalic acid (1 M and 0.05 M) and with [[89Zr][Zr(ox)4]4− within 1 day of production as well as 4 and 10 days post-production were used (Additional file 1: Tables S2–S3). Similar radiochemical yields were also obtained when two different sources of H3DFOsq-bisPhPSMA and H3DFOsq-TATE were used (one prepared in-house and one prepared by a commercial GMP manufacturer, Supporting Information, Table S2-S3). The procedures described here produced similar results with radioactivity ranging from 150 MBq to 1.02 GBq. As a final validation at least seven independent syntheses of each tracer were completed. Each independent synthesis was assessed by five parameters, radiochemical purity (by HPLC and radioTLC), radiochemical yield, visual appearance and pH (Additional file 1: Tables S2–S3). Each independent synthesis resulted in > 95% radiochemical purity at end of synthesis, as analyzed by both radio-HPLC and radio-iTLC, with radiochemical yields of 80–90%.
Discussion
In this work we developed a method for the synthesis of [89Zr]ZrCl4 that used an ion exchange cartridge (PS-HCO3 SAX) that is supplied in the bicarbonate form. This cartridge does not require pre-conditioning with acetonitrile, a significant advantage when compared to cartridges that use QMA resin in the chloride form. Following application of [[89Zr]ZrIV(ox)4]4− in oxalic acid to PS-HCO3 SAX cartridges, elution with a mixture of hydrochloric acid (0.05 M)/ sodium chloride (2.5 M) allowed isolation of [89Zr]ZrCl4 with recovery efficiencies of ~ 92%. The relatively low concentration of acid used allows the pH to be buffered with sodium acetate and does not require the addition of a stronger base (sodium carbonate). This pH neutralisation step is cumbersome and often performed manually by careful adjustment of pH to minimise the formation of zirconium hydroxide, and colloidal species (McInnes et al. 2017; Aja et al. 1995; Hu et al. 2013). It is possible that the weakly basic HCO3− counterion, which would react with hydrochloric acid to give carbon dioxide and water, coupled with the different ion-pairing and ion-exchange properties of HCO3− contribute to efficient elution with relatively low concentrations of hydrochloric acid/sodium chloride. In a separate investigation that evaluated the efficiency of eluting of [18F]F− from QMA cartridges with diaryliodonium salts the HCO3− form of the QMA cartridge out performed all other anions tested (Maisonial-Besset et al. 2018).
The PS-HCO3 SAX cartridge can be easily incorporated into an automated radiopharmaceutical production synthesiser and we developed an automated process using a process for producing [89Zr]ZrCl4 that was adapted to a commercial iPHASE MultiSyn synthesis module using disposable cassettes. Excellent trap** of [89Zr]ZrIV (> 95%) was observed on the PS-HCO3 cartridge and [89Zr]ZrCl4 could be recovered with ~ 90% efficiency when eluted with a mixture of NaCl (1.0 M) and HCl (0.1 M). This mixture can be used directly for radiolabeling reactions. For example, the [89Zr]ZrCl4 can be directly eluted into reaction vials pre-loaded with H3DFO conjugates such as H3DFOSq-bisPhPSMA dissolved in a aqueous buffer for direct radiolabelling. Our automated process allowed isolation of [89Zr]ZrDFOSq-bisPhPSMA in ~ 90% yield in greater than 97% RCP without the need of C18 SPE purification. For the automated synthesis of [89Zr]ZrDFOSq-TATE the radiochemical yields was optimized by the addition of higher quantities of ethanol and with an additional ethanol rinse step. The ethanol concentration can be reduced to ~ 10% by dilution with saline (Serdons et al. 2008). The automated processes reported here are amenable to scale-up for centralized multiple-dose preparation and validation to current good manufacturing practice (cGMP) standards. The automated methodology for the production of [89Zr]ZrCl4 presented here can also be used as precursor in the formation of [89Zr]ZrDFOSq-antibody conjugates and this will be reported in a separate publication.
Conclusion
Application of [89Zr][Zr(ox)]4− to a PS-HCO3 SAX cartridge followed by elution with a mixture of 0.1 M hydrochloric acid and sodium chloride allowed isolation of oxalate free [89Zr]ZrCl4. This method does not require removal of HCl by evaporation making this process relatively fast and efficient. This process has also been adapted to an automated procedure on a commercial synthesizer. The mixture of [89Zr]ZrCl4 in dilute acid can be neutralized with sodium acetate buffer allowing for direct reactions with ligands and has been used in a fully automated process for the synthesis of [89Zr]ZrDFOSq-bisPhPSMA and [89Zr]ZrDFOSq-TATE in high radiochemical yields with high radiochemical purity. The fully automated procedures for the production of [89Zr]ZrCl4 and its use in radiolabeling are well suited to support the centralized manufacture of multiple dose preparations of zirconium-89 based radiopharmaceuticals.
Methods
Manual [ 89 Zr]ZrCl 4 production using QMA cartridge and radiolabelling of H 3 DFOSq-bisPSMA
[[89Zr][Zr(ox)4]4− was purchased from Austin Health in 0.05 M Oxalic acid. Waters Sep-Pak Accell Plus QMA Plus Light Cartridge, 130 mg sorbent per cartridge, 37–55 µm was used to convert [[89Zr][Zr(ox)4]4− to [89Zr]ZrCl4. The cartridge was activated using 6 mL of acetonitrile, 10 mL of 0.9% saline and 10 mL Milliq Water. [[89Zr][Zr(ox)4]4− (100–200 µL in 0.05 M oxalic acid, 50 -150 MBq) was loaded onto a preactivated QMA cartridge. The cartridge was then washed with water (50 mL) to remove oxalic acid and then the activity was eluted as [89Zr]ZrCl4with 400 µL of 1.0 M HCl in a typical recovery of 80–90%. [89Zr]ZrCl4 (10–100 μL, 5 vol. equiv., 10–100 MBq, in 1 M HCl,) was neutralised to pH 4–5 with sodium acetate (3 M) then ethanol (1 vol. equiv.) was added to make final labelling mixture containing 20–25% ethanol and 0.6 M sodium acetate. H3DFOSq-bisPSMA (0.4 µg/MBq, in 1:1 MQ:ethanol) was added and the reaction mixture was agitated at 75 °C for 15 min. The reaction mixture was analysed by radio-HPLC. The traces showed > 95% radiochemical yield with a radiochemical purity of > 95%.
Manual [ 89 Zr]ZrCl 4 production using READI-CLING™ PS-HCO 3 SAX cartridge
READI-CLING™ PS-HCO3 SAX cartridge was obtained from Huayi Isotopes. The cartridge was conditioned with MilliQ water (10 mL) then [[89Zr][Zr(ox)4]4− solution in 0.05–1 M oxalic acid was passed through the cartridge. The cartridge was then washed with water (50 mL) to remove oxalic acid and then the activity was eluted as [89Zr]ZrCl4with 1 N NaCl/0.1 M HCl (1 mL) in > 90% radiochemical yield.
General method for Automated production of [89Zr]ZrDFOSq-bisPSMA/TATE
The manufacturing room was prepared in accordance with facility standard operating procedures. The reagents were either sourced commercially or freshly prepared in the lab, 0.1 M HCl solution in 1 M sodium chloride was drawn in a sterile 1 mL syringe provided with the disposable cassette, stored capped with sterile needle until required. H3DFOSq-bisPSMA/TATE precursors were dissolved in ethanol/water (1:1) to make a 5 mg/mL solution then added a volume of precursor solution corresponding to the mass of precursor required for the radiolabelling to a 1.2 mL 0.25 M sodium acetate vial. To the same vial, additional ethanol was added to make a final ethanol concentration of 20% (v/v) and 2.5% Na-gentisate (w/v). The disposable cassette MSH-3000 was modified and assembled as shown in Fig. 3a then mounted on the MultiSyn module Fig. 3b. The iPHASE Multisyn module was then switched on and the sequence scheme was loaded and prompts followed to perform initial leak checks. Following the sequence prompts, the 1 mL syringe containing 0.1 M hydrochloric acid in 1 M sodium chloride solution was mounted at position 1 (manifold 1), the vial containing 10 mL saline for injection was mounted at position 11 (manifold 4), 2 mL ethanol in a vial was mounted at position 10 (manifold 4), 100 mL water for injection bag was placed on a spike at position 5 (manifold 2), precursor solution was loaded into the center port of the reactor, the pre-prepared 15 mL sterile product collection vial in a shielded transport container was connect with the product delivery line at position 12 (manifold 4), and finally the calibrated sample of [[89Zr][Zr(ox)4]4− in oxalic acid in a 15 mL Falcon tube was connected to the device via a PEEK needle inlet at position 2 (manifold 1). The hot cell was closed and the sequence prompts were followed to commence radiosynthesis. The synthesis was completed in approximately 30 min. The product collection vial was removed and final product volume was calculated (12–15 mL), the product activity was recorded using a calibrated dose calibrator to calculate radiochemical yield (80–92%), and the product underwent QC analysis by radio-HPLC (95%), radio-TLC (> 95%), pH (5–7) and visual appearance testing (colorless and particulate free). The products were found to be stable up to 6 days from EOS when stored at room temperature.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its Additional file 1].
References
Aja SU, Wood SA, Williams-Jones AE. The aqueous geochemistry of Zr and the solubility of some Zr-bearing minerals. Appl Geochem. 1995;10:603–20.
Constable DJC, Jimenez-Gonzalez C, Henderson RK. Perspective on solvent use in the pharmaceutical industry. Org Process Res Dev. 2007;11:133–7.
De Feo MS, Pontico M, Frantellizzi V, Corica F, De Cristofaro F, De Vincentis G. 89Zr-PET imaging in humans: a systematic review. Clin Transl Imaging. 2022;10:23–36.
do Carmo SJC, Scott PJH, Alves F. Production of radiometals in liquid targets. EJNMMI Radiopharm Chem. 2020, 5: 2.
Felix D, Carsten K, Sergio Muñoz V, Thomas F, Heike E, Melanie H, Manuel R, Bernd N, Klaus S, Alexander ED, Markus D. An 89Zr-labeled PSMA tracer for PET/CT imaging of prostate cancer patients. J Nucl Med. 2022;63:573.
Grodowska K, Parczewski A. Organic solvents in the pharmaceutical industry. Acta Pol Pharm. 2010;67:3–12.
Heskamp S, Raavé R, Boerman O, Rijpkema M, Goncalves V, Denat F. 89Zr-immuno-positron emission tomography in oncology: state-of-the-Art 89Zr radiochemistry. Bioconjugate Chem. 2017;28:2211–23.
Holland JP, Sheh Y, Lewis JS. Standardized methods for the production of high specific-activity zirconium-89. Nucl Med Biol. 2009;36:729–39.
Hu Y-J, Knope KE, Skanthakumar S, Kanatzidis MG, Mitchell JF, Soderholm L. Understanding the role of aqueous solution speciation and its application to the directed syntheses of complex oxidic Zr chlorides and sulfates. J Am Chem Soc. 2013;135:14240–8.
ICH guideline Q3C (R8) on impurities: guideline for residual solvents Step 5. Published by Committee for Medicinal Products for Human Use, European Medicines Agency, 2022.
Jauw YWS, O’Donoghue JA, Zijlstra JM, Hoekstra OS, et al. 89Zr-Immuno-PET: toward a noninvasive clinical tool to measure target engagement of therapeutic antibodies in vivo. J Nucl Med. 2019;60:1825–32.
Maisonial-Besset A, Serre A, Ouadi A, Schmitt S, Canitrot D, Léal F, Miot-Noirault E, Brasse D, Marchand P, Chezal J-M. Base/cryptand/metal-free automated nucleophilic radiofluorination of [18F]FDOPA from iodonium salts: importance of hydrogen carbonate counterion. Eur J Org Chem. 2018;2018:7058–65.
McInnes LE, Rudd SE, Donnelly PS. Copper, gallium and zirconium positron emission tomography imaging agents: the importance of metal ion speciation. Coord Chem Rev. 2017;352:499–516.
Noor A, Van Zuylekom JK, Rudd SE, Waldeck K, Roselt PD, Haskali MB, Wheatcroft MP, Yan E, Hicks RJ, Cullinane C, Donnelly PS. Bivalent inhibitors of prostate-specific membrane antigen conjugated to desferrioxamine B squaramide labeled with zirconium-89 or gallium-68 for diagnostic imaging of prostate cancer. J Med Chem. 2020;63:9258–70.
Noor A, Van Zuylekom JK, Rudd SE, Roselt PD, Haskali MB, Yan E, Wheatcroft M, Hicks RJ, Cullinane C, Donnelly PS. Imaging somatostatin positive tumors with Tyr3-octreotate/octreotide conjugated to desferrioxamine B squaramide radiolabeled with either zirconium-89 or gallium-68. Bioconjugate Chem. 2021;32:1192–203.
Pandey MK, Engelbrecht HP, Byrne JF, Packard AB, DeGrado TR. Production of 89Zr via the 89Y(p, n)89Zr reaction in aqueous solution: effect of solution composition on in-target chemistry. Nucl Med Biol. 2014;41:309–16.
Pandya DN, Bhatt NB, Almaguel F, Rideout-Danner S, Gage HD, Sai KKS, Wadas TJ. 89Zr-chloride can be used for immuno-PET radiochemistry without loss of antigen reactivity in vivo. J Nucl Med. 2019;60:696–701.
Q3C(R8) Impurities: Guidance for Residual Solvents Guidance for Industry, 2021, Published by U.S. Department of Health and Human Services Food and Drug Administration, FDA-2020-D-1301.
Rosar F, Khreish F, Marlowe RJ, Schaefer-Schuler A, Burgard C, Maus S, Petto S, Bartholomä M, Ezziddin S. Detection efficacy of [89Zr]Zr-PSMA-617 PET/CT in [68Ga]Ga-PSMA-11 PET/CT-negative biochemical recurrence of prostate cancer. Eur J Nucl Med Mol Imaging. 2023;50:2899–909.
Serdons K, Verbruggen A, Bormans G. The presence of ethanol in radiopharmaceutical injections. J Nucl Med. 2008;49:2071–2071.
van Dongen GAMS, Beaino W, Windhorst AD, Zwezerijnen GJC, Oprea-Lager DE, Hendrikse NH, Kuijk CV, Boellaard R, Huisman MC, Vugts DJ. Fully automated 89Zr labeling and purification of antibodies. J Nucl Med. 2019; 60, 691.
van Dongen GAMS.; Beaino, W.; Windhorst, A. D.; Zwezerijnen, G. J. C.; Oprea-Lager, D. E.; Hendrikse, N. H.; Kuijk, C. v.; Boellaard, R.; Huisman, M. C.; Vugts, D. J. The Role of 89Zr-Immuno-PET in Navigating and Derisking the Development of Biopharmaceuticals. J Nucl Med. 2021, 62, 438–445.
Verel I, Visser GWM, Boellaard R, Walsum MS-V, Snow GB, van Dongen GAMS. 89Zr immuno-PET: comprehensive procedures for the production of 89Zr-labeled monoclonal antibodies. J Nucl Med. 2003;44:1271–81.
Wichmann CW, Ackermann U, Poniger S, Young K, Nguyen B, Chan G, Sachinidis J, Scott AM. Automated radiosynthesis of [68Ga]Ga-PSMA-11 and [177Lu]Lu-PSMA-617 on the iPHASE MultiSyn module for clinical applications. J Labelled Compd Radiopharm. 2021;64:140–6.
Wichmann CW, Poniger S, Guo N, Roselt P, Rudd SE, Donnelly PS, Blyth B, Van Zuylekom J, Rigopoulos A, Burvenich IJG, Morandeau L, Mohamed S, Nowak AK, Hegi-Johnson F, MacManus M, Scott AM. Automated radiosynthesis of [89Zr]Zr-DFOSq-Durvalumab for imaging of PD-L1 expressing tumours in vivo. Nucl Med Biol. 2023;120–121: 108351.
Yoon JK, Park BN, Ryu EK, An YS, Lee SJ. Current Perspectives on 89Zr-PET Imaging. Int J Mol Sci. 2020;21:4309.
Acknowledgements
The authors thank Catherine Fitzgerald (Telix/IMCRC) for their excellent support of this research program. Greg Santamaria and Cyclotek Pty Ltd are thanked for the provision of radiochemistry laboratories that supported this research.
Funding
This research was partially funded by the Innovative Manufacturing Cooperative Research Centre (IMCRC) for Centralized Manufacture of Molecularly Targeted Radiation (MTR) Drugs for Cancer Treatment. Aspects of this work were supported by equipment in the Mass Spectrometry and Proteomics Facility (MSPF) (Bio21 Institute, University of Melbourne) and by equipment supported by funding from the Australian Cancer Research Foundation.
Author information
Authors and Affiliations
Contributions
All authors: study conception and design. AN, PDR, ERM and SP: performed the experiments. All authors: Interpretation of results. AN and PSD: Wrote the manuscript with contributions from all authors. PSD and MPW: funding acquisition. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Asif Noor, Peter Roselt and Paul Donnelly are listed as inventors on intellectual property relating to aspects of this research. Paul Donnelly has financial interests in Telix Pharmaceuticals and Clarity Pharmaceuticals that are unrelated to this work. Michael Wheatcroft is an employee of Telix Pharmaceuticals. Stan Poniger is the director of iPHASE technologies Pty Ltd.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Tables S1–S3; Synthesis S1 and S2; Figures S1–S4.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Noor, A., Roselt, P.D., McGowan, E.R. et al. Automated synthesis of [89Zr]ZrCl4, [89Zr]ZrDFOSquaramide-bisPh(PSMA) and [89Zr]ZrDFOSquaramide-TATE. EJNMMI radiopharm. chem. 9, 39 (2024). https://doi.org/10.1186/s41181-024-00270-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41181-024-00270-2