Abstract
Mesenchymal stromal/stem cells (MSCs) are used in many studies due to their therapeutic potential, including their differentiative ability and immunomodulatory properties. These cells perform their therapeutic functions by using various mechanisms, such as the production of anti-inflammatory cytokines, growth factors, direct cell-to-cell contact, extracellular vesicles (EVs) production, and mitochondrial transfer. However, mechanisms related to immune checkpoints (ICPs) and their effect on the immunomodulatory ability of MSCs are less discussed. The main function of ICPs is to prevent the initiation of unwanted responses and to regulate the immune system responses to maintain the homeostasis of these responses. ICPs are produced by various types of immune system regulatory cells, and defects in their expression and function may be associated with excessive responses that can ultimately lead to autoimmunity. Also, by expressing different types of ICPs and their ligands (ICPLs), tumor cells prevent the formation and durability of immune responses, which leads to tumors' immune escape. ICPs and ICPLs can be produced by MSCs and affect immune cell responses both through their secretion into the microenvironment or direct cell-to-cell interaction. Pre-treatment of MSCs in inflammatory conditions leads to an increase in their therapeutic potential. In addition to the effect that inflammatory environments have on the production of anti-inflammatory cytokines by MSCs, they can increase the expression of various types of ICPLs. In this review, we discuss different types of ICPLs and ICPs expressed by MSCs and their effect on their immunomodulatory and therapeutic potential.
Similar content being viewed by others
Introduction
MSCs are a group of multipotent stem cells usually found in all body tissues [1]. These cells can usually be isolated and expanded in the laboratory from bone marrow, adipose tissue, umbilical cord, etc. [2]. According to past studies, it has been shown that these cells can be used in the treatment of various types of diseases, such as autoimmune and infectious, as well as in treatments based on tissue regeneration due to their multiple characteristics [3, 4]. It has also been shown that the use of MSCs in live, apoptotic, and dead states can have different therapeutic effects [5, 6]. One of the features that has led to a lot of attention to these cells is their ability to self-renew and differentiate [7, 8]. MSCs in therapeutic applications, after being injected into animal models or patients, maintain their self-renewal ability for an acceptable period, proliferate and differentiate in the damaged tissue-related cells [9], and help restore the damaged tissue [10, 11]. These cells produce various growth factors, including VEGF, PDGF, EGF, HGF, IGF-1, FGF-4, FGF-2, FGF-7, BMP-7, and FGF-9 [12, 13], which leads to the growth, proliferation, and differentiation of healthy cells in the tissues also increase and prevent the destruction of other tissue cells [14, 15]. It is also known that MSCs have a high immunomodulatory potential [16,17,18]. Investigations to find the reasons for this kind of therapeutic effect of MSCs have shown that MSCs perform this action by producing anti-inflammatory cytokines, including IL-13, IL-16, IL-10, and TGF-β [19]. In addition, various chemokines are produced by MSCs, which can increase the recruitment of immune system regulatory cells to the injury site [20, 21]. In addition to the mentioned mechanisms, studies have shown that MSCs can perform their therapeutic functions by producing extracellular vesicles (EVs), especially exosomes (EXOs) [22,23,24]. EXOs are secreted from the cell after formation in the multivesicular body (MVB) [25]. These vesicles contain various substances, including proteins (enzymes, cytokines, growth factors, chemokines), nucleic acids (single-stranded and double-stranded DNA, mRNA, miRNA, lncRNA, and circRNA), and lipids [26,27,28]. Also, novel studies have shown that exosomes can carry whole mitochondria or mitochondria-related subunits to the damaged cells and restore their functions [29]. However, many studies state that changes in the culture conditions of MSCs can increase their therapeutic ability [2, 30, 31]. For example, 3D culture of MSCs can increase their immunomodulatory capacity by increasing cytokine and exosome production [32]. Also, placing them in conditions such as hypoxia [33], cultivation in inflammatory conditions [34], and using genetic engineering methods [35] can increase these cells' theraputic efficacy. But how this modifications (culture in inflammatory condition) takes place was questioned and debated for years. Some studies have highlighted the importance of exosomes and EVs derived from MSCs in their therapeutic potential [36]. However, some other studies have shown that the therapeutic effects of MSCs occur through cell-to-cell interaction [37,38,39,40] (Table 1).
Molecules belonging to a family called ICPs play an important role in regulating immune system responses induced by regulatory cells, including Tregs [73, 74]. Therefore, in recent years, researchers have investigated whether these molecules and their ligands are expressed on the surface of MSCs and their role in regulating immunity induced by these cells. The results show that MSCs express different types of ICPs and their ligands, and they can be called quasi-immune regulatory cells. Studies show that the functions of ICPs and their ligands play a role in the therapeutic ability of MSCs in the treatment of inflammatory diseases such as autoimmune. In this review, we collect published information about the expression of ICPs and their ligands (ICPLs) on the MSCs' surface. First, we will talk about ICPs, their functions, and their role in disease and treatment, and then we will talk in detail about the studies on each of the ICPs expressed by MSCs.
Immune checkpoints (ICPs) in disease and treatment
ICPs are usually considered as membrane receptors present on immune cells, while the ICPLs are expressed on the so-called “target cells” (namely, tumor cells, APCs, and stromal cells of different origins). This notion was modified markedly with the finding that CTLA-4, the major immune checkpoint molecule expressed on CTLs, can be expressed not only in lymphocytes but also by tumor cells [75, 76] and DCs [77]. As mentioned, the primary function of ICPs is the immune system response homeostasis (especially T cell responses) and preventing inappropriate responses [78]. ICPs play an essential role in peripheral tolerance and prevent the development of autoimmunity [79,80,81]. At the same time, tumor cells can express ICPs and prevent immune responses against these cells during tumor growth and development [82, 83]. Table 2 briefly shows the different types of ICPs, their expressing cells, and their roles (Table 2).
The expression of ICPs by tumor cells and vesicles produced by them (exosomes and MVs) lead to the differentiation of T cells to Treg [140, 141], the differentiation of macrophages to the M2 phenotype [142, 143], the reduction of the cytotoxic T lymphocytes (CTLs) functions [144, 145], the reduction of the immune cells recruitment to the tumor site, the increase of the recruitment of myeloid-derived suppressor cells (MDSCs) and Tregs [146], and induces of exhaustion [147], senescence and apoptosis in immune cells [140, 148]. It has also been shown that ICPs play an important role in the pathogenesis and persistence of infections related to malaria [149], human immunodeficiency virus (HIV) [150], and hepatitis B virus (HBV) [151]. Studies have shown that CD4+ and CD8+ T cells of patients infected with P. falciparum express PD-1 to a large extent [152]. This action can contribute to the immune evasion mechanism induced by P. falciparum [153]. Also, the infection of immune cells with HIV leads to an increase in the expression of ICPs such as CTLA-4, PD-1, LAG-3, and TIM-3, which disrupts the functions of NK cells and CTLs leads to preventing the removal of virus-infected cells [153, 154]. Therefore, ICPs can act as a double-edged sword based on which cells are expressed and at which stage of immune cells development ICPs interact with them [155].
During the past decades, due to the role defined for ICPs in the pathogenesis of infectious diseases and cancer, blocking the function of these molecules has been proposed to treat these diseases. In many studies, antibody-based immune checkpoint blockades (ICBs) have been used to inhibit the function of ICPs and have had promising results [156, 157]. However, it seems that blocking the function of one of the ICPs leads to a compensatory increase in the expression of other ICPs on the surface of tumor and infected cells [158, 159]. Table 3 summarizes 10 FDA-approved ICBs that are used in different types of cancers. It has also been shown that drugs such as metformin [160], curcumin [161], etoposide [162], etc., can affect the expression of ICPs and their ligands. In addition, nanobodies [163, 164] and small molecules [165] have also been used to block the functions of ICPs.
However, as we said, the function of ICPs in physiological conditions is essential for health, and defects in the expression of these molecules can lead to various autoimmunities [166]. Based on this and considering the immunomodulatory role of MSCs, it is suggested that ICPs have a vital role in the therapeutic potential and immunosuppression induced by these cells. Various studies have shown that MSCs express different types of ICPs and their ligands and thus can influence the responses of T cells, macrophages, NK cells, and other innate and adaptive immune system cells. Also, the expression of ICPs affects the MSCs' regenerative potential and migration ability. In addition, knowing the ICPs of MSCs can help to decide on their selection as an appropriate treatment option for various diseases.
Cytotoxic T-lymphocyte associated protein 4 (CTLA-4) expression by MSCs
CTLA-4, one of the most important immune checkpoints expressed on immune system regulatory cells, including Treg cells, plays an important role in immunomodulation [167]. A defect or mutation in the expression of this molecule can lead to inflammatory responses and various autoimmune diseases [168,169,170]. New studies have shown that MSCs can also express CTLA-4 and thus play a role in immune regulation [171]. Studies show that cells express different isoforms of CTLA-4 in different conditions. Also, CTLA-4 expressed by MSCs through alternating splicing can be in the form of 4 isoforms [171], which include 1) the full-length version (flCTLA-4) that has all the regions related to binding to the ligand, transmembrane, transduction intracytoplasmic domain [172], 2) the type that lacks the ligand binding domain (liCTLA-4), 3) lacks the transmembrane domain (sCTLA-4) and is secreted into the extracellular environment, and the fourth type that lacks both ligand binding and transmembrane domain (1/4 of CTLA-4) [172].
Further investigations of MSCs through qPCR analysis show that the expression level of sCTLA-4 is higher than that of flCTLA-4 [171]. The critical point is that hypoxia can increase the expression of sCTLA-4 in MSCs [171]. sCTLA-4 produced by MSCs can be detected in the supernatant, and the therapeutic uses of the MSCs-derived supernatant [173] play an essential role in induced anti-inflammatory responses [85]. In a study, it has been shown that the addition of anti-CTLA4 antibody to the coculture of MSCs and PBMCs stimulated with phytohemagglutinin (PHA), both in hypoxic and normoxic conditions, leads to the suppression of anti-inflammatory responses induced by MSCs [171]. These results show the importance of sCTLA-4 and flCTLA-4 associated with MSCs in their anti-inflammatory responses.
Another study showed that anti-CTLA-4 antibodies could not reverse the MSCs-induced anergy in T cells [174]. Therefore, it seems this type of anergy, which results from the co-culture of T cells with MSCs, is through a pathway independent of CTLA-4 [175]. Also, in another study, this issue has been confirmed and shown that MSCs independent of CTLA-4 increase the frequency and differentiation of Treg and lead to the reduction of Th17 cells [176].
Programmed cell death ligand (PD-L) expression by MSCs
Programmed cell death-1 (PD-1) and its ligands PD-L1 and PD-L2 are crucial in controlling immune responses in the hemostasis phase. PD-L1 and PD-L2 are expressed on the surface of tumor cells and lead to immune deviation [177]. MSCs express PD-1, PD-L1, and PD-L2 and bind to their ligands on the surface of B cells, T-cells, and other cells [178]. PD-1/PD-L1 interaction can suppress T cell functions through different mechanisms. One of these mechanisms is the suppression of proliferation in T cells [179]. As mentioned in various studies, the presence of IFN-γ can lead to an increase in the immunosuppressive ability of MSCs, which does this by increasing the expression of PD-L1. According to new studies, it has been shown that binding of IFN-γ to the IFN-γR in MSCs leads to the activation of the JAK/STAT1/IRF1 pathway, and by binding the IRF1 to the PD-L1 promoter increases its expression. On the other hand, TNF-α, as another pro-inflammatory cytokine, does this by activating the NF-κB transcription factor. The important point is that TNF-α alone is not able to increase the expression of PD-L1, and it does this effect synergistically in combination with IFN-γ. In this way, the NF-κB transcription factor helps in this process by increasing the expression of IFN-γR by MSCs [180].
In such a way that in the case of MSCs culture in the presence of anti-PD-L1 siRNA or MSCs co-culture with active lymphocytes derived from IFNγ−/−, it cannot stimulate the suppressive function of MSCs [181]. Polyinosinic-polycytidylic acid (polyI:C), as a synthetic ligand of Toll-like receptor 3 (TLR3), increases PD-L1 expression in tonsil-derived MSCs [182]. The results of a study that used Poly I:C pretreated MSCs show that their co-culture with T cells isolated from the spleen leads to the suppression of the differentiation of naive T cells into Th1, Th2, and Th17 [182]. However, this inhibition seems to be stronger for Th17 than others. Based on the results of this study, when MSCs are used for their immunomodulatory properties, manipulating them, such as adding Poly I:C, can increase these properties and achieve better treatment results. As we know, MSCs reduce the differentiation of inflammatory cells and improve the differentiation and function of Treg cells [183]. The results of Fei Gao et al.'s study showed that MSCs perform this action at least partially by expressing PD-L1 and by inhibiting the Akt/mTOR signaling pathway [184]. Because the use of siRNA against PD-L1 can suppress the function of MSCs in stimulating Treg differentiation to some extent. The use of MSCs expressing PD-L1 in TNBS-induced colitis rats leads to the improvement of disease symptoms [184]. Also, PD-1/PD-L1 interaction between adipose tissue-derived MSCs and PBMCs can suppress TCD8+ and TCD4+ cell responses through the negative regulation of NF-κB function [185]. The results of the study conducted by Kaijian Zhou et al. show that the co-culture of MSCs and PBMCs significantly reduces the phosphorylation of NF-κB, which is a critical step in the migration of this factor to the nucleus for transcription, and this action is in the presence of anti-PD- L1 antibodies is inhibited [185].
New studies also confirm the results of previous studies. In a study conducted by Rosanna Di Tincoet et al. in 2021, it has been shown that the co-culture of MSCs derived from dental pulp with PBMCs can lead to a significant decrease in mRNA levels of IL-10, IFNγ, CXCL10, TNF-α, IL-2, and CCL5 [186]. This co-culture also led to a significant increase in PD-L1 expression in MSCs. However, the study's results show that using PD-L1 antibodies and their blockade does not lead to losing the immunosuppressive effect of MSCs [187]. Therefore, it is suggested that MSCs use alternative methods to suppress the immune response. In this study, it has been shown that the PD1/PD-L1 pathway coordinates with the Fas/FasL pathway by increasing the expression of FasL (in the presence of PD-L1 blockade) by MSCs to modulate immune system responses in PBMCs [188]. PD-L1 expressed by MSCs modulates the responses of PBMCs but also helps MSCs maintain immunomodulatory properties [186]. In addition to the expression of PD-L1 on the surface of MSCs, these cells can secrete PD-L1 into the extracellular environment. Inflammatory licensing for MSCs through an N-glycosylation-dependent post-translational regulatory mechanism leads to increased expression and secretion of PD-L1 by MSCs [189]. Also, the level of PD-L1 expression has a strong relationship with their therapeutic and immunomodulatory potentials in the mouse model of autoimmune hepatitis [190].
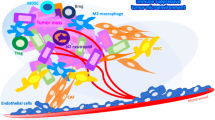
It is also possible that in addition to PD-L1 expressed on the surface and secreted into the extracellular environment by MSCs, this molecule is transferred to the target cell by small extracellular vesicles (sEVs) and exerts its immunoinhibitory function. Studies show that in patients with aGvHD, the amount of PD-L1 containing sEV in blood plasma increases after infusion of Wharton jelly-derived MSCs. It has also been shown that the amount of this sEV is entirely related to the time of injection, and 30 min after the injection, the increase in the amount of PD-L1 containing sEV can be evaluated (Fig. 1). These extracellular vesicles can suppress T-cell responses in a TCR-dependent manner. Meanwhile, using Wharton's jelly MSCs genetically modified by the CRISPR/CAS9 system to not express PD-L1 produces sEVs that cannot suppress T-cell responses [191]. Finally, it has been shown that the use of Wharton jelly derived-MSCs can lead to the improvement of patients with aGvHD symptoms and conditions. This study also reports that, in addition to the fact that, like previous studies [192], IFNγ increases the expression of PD-L1 by MSCs but also leads to an increase in the secretion of sEV-PD-L1 from these cells [193].
PD-L1 mediated immunomodulatory and therapeutic potential of mesenchymal stromal/stem cells (MSCs). PD-L1 expressed by MSCs can interact with PD1 on the surface of T cells in three mechanisms. In the first option, PD-L1 is transferred to the cell plasma membrane and, in this way, inhibits the functions of T cells through direct cell-to-cell interaction. In the second mechanism, PD-L1 is secreted into the extracellular environment. For this reason, MSCs-derived supernatant can induce PD-L1-mediated immunomodulatory functions in co-culture with T cells or PBMCs. In the third method, MSCs affect the response of T cells by transferring PD-L1 to extracellular vesicles (EVs) such as exosomes. The interaction between PD-1 and PD-L1 leads to the creation of signaling cascades in T cells, which inhibits their proliferation and differentiation into inflammatory populations, including Th1, Th2, and Th17, as well as the production of inflammatory cytokines. It has also been found that this interaction also inhibits the function of TCD8 + cells. However, this interaction increases the differentiation of T cells towards regulatory T cells. In addition, it has been shown that signal transmission from PD-1 leads to positive feedback to PD-L1+ MSCs and enhances their immunomodulatory functions. It has also been shown that the use of inflammatory environments and pretreatment of MSCs with TLR ligands, including poly I:C, can lead to an increase in the immunomodulatory potential of MSCs by increasing the expression and production of PD-L1. This is while the use of anti-PD-L1 antibodies and siRNA inhibiting its expression and translation can reduce the immunomodulatory potential of MSCs. Therefore, the expression of PD-L1 plays an essential role in the immunomodulatory potential of MSCs, which leads to an increase in its therapeutic potential in autoimmune colitis and psoriasis animal models
Inducible costimulator ligand (ICOSL) expression by MSCs
ICOSL is a member of the B7 family and plays a vital role in follicular helper T cell interaction and high-affinity antibody production [194]. According to the results of the studies, it has been shown that blocking the ICOS-ICOSL interaction aggravates the experimentally induced allergic encephalomyelitis in the model [195]. Therefore, the inhibitory signaling resulting from this interaction seems to negatively affect the immune responses. ICOS is expressed by various types of cells, including tumor cells, antigen-presenting cells, and epithelial cells, and is very important for the function of Treg cells [196, 197]. Studies have shown that placing MSCs in inflammatory conditions leads to increased expression of ICOSL by these cells [198].
The results of the study conducted by Lee et al. show that, in addition to other mechanisms, the co-culture of MSCs with T cells increases the differentiation of Treg cells through the ICOS-ICOSL interaction-dependent pathway. It has also been shown that blocking ICOSL expressed on the surface of MSCs reduces their ability to induce Treg cell responses [199].
Another study conducted in 2021 showed that the use and coculture of MSCs with PBMCs leads to the inhibition of type 2 responses by inhibiting the differentiation of Th2 cells and type 2 innate lymphoid cells (ILC2) [200]. A significant point about these cells is that the direct cell-to-cell contact of MSCs with ILC2 mediated by ICOS-ICOSL interaction leads to increased ILC2 activity [201]. The results show that MSCs exert their inhibitory effects on ILC2 functions through the induction of Treg cells [201]. Tregs alone cannot inhibit the responses of ILC2s, but after co-culture with MSCs, they acquire this ability [201]. Further investigations to find the mechanism of MSCs' influence on the suppressive responses of Tregs show that ICOS-ICOSL interaction is one of the main factors [201]. Therefore, it was demonstrated that the co-culture of Tregs with MSCs through ICOS-ICOSL interaction increases the ability of Tregs to suppress the functions of ILC2 and leads to a significant decrease in the production of IL-13, IL-9, and IL-5 cytokines by ILC2 [201]. Studies show that Tregs do this by producing IL-10 induced by ICOS-ICOSL interaction [201] (Fig. 2).
ICOSL mediated immunomodulatory of mesenchymal stromal/stem cells (MSCs) on T cells and ILC2. MSCs express a low amount of ICOSL in the naive state. However, the co-culture of MSCs by TCD4+ cells in the regulatory T cell-inducing conditions increases ICOSL expression up to tenfold according to qPCR results. Also, this co-culture leads to an increase in the number and percentage of regulatory T cells compared to the culture of TCD4.+ cells only under regulatory induction conditions (as the control group). It has been shown that the co-culture of MSCs with PBMCs also leads to the expansion and function of the regulatory T-cell population. The effect of MSCs on ILC2 functions is direct and indirect. In indirect conditions, the co-culture of MSCs with PBMCs suppresses the functions of ILC2 by expanding the population of regulatory T cells and their IL-10 production. Meanwhile, the direct co-cultivation of ILC2 with MSCs increases the functions of ILC2 through ICOS-ICOSL interaction. ILC-2 that have been directly co-cultured with MSCs have a high survival rate and highly produce cytokines such as IL-5, IL-10, and IL-33
CD39 and CD73 expression by MSCs
As we know, adenosine is one of the suppressors of the immune system, which performs its function by binding to the A2A receptor (ADORA2A) [202]. Extracellular adenosine is usually produced from ATP by two molecules, CD39 and CD73 [203]. During this process, the adenosine deaminase converts adenosine into inosine [204]. Extracellular adenosine can suppress the proliferation and responses of T cells [205]. The results of various studies have shown that the co-culture of T cells with MSCs can suppress T cell responses [9, 60]. Therefore, considering the importance of this molecule (CD39) in T-cell responses, the researchers investigated the expression of CD39 on the surface of MSCs and its effect on modulating immunity induced by MSCs. Various methods, including flow cytometry, have shown that CD39 has a permanent expression on the surface of MSCs [206]. However, when MSCs are exposed to inflammatory conditions, the level of CD39 expression increases from 15 to 35%. Also, the amount of adenosine production, evaluated by high-pressure liquid chromatography (HPLC), was associated with an increase of 2 times in co-culture with activated T cells [206]. Therefore, in inflammatory conditions, these cells can produce more adenosine and suppress the immune system's responses more strongly. It has been shown that the co-culture of human MSCs with activated T cells leads to a threefold increase in MSCs double positive for CD39 and CD73 [206]. On the other hand, this co-culture affects the responses of T cells and is associated with a significant increase in ADORA2A in these cells, which is associated with a decrease in the proliferative activity of these cells [206]. Also, the use of ADORA2A antagonist (ZM 241385) [207] in this co-culture system led to a significant increase in proliferation in T cells, which shows the importance of adenosine in suppressing the proliferative responses of T cells [206].
Mouse studies have also shown that CD39 and CD73 are simultaneously expressed on the surface of MSCs and perform essential inhibitory functions on the immune system [208]. Many studies have suggested that soluble factors produced and secreted by MSCs, including TGF-β and HGF, lead to decreased proliferation in activated T cells [209, 210]. However, the study conducted by Sattler et al. showed that using monoclonal antibodies against HGF and TGF-β receptors does not significantly increase T cell proliferation [208]. Also, kynurenine, a tryptophan metabolite through the action of IDO enzyme [211], was not observed in the MSC supernatant. Therefore, they proposed another factor responsible for this proliferation suppression [208]. CD39 expressed on the surface of MSCs leads to the production of adenosine, suppressing the proliferation of T cells [208]. It has been shown that the use of SCH58261, an antagonist of ADORA2A [212], and the use of polyoxotungstate 1 (POM-1) as a CD39 inhibitor [213] separately lead to the reversal of the suppression induced by MSCs in coculture conditions [208].
In addition, MSCs can suppress the function and differentiation of Th17 cells through CD39 [214]. Co-cultivation of T cells with MSCs leads to a decrease in the production of IL-17A/IFN-γ and an increase in the expression of CD39 and CD73 on the surface of T cells [214]. Using monoclonal antibodies against CD39 can significantly reduce the inhibition applied to Th17 differentiation. So, it can be concluded that MSCs, through a CD39-dependent pathway, inhibit Th17 function and proliferation [214]. Also, mass spectrometry analysis showed that the amount of adenosine in the supernatant increases during the co-culture of Th17 cells and bone marrow-derived MSCs [214]. This is while the use of monoclonal antibodies against CD39 leads to a significant decrease in the production of adenosine and its amount in the supernatant of the coculture system.
Considering the importance of the expression of these molecules, in the study conducted by Tan et al., it was shown that MSCs isolated from C57BL/6 mice adipose tissue include two populations in terms of CD73 expression. One of these populations expresses CD73 at a low level (CD73low), and the other expresses a high level of CD73 (CD73high) [215]. These cells differ from each other in terms of function and therapeutic potential. Examining the ability of these cells to repair myocardial infarction (MI) damages in a model murine induced by 2OA-BSA [216] and transplanting both MSCs subpopulations show that the CD73high subpopulation has a higher ability to repair and can lead to improving the structure and function of the heart [215]. The transplantation of these two types of cells seems to have no difference in the amount and the type of recruited immune cell population to the MI heart tissue. However, the results of the study show that the CD73high subpopulation leads to the reduction of inflammation and the modulation of immune system responses through the positive regulation of the expression and production of several anti-inflammatory cytokines, including IL-4 and IL-10, and the reduction of the expression and production of inflammatory cytokines such as TNF-α. In addition to the effect on cytokines, these cells affect the expression of other anti-inflammatory molecules, including TGM-2 and arginase-1 (Arg-1), and reduce the expression of NOS2 [215]. Therefore, it seems that the immunomodulatory efficiency of CD73high MSCs is higher than the CD73low subpopulation. In this way, it helps to improve the functions and prevent damaging inflammations to the heart tissue after MI. The results of the in vitro studies also show that compared to the CD73low subpopulation, CD73high MSCs significantly lead to the differentiation of macrophages to the M2 phenotype and functions related to the regeneration of damaged tissues [215]. The importance of the effect of the expression of these molecules and the axis induced by them, that is, CD39/CD73/adenosine, in the therapeutic potential of MSCs in other diseases has also been investigated. For example, in a study where MSCs were used to treat autoimmune arthritis, the role of CD39/CD73/adenosine was evaluated [217]. Molecular studies show that MSCs reduce the expression of NF-kB and p65/p50 in vitro conditions and lead to decreased osteoclastogenesis [218]. Also, studies showed that transplantation of MSCs into autoimmune arthritis DBA/1J mice model led to decreased RANKL expression in synovial tissue and osteoclast formation [217]. Using inhibitors and blocking the function of CD39 through POM1, blocking CD73 by APCP, or inhibiting the functions of adenosine by inhibiting its receptor, i.e., adenosine A2A receptor inhibitor (SCH58261) or adenosine A2B receptor inhibitor (Alloxazine), lead to reverted treatment outcome induced by MSCs [217].
In addition to the role of markers such as CD73 and CD39 in modulating immune responses induced by MSCs, these molecules also perform other functions. Studies show that MSCs have procoagulant and anticoagulant activity [219], but their supernatant showed to cannot do phenomenon [220]. Therefore, MSCs perform this action through cell–cell interaction. It has also been shown that this inhibition of activation is independent of the molecules related to the activation of platelets, i.e., P-selectin and cyclooxygenase [220]. The study conducted by P. Netsch and his colleagues shows that MSCs isolated from different sources do this through the CD73-produced adenosine-dependent mechanism and lead to preventing the platelets activation and their aggregation. In this study, the use of adenosine deaminase (ADA), which converts adenosine to inosine [221], led to the removal of the inhibition induced by MSCs, which confirms the role of CD73 in inhibiting platelet activation [220] (Fig. 3).
CD39, CD73, and adenosine mediated immunomodulatory of mesenchymal stromal/stem cells (MSCs) on T cells, macrophages, and osteoclasts. Extracellular ATP is first converted to ADP and then to AMP through CD39. The produced AMP is used as a substrate for the function of CD73 and leads to the production of adenosine. Adenosine receptor (A2AR) can be expressed on the surface of various cells, including immune cells and tumor cells. The binding of extracellular-produced adenosine to A2AR on the surface of tumor cells can increase their survival and proliferation of tumor cells and facilitate epithelial-mesenchymal transition (EMT). Also, its binding to immune cells expressed A2AR can lead to reduced survival and production of inflammatory cytokines by inhibiting pathways related to mTORC and NF-κB. It seems that tumor cells, by expressing CD39 and CD73, help their immune escape. MSCs also express CD39 and CD73, and by producing adenosine, they affect the functions of immune cells, including T cells, macrophages, and osteoclasts. Adenosine produced by MSCs inhibits the functions of Th17 and M1 macrophages while they increase the functions of regulatory T cells and M2 macrophages. Adenosine can also reduce the function and proliferation of osteoclasts. It has also been shown that MSCs that produce adenosine can reduce the aggregation and activation of platelets
Galectins expression by MSCs
Galectins are a group of molecules related to lectins that exert their biological effects by binding to galactoside (in the receptor's structure) [222]. The results of the studies have shown that this ligand, by binding to its receptor, T cell immunoglobulin and mucin domain-containing protein 3 (TIM3), performs actions such as homeostasis of immune system responses [223]. Studies have shown that treating diseases such as autoimmune disorders and patients who have received allograft transplants leads to the improvement of the patients and increases the survival of the transplanted tissue [224]. TIM3 plays an important role in suppressing the responses of Th1 cells and prevents the production of inflammatory cytokines such as IFN-γ and TNF-α [225]. Also, the interaction of galectin-9 (Gal-9) with TIM3 leads to suppressing the functions of Th17 and cytotoxic T cells [226].
Various studies show that MSCs suppress immune system responses through Gal-9 production and have a role in their therapeutic potential. MSCs culture in IFN-γ containing medium leads to increased production of soluble Gal-9 by MSCs [227]. Therefore, it can be concluded that the production of Gal-9 from MSCs depends on the STAT and JNK signaling pathway. A study published in 2018 showed that the transplanted MSCs suppress the proliferation and differentiation of Th1 and Th17, leading to the reduction of liver inflammation in the autoimmune cholangitis mice model [228]. After transplanting MSCs to autoimmune cholangitis model mice, the level of Gal-9 in the liver and serum increases significantly. Since Gal-9 is secreted by MSCs, the supernatant of these cells can perform Gal-9-mediated therapeutic and immune suppression functions [228]. The use of α-lactose to inhibit the function of Gal-9 in MSCs-CM leads to the reduction of the therapeutic potential and reverses the suppression of the proliferation of CD4+ T cells. Therefore, it is suggested that the soluble Gal-9 produced by MSCs is one of the immunosuppressive mechanisms mediated by these cells. It has also been shown that the therapeutic potential of MSCs that express high levels of Gal-9 is significantly higher than Gal-9 blocking MSCs in endotoxemia induced by LPS [229]. Examining the spleen cells of Gal-9 highly expressed MSCs recipients shows that the amount of M2 macrophages and Treg in them is associated with a significant increase compared to other groups [229]. The therapeutic use of MSCs in septic mice has also shown that these cells can improve kidney functions in septic mice through Gal-9 production and Th1/Th2 balance adjustment. Also, the results of this study showed that the use of MSCs can affect the Th17/Treg axis by TIM3/Gal-9 interaction. Considering that the use of whole-soluble TIM3 can reverse the therapeutic effects of MSCs, it seems that Gal-9 is one of the effective immunomodulatory factors produced by these cells [230].
Another study showed that MSCs lead to the differentiation of tolerogenic DCs through the production of Gal-1 [231]. Co-cultivation of MSCs with DCs leads to a decrease in the expression of MHC-II and co-stimulatory molecules such as CD80, CD83, and CD86 on the surface of DCs. Also, this co-culture leads to an increase in the production of IL-10, IL-12, and Gal-1 in the supernatant. Further investigations show that Gal-1 produced from MSCs through inhibiting the p38 MAPK signaling pathway leads to suppression of proliferation and increased anti-inflammatory activity in DCs [231]. In addition, the study conducted by Yoo** Seo et al. showed that in the co-culture system, MSCs that produced Gal-1 to the supernatant can prevent the differentiation of microglia to M1 pro-inflammatory phenotype. This function of MSCs is suppressed through a selective Gal-1 inhibitor (OTX008) [232]. Also, MSCs can affect the alloreactive CD4+ and CD8+ T cell responses through the production of Gal-1 and inhibit their function. The result of the study conducted by Gieseke et al. shows that MSCs can reduce the proliferation of CD4+ T cells in a Gal-1-dependent manner. However, MSCs produced Gal-1 do not seem to play a role in modulating NK cell responses [233].
Therefore, it seems that MSCs produced Gal-1 play an important role in their immunomodulatory potential by affecting DCs, MQ, and T cell functions.
CD155 (Poliovirus receptor) expression by MSCs
CD155, also known as poliovirus receptor (PVR), is a ligand expressed on the surface of different cells, and its receptor expressed on the surface of T and NK cells called T cell immunoreceptor with immunoglobulin and ITIM domain (TIGIT) [234]. The interaction of CD155 with TIGIT leads to the initiating of inhibitory responses in TIGIT-expressing cells. For example, this interaction leads to a decrease in cytotoxic activity, the production of cytokines, and a reduction in the degranulation of NK cells [235]. In addition, CD226 (DNAM-1) can also bind to CD155, but the affinity of TIGIT for CD155 is higher than for CD226 [236, 237]. Also, new studies have shown that CD155 is highly expressed on the surface of MSCs and can be responsible for a part of the immune inhibition induced by these cells. NK cells express both TIGIT and CD226, which are CD155 receptors [238]. The binding of CD155 to CD226 leads to increased activity of NK cells, and its binding to TIGIT leads to functional inhibition of T cells [239]. As mentioned, the affinity of TIGIT to CD155 is higher than the binding affinity of CD226 to CD155, and therefore, due to the possible simultaneous involvement of both inhibitory and stimulatory receptors in the presence of CD155, the function of the inhibitory receptor is overcome and leads to the suppression of NK cell responses [237, 240].
The results of the studies show that in myelodysplastic syndrome (MDS), the activity of NK cells becomes abnormal, and a decrease accompanies the antibody-dependent cytotoxic activity and cytolytic activity [241, 242]. Considering the presence of MSCs in the bone marrow, it seems that these cells play a role in the progression of the disease. MSCs affect the expression and secretion of factors related to hematopoiesis and play a role in immune regulation by producing cytokines, growth hormones, intercellular communication, and exosomes [9]. The results show that the culture of MSCs in inflammatory conditions increases the amount of CD155 on the surface of these cells [243]. However, the exact mechanism of how MSCs work in different diseases is different, and depending on the type of tumor and disease, they may use different main mechanisms. Investigations to search for ligands related to NK inhibitory receptors, including CD155, CD112, and CD113, show that the expression of CD155 on the surface of MDS patients isolated MSCs increased significantly compared to MSCs isolated from healthy controls [244]. It has also been shown that blocking TIGIT and activating CD226 can reverse the inhibitory effect induced by MSCs on NK cells. Therefore, the in vitro results indicate that MSCs can suppress the inflammatory functions of NK cells through the CD155/TIGIT pathway [244, 245]. It is also known that the CD155/TIGIT pathway can lead to the exhaustion of NK cells [246]. The results of examining NK cells isolated from multiple myeloma (MM) patients also show that these cells have exhausting markers, and the level of TIGIT in them is associated with an increase compared to healthy control [234, 247]. In addition, MSCs isolated from multiple myeloma patients also have a significant increase in CD155 expression. The in vitro results conducted by Yun Liu et al. show that the co-culture of NK cells with MSCs leads to CD155/TIGIT interaction and induction of exhaustion in NK cells [234]. So, it proposed that blocking TIGIT can restore NK cell exhaustion and provide a potential avenue for antitumor immunotherapy for multiple myeloma patients.
Herpes virus entry mediator (HVEM) expression by MSCs
HVEM is another ligand that binds to immune checkpoints family family-related receptors [248]. The receptor of this molecule (HVEM) is B and T Lymphocyte attenuator (BTLA), which binds to it and inhibits the functions of lymphocytes [249]. BTLA has been identified as the third immune checkpoint after PD-1 and CTLA-4, and having 2 ITIM motifs leads to the calling of SHP2, which inhibits signaling events related to inflammatory responses [250, 251]. HVEM is expressed by various types of tumor cells, including melanoma cells, and in this way, they suppress the antitumor responses of CD8+ T cells [252]. It has also been shown that HVEM has a broader expression than PD-L1 in melanoma cells, and its expression level is associated with a poor prognosis [252, 253]. Therefore, it is imperative to investigate the cells that express this molecule and its functions.
The results of published studies have shown that HVEM is highly expressed on the surface of MSCs and is responsible for part of the anti-inflammatory functions of MSCs in treating inflammatory diseases [254]. In LPS-stimulated mice, alveolar macrophages have been shown to accumulate near lung tissue-resident MSCs (LRMSCs) [255]. Therefore, LRMSCs may be responsible for inducing anti-inflammatory responses in these macrophages. The results of RNA sequencing obtained from co-culture of LRMSCs with alveolar macrophages incubated with LPS show the downregulation of the expression of molecules involved in various inflammatory signaling pathways related to TLRs, TNF, JAK-STAT and PI3K-Akt in them [255]. Also, as a result, the level of expression of inflammatory cytokines in alveolar macrophages decreases. Also, the co-culture of LRMSCs with splenocytes in LPS-stimulated conditions reduces inflammatory responses [255]. Therefore, LRMSCs can suppress the production of inflammatory cytokines from innate and adaptive immune cells. Injection of LRMSCs into LPS-induced ARDS model rats also had similar results to in vitro. Considering the importance of HVEM, its expression level in LRMSCs isolated from LPS-induced ARDS model rats was five times higher than in LRMSCs isolated from the lungs of the sham group [255]. Further studies showed that MSCs overexpressing HVEM have a greater ability to suppress immune cell responses than MSCs with low HVEM expression. It has also been shown that the expression of BTLA on the surface of immune cells is required to induce the increased anti-inflammatory response of MSCs by HVEM.
HVEM expression has also been observed in MSCs isolated from other tumor tissues. Considering that MSCs are present in the tumor microenvironment as one of the cell populations [256], it has been shown that these cells play an important role in the chemoresistance of tumor cells in intrahepatic cholangiocarcinoma (ICC) [257]. Compared to other tissues (for example, umbilical cord-derived MSCs), MSCs isolated from ICC tissue have a high level of HVEM expression. In this way, they help the survival of tumor cells and prevent their apoptosis [257]. MSCs activate AMPK/mTOR-mediated autophagy in cholangiocarcinoma cells by overexpressing HVEM and producing IL-6 [257, 258].
Tumor necrosis factor receptor 2 (TNFR2)
As a pro-inflammatory cytokine in soluble and membrane-bound forms [259], TNF-α plays an important role in forming immune system responses [260]. This cytokine has two separate receptors, including TNFR1 and TNFR2 [261]. However, it seems that the affinity of TNFR1 for both soluble and membrane-bound forms of TNF-α is higher than TNFR2 [262, 263]. TNFR1 has a wide expression on different cells [264]. After binding to TNF-α, it can increase proliferation, differentiation, and survival by activating signaling pathways related to NF-kB and MAPK or by using the death domain (DD) and activating related RIP1-dependent and RIP1-independent leads to the activation of caspase 8 and the initiation of apoptosis in cells [263, 265]. It seems that the choice between whether the cell with TNFR1 receptor after binding to TNF-α is selected for survival or apoptosis depends on the responding cell cellular stress and metabolic state [266]. Although TNFR1, TNFR2 is expressed in some cells, including immune cells, endothelial cells, neurons, and MSCs [265]. TNFR2 signaling leads to the proliferation and differentiation of Treg cells as well as the proliferation and survival of tumor cells [267, 268]. The results of the studies have shown that antibodies against TNFR2 can be a potential treatment for patients with ovarian cancer by inducing Treg cells and apoptosis in tumor cells [269]. Therefore, today, TNFR2 is called as one of the emerging immune checkpoints [270]. Considering the therapeutic role of immunomodulatory functions in MSCs-mediated treatments, it has been shown that TNF-α /TNFR2 signaling in MSCs can lead to an increase in their therapeutic efficiency. MSCs isolated from genetically engineered mice lacking TNFR2 had less therapeutic ability than MSCs isolated from normal mice [271, 272].
In a study conducted in 2020 by Ghada Beldi et al., co-culture of WT or TNFR2 KO-MSCs with mouse T cells was used to investigate the role of TNFR2 in the immunomodulatory function of MSCs [273]. Then, T cells were stimulated by antibodies against CD3 and CD28, and by immunostaining, the cytokines related to the stimulated T cell population were evaluated. Also, different Treg markers were examined to check the percentage of these cells in each test group. The results showed the importance of the presence of TNFR2 on the immunomodulatory properties of MSCs. However, the absence of TNFR2 does not eliminate the immunosuppressive potential of MSCs [273]. It was also shown that the production of pro-inflammatory cytokines dependent on TNFR2 was associated with a significant decrease in the groups co-cultured with WT-MSCs [273].
Regarding the status and percentage of Treg cells in different experimental groups, the percentage of T cells induced to Treg was higher in the groups co-cultured with WT-MSCs, and it showed that MSCs expressing TNFR2 have higher Foxp3+ Treg induction capacity [273, 274]. Therefore, MSCs can reduce the proliferation, activation, and production of inflammatory cytokines in T cells by expressing TNFR2 [273]. Consequently, it is imperative to investigate the mechanisms related to weakened immunomodulatory in TNFR2 KO-MSCs. In another study to investigate this issue, it was reported that knocking out TNFR2 expression, in addition to decreasing the immunomodulatory ability of MSCs, leads to a decrease in specific markers characterizing these cells (except CD44) [275]. Also, the expression of inflammatory cytokines such as TNF-α, IL-6, and IFN-γ increases significantly in TNFR2 KO-MSCs [275]. This is while the expression of anti-inflammatory cytokines such as IL-10 and TGFβ decreases [275]. The comparison of nitric oxide production by two types of WT-MSCs and TNFR2 KO-MSCs also indicates that nitric oxide production in WT-MSCs expressing TNFR2 is higher than TNFR2 KO-MSCs [275]. Also, knocking out TNFR2 as an immune checkpoint on MSCs can decrease the ability of these cells to migrate and heal wounds [275].
Concluding and future perspectives
One of the main features that lead to the therapeutic applications of MSCs is their immunomodulatory potential. One of the most recent suggestions for this function of MSCs is the immune checkpoint-related mechanisms. As mentioned in this article, MSCs express different types of ICPs and their ligands. Previously, it was mentioned in many studies that pretreated MSCs in inflammatory conditions, such as adding TNF-α and IFN-γ to the culture medium, can increase their therapeutic potential by improving their immunomodulatory ability (Table 4). The exact mechanism of this issue was not clear. However, in this review, we conclude that in addition to the effect of these pro-inflammatory environments on the production of anti-inflammatory cytokines and exosomes by MSCs, inflammatory conditions by affecting different signaling pathways lead to increased ICPs and their ligands expression on the surface of MSCs and their secretion and thereby increase these cells immunomodulatory potential.
Each of the ICPs and their ligands expressed by MSCs plays an important role in their immunomodulatory ability. A very important point that came to the opinion of the authors during the review of various articles is that in studies that investigated the role of different immune checkpoints expressed by MSCs, the suppression of the pathway related to the studied ICPs led to the loss of MSCs' therapeutic potential. This is while other mechanisms associated with the therapeutic potential of MSCs, such as the production of exosomes, cytokines, growth factors, and mitochondrial transfer, are not affected by the inhibition of the ICPs and their ligands' expression or function. Also, inhibiting the expression or function of one type of ICP and its ligands on the surface of MSCs does not affect other ICPs and their ligands' functions.
In addition, it has been shown in some studies that inhibiting the functions of PD-1 and its ligand leads to a compensatory increase in the expression of proteins related to other ICPs and suppresses the inhibition induced by the treatment. It seems that in some studies, the inhibition of the immunomodulatory function of MSCs by the inhibition of an ICP has been exaggerated to some extent. As discussed in this article, it has been pointed out in some other studies that the inhibition of an ICP and its ligands can lead to a decrease in the therapeutic potential of MSCs. Still, it does not lead to its complete inhibition.
Some studies have pointed out the importance of cancer-associated MSCs (CA-MSCs) in tumor progression using various mechanisms such as immune modulation, increased metastasis, drug resistance, angiogenesis, and, finally, increased tumor growth. However, none of these studies have investigated the importance of the ICPs and their ligands' role expressed or secreted by CA-MSCs. In many tumor treatments using ICBs, only the changed responses in tumor cells and immune cells are examined, and the importance of MSCs in this type of treatment is ignored. It seems that examining the functions of CA-MSCs after treatment with ICBs can help our understanding of the effect of this type of treatment on the interaction between CA-MSCs and tumor cells.
Considering the importance of the expression of ICPs and their ligands by MSCs, various methods, including CRISPR/CAS technology, can be used to produce MSCs with high expression levels of one or more types of ICPs and ligands. Also, considering the role of 3D culture in increasing the therapeutic potential of MSCs, it seems that they affect the expression of ICPs and ligands. Therefore, studies can be designed to investigate this issue. Studies on ICPs and their ligands expressed on the surface of MSCs are low, and it seems that this field is in its early stages, and more studies are needed to reveal all its aspects.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
- MSCs:
-
Mesenchymal stromal/stem cells
- EVs:
-
Extracellular vesicles
- MSCs-EVs:
-
MSCs-derived EVs
- MSCs-CM:
-
MSCs-derived condition medium
- MVs:
-
Microvesicles
- EXOs:
-
Exosomes
- sEVs:
-
Small extracellular vesicles
- ICPs:
-
Immune checkpoints
- ICPLs:
-
Immune checkpoints ligands
- ICBs:
-
Immune checkpoint blockades
- HIV:
-
Human immunodeficiency virus
- NF-κB:
-
Nuclear factor-kappa B
- TNF-α:
-
Tumour necrosis factor-α
- Th:
-
T helper cell
- Treg:
-
Regulatory T
- IL:
-
Interleukin
- LPS:
-
Lipopolysaccharides
- MDSC:
-
Myeloid-derived suppressor cells
- CTLA-4:
-
Cytotoxic T-lymphocyte associated protein 4
- PBMCs:
-
Peripheral blood mononuclear cells
- NK cells:
-
Natural killer cells
- PD-1:
-
Programmed cell death-1
- PD-L:
-
Programmed cell death-ligand
- IFN-γ:
-
Interferon-gamma
- TLR:
-
Toll-like receptor
- polyI:C:
-
Polyinosinic-polycytidylic acid
- ICOSL:
-
Inducible costimulator ligand
- ILC2:
-
Type 2 innate lymphoid cells
- ADORA2A:
-
A2A receptor
- TGF:
-
Transforming growth factor
- IDO:
-
Indoleamine 2,3-dioxygenase
- Arg-1:
-
Arginase-1
- Gal-9:
-
Galectin-9
- TIM3:
-
T cell immunoglobulin and mucin domain-containing protein 3
- MAPK:
-
Mitogen-activated protein kinase
- TIGIT:
-
T cell immunoreceptor with immunoglobulin and ITIM domain
- MDS:
-
Myelodysplastic syndrome
- MM:
-
Multiple myeloma
- HVEM:
-
Herpes virus entry mediator
- BTLA:
-
B and T lymphocyte attenuator
- ITIM:
-
Immunoreceptor tyrosine-based inhibitory motif
- SHP2:
-
Src homology region 2 domain-containing phosphatase-2
- LRMSCs:
-
Lung tissue-resident MSCs
- ICC:
-
Intrahepatic cholangiocarcinoma
- AMPK:
-
AMP-activated protein kinase
- TNFR2:
-
Tumor necrosis factor receptor 2
- WT-MSCs:
-
Wild type MSCs
- NO:
-
Nitric oxide
References
Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Communication and Signaling. 2011;9(1):1–14.
Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regenerative medicine. 2019;4(1):22.
Li P, Ou Q, Shi S, Shao C. Immunomodulatory properties of mesenchymal stem cells/dental stem cells and their therapeutic applications. Cell Mol Immunol. 2023;20(6):558–69.
Zhou J, Shi Y. Mesenchymal stem/stromal cells (MSCs): origin, immune regulation, and clinical applications. Cell Mol Immunol. 2023;20:555–7.
Giacomini C, Granéli C, Hicks R, Dazzi F. The critical role of apoptosis in mesenchymal stromal cell therapeutics and implications in homeostasis and normal tissue repair. Cell Mol Immunol. 2023;20:570–82.
Weiss ARR, Dahlke MH. Immunomodulation by mesenchymal stem cells (MSCs): mechanisms of action of living, apoptotic, and dead MSCs. Front Immunol. 2019;10:1191.
Malekpour K, Hazrati A, Zahar M, Markov A, Zekiy AO, Navashenaq JG, et al. The potential use of mesenchymal stem cells and their derived exosomes for orthopedic diseases treatment. Stem cell reviews and reports. 2022;18(3):933–51.
Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells: biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9(1):204.
Hazrati A, Malekpour K, Soudi S, Hashemi SM. Mesenchymal stromal/stem cells and their extracellular vesicles application in acute and chronic inflammatory liver diseases: emphasizing on the anti-fibrotic and immunomodulatory mechanisms. Front Immunol. 2022;13: 865888.
Petri RM, Hackel A, Hahnel K, Dumitru CA, Bruderek K, Flohe SB, et al. Activated tissue-resident mesenchymal stromal cells regulate natural killer cell immune and tissue-regenerative function. Stem Cell Reports. 2017;9(3):985–98.
Liu Y, Wang L, Kikuiri T, Akiyama K, Chen C, Xu X, et al. Mesenchymal stem cell–based tissue regeneration is governed by recipient T lymphocytes via IFN-γ and TNF-α. Nat Med. 2011;17(12):1594–601.
Murakami J, Ishii M, Suehiro F, Ishihata K, Nakamura N, Nishimura M. Vascular endothelial growth factor-C induces osteogenic differentiation of human mesenchymal stem cells through the ERK and RUNX2 pathway. Biochem Biophys Res Commun. 2017;484(3):710–8.
Han Y, Yang J, Fang J, Zhou Y, Candi E, Wang J, et al. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct Target Ther. 2022;7(1):92.
Costa LA, Eiro N, Fraile M, Gonzalez LO, Saá J, Garcia-Portabella P, et al. Functional heterogeneity of mesenchymal stem cells from natural niches to culture conditions: implications for further clinical uses. Cell Mol Life Sci. 2021;78:447–67.
Suga H, Eto H, Shigeura T, Inoue K, Aoi N, Kato H, et al. IFATS collection: Fibroblast growth factor-2-induced hepatocyte growth factor secretion by adipose-derived stromal cells inhibits postinjury fibrogenesis through a c-Jun N-terminal kinase-dependent mechanism. Stem cells. 2009;27(1):238–49.
Gao F, Chiu S, Motan D, Zhang Z, Chen L, Ji H, et al. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 2016;7(1): e2062.
Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32(3):252–60.
Liu Q, Zheng H, Chen X, Peng Y, Huang W, Li X, et al. Human mesenchymal stromal cells enhance the immunomodulatory function of CD8+ CD28− regulatory T cells. Cell Mol Immunol. 2015;12(6):708–18.
Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14(8):493–507.
Shi Y, Hu G, Su J, Li W, Chen Q, Shou P, et al. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res. 2010;20(5):510–8.
Wang M, Yuan Q, **e L. Mesenchymal stem cell-based immunomodulation: properties and clinical application. Stem cells international. 2018;2018:3057624.
Malekpour K, Hazrati A, Soudi S, Roshangar L, Pourfathollah AA, Ahmadi M. Combinational administration of mesenchymal stem cell-derived exosomes and metformin reduces inflammatory responses in an in vitro model of insulin resistance in HepG2 cells. Heliyon. 2023;9(5): e15489.
Ni Z, Zhou S, Li S, Kuang L, Chen H, Luo X, et al. Exosomes: roles and therapeutic potential in osteoarthritis. Bone research. 2020;8(1):25.
Akyurekli C, Le Y, Richardson RB, Fergusson D, Tay J, Allan DS. A systematic review of preclinical studies on the therapeutic potential of mesenchymal stromal cell-derived microvesicles. Stem Cell Reviews and Reports. 2015;11:150–60.
Hazrati A, Soudi S, Malekpour K, Mahmoudi M, Rahimi A, Hashemi SM, et al. Immune cells-derived exosomes function as a double-edged sword: role in disease progression and their therapeutic applications. Biomarker Research. 2022;10(1):30.
Tan Y, Luo X, Lv W, Hu W, Zhao C, **ong M, et al. Tumor-derived exosomal components: the multifaceted roles and mechanisms in breast cancer metastasis. Cell Death Dis. 2021;12(6):547.
Lou R, Chen J, Zhou F, Wang C, Leung C-H, Lin L. Exosome-cargoed microRNAs: Potential therapeutic molecules for diabetic wound healing. Drug Discovery Today. 2022;27(10): 103323.
Schmittgen TD. Exosomal miRNA cargo as mediator of immune escape mechanisms in neuroblastoma. Can Res. 2019;79(7):1293–4.
Malekpour K, Hazrati A, Soudi S, Hashemi SM. Mechanisms behind therapeutic potentials of mesenchymal stem cell mitochondria transfer/delivery. J Control Release. 2023;354:755–69.
Ahmed L, Al-Massri K. New approaches for enhancement of the efficacy of mesenchymal stem cell-derived exosomes in cardiovascular diseases. Tissue Engineering and Regenerative Medicine. 2022;19(6):1129–46.
Chen S, Sun F, Qian H, Xu W, Jiang J. Preconditioning and engineering strategies for improving the efficacy of mesenchymal stem cell-derived exosomes in cell-free therapy. Stem Cells International. 2022;2022:1779346.
Hazrati A, Malekpour K, Soudi S, Hashemi SM. Mesenchymal stromal/stem cells spheroid culture effect on the therapeutic efficacy of these cells and their exosomes: A new strategy to overcome cell therapy limitations. Biomed Pharmacother. 2022;152: 113211.
Yang Y, Lee EH, Yang Z. Hypoxia-conditioned mesenchymal stem cells in tissue regeneration application. Tissue Eng Part B Rev. 2022;28(5):966–77.
Park WS, Ahn SY, Sung SI, Ahn J-Y, Chang YS. Strategies to enhance paracrine potency of transplanted mesenchymal stem cells in intractable neonatal disorders. Pediatr Res. 2018;83(1):214–22.
Hazrati A, Malekpour K, Soudi S, Hashemi SM. CRISPR/Cas9-engineered mesenchymal stromal/stem cells and their extracellular vesicles: A new approach to overcoming cell therapy limitations. Biomed Pharmacother. 2022;156: 113943.
Guo Y, Yu Y, Hu S, Chen Y, Shen Z. The therapeutic potential of mesenchymal stem cells for cardiovascular diseases. Cell Death Dis. 2020;11(5):349.
Jiang W, Xu J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020;53(1): e12712.
Burr SP, Dazzi F, Garden OA. Mesenchymal stromal cells and regulatory T cells: the Yin and Yang of peripheral tolerance? Immunol Cell Biol. 2013;91(1):12–8.
Cammarota F, Laukkanen MO. Mesenchymal stem/stromal cells in stromal evolution and cancer progression. Stem Cells International. 2016;2016:4824573.
English K, French A, Wood KJ. Mesenchymal stromal cells: facilitators of successful transplantation? Cell Stem Cell. 2010;7(4):431–42.
Askenase PW. COVID-19 therapy with mesenchymal stromal cells (MSC) and convalescent plasma must consider exosome involvement: Do the exosomes in convalescent plasma antagonize the weak immune antibodies? Journal of extracellular vesicles. 2020;10(1): e12004.
Hazrati A, Soudi S, Hashemi SM. Wharton’s jelly mesenchymal stem cells-derived exosomes and imipenem in combination reduce apoptosis and inflammatory responses in E. Coli-infected HepG2 cells. Iranian Journal of Allergy, Asthma and Immunology. 2022;21(3):273–86.
Chen J-y, An R, Liu Z-j, Wang J-j, Chen S-z, Hong M-m, et al. Therapeutic effects of mesenchymal stem cell-derived microvesicles on pulmonary arterial hypertension in rats. Acta Pharmacologica Sinica. 2014;35(9):1121–8.
Kholodenko IV, Kholodenko RV, Majouga AG, Yarygin KN. Apoptotic MSCs and MSC-Derived Apoptotic Bodies as New Therapeutic Tools. Curr Issues Mol Biol. 2022;44(11):5153–72.
Liu J, Qiu X, Lv Y, Zheng C, Dong Y, Dou G, et al. Apoptotic bodies derived from mesenchymal stem cells promote cutaneous wound healing via regulating the functions of macrophages. Stem Cell Res Ther. 2020;11(1):507.
Chamberlain G, Smith H, Rainger GE, Middleton J. Mesenchymal stem cells exhibit firm adhesion, crawling, spreading and transmigration across aortic endothelial cells: effects of chemokines and shear. PLoS ONE. 2011;6(9): e25663.
Hatzistergos KE, Saur D, Seidler B, Balkan W, Breton M, Valasaki K, et al. Stimulatory effects of mesenchymal stem cells on cKit+ cardiac stem cells are mediated by SDF1/CXCR4 and SCF/cKit signaling pathways. Circ Res. 2016;119(8):921–30.
Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, et al. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 2011;34(4):590–601.
Zheng Z, Jia S, Shao C, Shi Y. Irradiation induces cancer lung metastasis through activation of the cGAS–STING–CCL5 pathway in mesenchymal stromal cells. Cell Death Dis. 2020;11(5):326.
Huang L, Xu G, Guo J, **e M, Chen L, Xu W. Mesenchymal stem cells modulate light-induced activation of retinal microglia through CX3CL1/CX3CR1 signaling. Ocul Immunol Inflamm. 2016;24(6):684–92.
Togel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. American Journal of Physiology-Renal Physiology. 2007;292(5):F1626–35.
Wang H, Zheng R, Chen Q, Shao J, Yu J, Hu S. Mesenchymal stem cells microvesicles stabilize endothelial barrier function partly mediated by hepatocyte growth factor (HGF). Stem Cell Res Ther. 2017;8:1–10.
An SY, Jang YJ, Lim H-J, Han J, Lee J, Lee G, et al. Milk fat globule-EGF factor 8, secreted by mesenchymal stem cells, protects against liver fibrosis in mice. Gastroenterology. 2017;152(5):1174–86.
Jia Y, Cao N, Zhai J, Zeng Q, Zheng P, Su R, et al. HGF mediates clinical-grade human umbilical cord-derived mesenchymal stem cells improved functional recovery in a senescence-accelerated mouse model of Alzheimer’s disease. Advanced Science. 2020;7(17):1903809.
Nguyen DC, Garimalla S, **ao H, Kyu S, Albizua I, Galipeau J, et al. Factors of the bone marrow microniche that support human plasma cell survival and immunoglobulin secretion. Nat Commun. 2018;9(1):3698.
Nemoto Y, Kanai T, Takahara M, Oshima S, Nakamura T, Okamoto R, et al. Bone marrow-mesenchymal stem cells are a major source of interleukin-7 and sustain colitis by forming the niche for colitogenic CD4 memory T cells. Gut. 2013;62(8):1142–52.
Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12(5):383–96.
McGuire JJ, Frieling JS, Lo CH, Li T, Muhammad A, Lawrence HR, et al. Mesenchymal stem cell-derived interleukin-28 drives the selection of apoptosis resistant bone metastatic prostate cancer. Nat Commun. 2021;12(1):723.
Nasef A, Mazurier C, Bouchet S, François S, Chapel A, Thierry D, et al. Leukemia inhibitory factor: Role in human mesenchymal stem cells mediated immunosuppression. Cell Immunol. 2008;253(1–2):16–22.
Yang S-H, Park M-J, Yoon I-H, Kim S-Y, Hong S-H, Shin J-Y, et al. Soluble mediators from mesenchymal stem cells suppress T cell proliferation by inducing IL-10. Exp Mol Med. 2009;41(5):315–24.
Park HJ, Oh SH, Kim HN, Jung YJ, Lee PH. Mesenchymal stem cells enhance α-synuclein clearance via M2 microglia polarization in experimental and human parkinsonian disorder. Acta Neuropathol. 2016;132(5):685–701.
Nemeth K, Keane-Myers A, Brown JM, Metcalfe DD, Gorham JD, Bundoc VG, et al. Bone marrow stromal cells use TGF-β to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci. 2010;107(12):5652–7.
English K, Ryan J, Tobin L, Murphy M, Barry FP, Mahon BP. Cell contact, prostaglandin E2 and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+ CD25Highforkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009;156(1):149–60.
Sharma P, Bolten ZT, Wagner DR, Hsieh AH. Deformability of human mesenchymal stem cells is dependent on vimentin intermediate filaments. Ann Biomed Eng. 2017;45:1365–74.
Sioud M, Mobergslien A, Boudabous A, Fløisand Y. Mesenchymal stem cell-mediated T cell suppression occurs through secreted galectins. Int J Oncol. 2011;38(2):385–90.
Mbongue JC, Nicholas DA, Torrez TW, Kim N-S, Firek AF, Langridge WH. The role of indoleamine 2, 3-dioxygenase in immune suppression and autoimmunity. Vaccines. 2015;3(3):703–29.
Zhai L, Bell A, Ladomersky E, Lauing KL, Bollu L, Sosman JA, et al. Immunosuppressive IDO in cancer: mechanisms of action, animal models, and targeting strategies. Front Immunol. 2020;11:85.
Cinelli MA, Do HT, Miley GP, Silverman RB. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med Res Rev. 2020;40(1):158–89.
Antonioli L, Pacher P, Vizi ES, Haskó G. CD39 and CD73 in immunity and inflammation. Trends Mol Med. 2013;19(6):355–67.
Moesta AK, Li X-Y, Smyth MJ. Targeting CD39 in cancer. Nat Rev Immunol. 2020;20(12):739–55.
Cloer CM, Givens CS, Buie LK, Rochelle LK, Lin Y-T, Popa S, et al. Mitochondrial transplant after ischemia reperfusion promotes cellular salvage and improves lung function during ex-vivo lung perfusion. J Heart Lung Transplant. 2023;42(5):575–84.
Lin H-Y, Liou C-W, Chen S-D, Hsu T-Y, Chuang J-H, Wang P-W, et al. Mitochondrial transfer from Wharton’s jelly-derived mesenchymal stem cells to mitochondria-defective cells recaptures impaired mitochondrial function. Mitochondrion. 2015;22:31–44.
Cao Y, Wang X, ** T, Tian Y, Dai C, Widarma C, et al. Immune checkpoint molecules in natural killer cells as potential targets for cancer immunotherapy. Signal Transduct Target Ther. 2020;5(1):250.
Tang W, Chen J, Ji T, Cong X. TIGIT, a novel immune checkpoint therapy for melanoma. Cell Death Dis. 2023;14(7):466.
Pistillo MP, Tazzari PL, Palmisano GL, Pierri I, Bolognesi A, Ferlito F, et al. CTLA-4 is not restricted to the lymphoid cell lineage and can function as a target molecule for apoptosis induction of leukemic cells. Blood, The Journal of the American Society of Hematology. 2003;101(1):202–9.
Laurent S, Queirolo P, Boero S, Salvi S, Piccioli P, Boccardo S, et al. The engagement of CTLA-4 on primary melanoma cell lines induces antibody-dependent cellular cytotoxicity and TNF-α production. J Transl Med. 2013;11(1):108.
Laurent S, Carrega P, Saverino D, Piccioli P, Camoriano M, Morabito A, et al. CTLA-4 is expressed by human monocyte—derived dendritic cells and regulates their functions. Hum Immunol. 2010;71(10):934–41.
Riva A, Chokshi S. Immune checkpoint receptors: homeostatic regulators of immunity. Hep Intl. 2018;12(3):223–36.
Korman AJ, Garrett-Thomson SC, Lonberg N. The foundations of immune checkpoint blockade and the ipilimumab approval decennial. Nat Rev Drug Discovery. 2022;21(7):509–28.
Triggianese P, Novelli L, Galdiero MR, Chimenti MS, Conigliaro P, Perricone R, et al. Immune checkpoint inhibitors-induced autoimmunity: the impact of gender. Autoimmun Rev. 2020;19(8): 102590.
Xu W, Hiếu T, Malarkannan S, Wang L. The structure, expression, and multifaceted role of immune-checkpoint protein VISTA as a critical regulator of anti-tumor immunity, autoimmunity, and inflammation. Cell Mol Immunol. 2018;15(5):438–46.
Creelan BC, Antonia SJ. The NKG2A immune checkpoint—a new direction in cancer immunotherapy. Nat Rev Clin Oncol. 2019;16(5):277–8.
Preusser M, Lim M, Hafler DA, Reardon DA, Sampson JH. Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nat Rev Neurol. 2015;11(9):504–14.
Kennedy A, Waters E, Rowshanravan B, Hinze C, Williams C, Janman D, et al. Differences in CD80 and CD86 transendocytosis reveal CD86 as a key target for CTLA-4 immune regulation. Nat Immunol. 2022;23(9):1365–78.
Oyewole-Said D, Konduri V, Vazquez-Perez J, Weldon SA, Levitt JM, Decker WK. Beyond T-cells: functional characterization of CTLA-4 expression in immune and non-immune cell types. Front Immunol. 2020;11: 608024.
Van Coillie S, Wiernicki B, Xu J. Molecular and cellular functions of CTLA-4. Regulation of Cancer Immune Checkpoints: Molecular and Cellular Mechanisms and Therapy. 2020:7–32.
Yang R, Sun L, Li C-F, Wang Y-H, Yao J, Li H, et al. Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat Commun. 2021;12(1):832.
Simon S, Labarriere N. PD-1 expression on tumor-specific T cells: Friend or foe for immunotherapy? Oncoimmunology. 2018;7(1): e1364828.
Kim MJ, Ha S-J. Differential role of PD-1 expressed by various immune and tumor cells in the tumor immune microenvironment: expression, function, therapeutic efficacy, and resistance to cancer immunotherapy. Frontiers in Cell and Developmental Biology. 2021;9: 767466.
**ng X, Guo J, Ding G, Li B, Dong B, Feng Q, et al. Analysis of PD1, PDL1, PDL2 expression and T cells infiltration in 1014 gastric cancer patients. Oncoimmunology. 2018;7(3): e1356144.
Zhu C, Anderson AC, Schubart A, **ong H, Imitola J, Khoury SJ, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6(12):1245–52.
Thommen DS, Koelzer VH, Herzig P, Roller A, Trefny M, Dimeloe S, et al. A transcriptionally and functionally distinct PD-1+ CD8+ T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med. 2018;24(7):994–1004.
Das M, Zhu C, Kuchroo VK. Tim-3 and its role in regulating anti-tumor immunity. Immunol Rev. 2017;276(1):97–111.
Wolf Y, Anderson AC, Kuchroo VK. TIM3 comes of age as an inhibitory receptor. Nat Rev Immunol. 2020;20(3):173–85.
Goldberg MV, Drake CG. LAG-3 in cancer immunotherapy. Cancer immunology and immunotherapy. 2011;344:269–78.
Mao X, Ou MT, Karuppagounder SS, Kam T-I, Yin X, **ong Y, et al. Pathological α-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science. 2016;353(6307):aah3374.
Belkina AC, Starchenko A, Drake KA, Proctor EA, Pihl RM, Olson A, et al. Multivariate computational analysis of gamma delta T cell inhibitory receptor signatures reveals the divergence of healthy and ART-suppressed HIV+ aging. Front Immunol. 2018;9:2783.
Graydon CG, Mohideen S, Fowke KR. LAG3’s enigmatic mechanism of action. Front Immunol. 2021;11: 615317.
Maruhashi T, Sugiura D, Okazaki I-m, Okazaki T. LAG-3: from molecular functions to clinical applications. J Immunother Cancer. 2020;8(2):e001014.
Aggarwal V, Workman CJ, Vignali DA. LAG-3 as the third checkpoint inhibitor. Nat Immunol. 2023;24:1415–22.
Chauvin J-M, Zarour HM. TIGIT in cancer immunotherapy. J Immunother Cancer. 2020;8(2): e000957.
Dougall WC, Kurtulus S, Smyth MJ, Anderson AC. TIGIT and CD 96: new checkpoint receptor targets for cancer immunotherapy. Immunol Rev. 2017;276(1):112–20.
Reches A, Ophir Y, Stein N, Kol I, Isaacson B, Amikam YC, et al. Nectin4 is a novel TIGIT ligand which combines checkpoint inhibition and tumor specificity. J Immunother Cancer. 2020;8(1): e001294.
Joller N, Lozano E, Burkett PR, Patel B, **ao S, Zhu C, et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity. 2014;40(4):569–81.
Iellem A, Mariani M, Lang R, Recalde H, Panina-Bordignon P, Sinigaglia F, et al. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4+ CD25+ regulatory T cells. J Exp Med. 2001;194(6):847–54.
Li X, Xu Z, Cui G, Yu L, Zhang X. BTLA expression in stage I-III non–small-cell lung cancer and its correlation with PD-1/PD-L1 and clinical outcomes. Onco Targets Ther. 2020;13:215–24.
Peggs KS, Quezada SA, Allison JP. Cell intrinsic mechanisms of T-cell inhibition and application to cancer therapy. Immunol Rev. 2008;224(1):141–65.
Paulos CM, June CH. Putting the brakes on BTLA in T cell–mediated cancer immunotherapy. J Clin Investig. 2010;120(1):76–80.
Zhang J-A, Lu Y-B, Wang W-D, Liu G-B, Chen C, Shen L, et al. BTLA-Expressing Dendritic Cells in Patients with Tuberculosis Exhibit Reduced Production of IL-12/IFN-α and Increased Production of IL-4 and TGF-β, Favoring Th2 and Foxp3+ Treg Polarization. Front Immunol. 2020;11:518.
Chevalier MF, Bohner P, Pieraerts C, Lhermitte B, Gourmaud J, Nobile A, et al. Immunoregulation of dendritic cell subsets by inhibitory receptors in urothelial cancer. Eur Urol. 2017;71(6):854–7.
Sun C, Wang B, Hao S. Adenosine-A2A receptor pathway in cancer immunotherapy. Front Immunol. 2022;13: 837230.
Borroto-Escuela DO, Hinz S, Navarro G, Franco R, Müller CE, Fuxe K. Understanding the role of adenosine A2AR heteroreceptor complexes in neurodegeneration and neuroinflammation. Front Neurosci. 2018;12:43.
Yin S-S, Gao F-H. Molecular mechanism of tumor cell immune escape mediated by CD24/Siglec-10. Front Immunol. 2020;11:1324.
Fang X, Zheng P, Tang J, Liu Y. CD24: from A to Z. Cell Mol Immunol. 2010;7(2):100–3.
Panagiotou E, Syrigos NK, Charpidou A, Kotteas E, Vathiotis IA. CD24: A novel target for cancer immunotherapy. Journal of Personalized Medicine. 2022;12(8):1235.
Altevogt P, Sammar M, Hüser L, Kristiansen G. Novel insights into the function of CD24: A driving force in cancer. Int J Cancer. 2021;148(3):546–59.
Takahashi S. Molecular functions of SIRPα and its role in cancer. Biomedical reports. 2018;9(1):3–7.
Kaur S, Isenberg JS, Roberts DD. CD47 (cluster of differentiation 47). Atlas of genetics and cytogenetics in oncology and haematology. 2021;25(2):83.
van Duijn A, Van der Burg SH, Scheeren FA. CD47/SIRPα axis: bridging innate and adaptive immunity. J Immunother Cancer. 2022;10(7): e004589.
Kang X, Kim J, Deng M, John S, Chen H, Wu G, et al. Inhibitory leukocyte immunoglobulin-like receptors: Immune checkpoint proteins and tumor sustaining factors. Cell Cycle. 2016;15(1):25–40.
Chen H, Chen Y, Deng M, John S, Gui X, Kansagra A, et al. Antagonistic anti-LILRB1 monoclonal antibody regulates antitumor functions of natural killer cells. J Immunother Cancer. 2020;8(2): e000515.
Colonna M, Navarro F, Bellón T, Llano M, García P, Samaridis J, et al. A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J Exp Med. 1997;186(11):1809–18.
Naji A, Menier C, Maki G, Carosella E, Rouas-Freiss N. Neoplastic B-cell growth is impaired by HLA-G/ILT2 interaction. Leukemia. 2012;26(8):1889–92.
Mori Y, Tsuji S, Inui M, Sakamoto Y, Endo S, Ito Y, et al. Inhibitory immunoglobulin-like receptors LILRB and PIR-B negatively regulate osteoclast development. J Immunol. 2008;181(7):4742–51.
Abdallah F, Coindre S, Gardet M, Meurisse F, Naji A, Suganuma N, et al. Leukocyte immunoglobulin-like receptors in regulating the immune response in infectious diseases: A window of opportunity to pathogen persistence and a sound target in therapeutics. Front Immunol. 2021;12: 717998.
Lines JL, Pantazi E, Mak J, Sempere LF, Wang L, O’Connell S, et al. VISTA is an immune checkpoint molecule for human T cells. Can Res. 2014;74(7):1924–32.
Mulati K, Hamanishi J, Matsumura N, Chamoto K, Mise N, Abiko K, et al. VISTA expressed in tumour cells regulates T cell function. Br J Cancer. 2019;120(1):115–27.
Huang X, Zhang X, Li E, Zhang G, Wang X, Tang T, et al. VISTA: an immune regulatory protein checking tumor and immune cells in cancer immunotherapy. J Hematol Oncol. 2020;13:1–13.
Nowak EC, Lines JL, Varn FS, Deng J, Sarde A, Mabaera R, et al. Immunoregulatory functions of VISTA. Immunol Rev. 2017;276(1):66–79.
**e X, Chen C, Chen W, Jiang J, Wang L, Li T, et al. Structural basis of VSIG3: The ligand for VISTA. Front Immunol. 2021;12: 625808.
Yuan L, Tatineni J, Mahoney KM, Freeman GJ. VISTA: a mediator of quiescence and a promising target in cancer immunotherapy. Trends Immunol. 2021;42(3):209–27.
Campbell KS, Purdy AK. Structure/function of human killer cell immunoglobulin-like receptors: lessons from polymorphisms, evolution, crystal structures and mutations. Immunology. 2011;132(3):315–25.
Pende D, Falco M, Vitale M, Cantoni C, Vitale C, Munari E, et al. Killer Ig-like receptors (KIRs): their role in NK cell modulation and developments leading to their clinical exploitation. Front Immunol. 2019;10:1179.
Mora-Bitria L, Asquith B. Innate receptors modulating adaptive T cell responses: KIR-HLA interactions and T cell-mediated control of chronic viral infections. Immunogenetics. 2023;75(3):269–82.
Kaifu T, Yabe R, Maruhashi T, Chung S-H, Tateno H, Fujikado N, et al. DCIR and its ligand asialo-biantennary N-glycan regulate DC function and osteoclastogenesis. J Exp Med. 2021;218(12): e20210435.
Bermejo-Jambrina M, Eder J, Helgers LC, Hertoghs N, Nijmeijer BM, Stunnenberg M, et al. C-type lectin receptors in antiviral immunity and viral escape. Front Immunol. 2018;9:590.
van Houtum EJ, Büll C, Cornelissen LA, Adema GJ. Siglec signaling in the tumor microenvironment. Front Immunol. 2021;12: 790317.
Egan H, Treacy O, Lynch K, Leonard NA, O’Malley G, Reidy E, et al. Targeting stromal cell sialylation reverses T cell-mediated immunosuppression in the tumor microenvironment. Cell Rep. 2023;42(5): 112475.
Perdicchio M, Ilarregui JM, Verstege MI, Cornelissen LA, Schetters ST, Engels S, et al. Sialic acid-modified antigens impose tolerance via inhibition of T-cell proliferation and de novo induction of regulatory T cells. Proc Natl Acad Sci. 2016;113(12):3329–34.
RodrÍguez E, Schetters ST, van Kooyk Y. The tumour glyco-code as a novel immune checkpoint for immunotherapy. Nat Rev Immunol. 2018;18(3):204–11.
Di Pilato M, Kim EY, Cadilha BL, Prüßmann JN, Nasrallah MN, Seruggia D, et al. Targeting the CBM complex causes Treg cells to prime tumours for immune checkpoint therapy. Nature. 2019;570(7759):112–6.
Zhong Z, Vong CT, Chen F, Tan H, Zhang C, Wang N, et al. Immunomodulatory potential of natural products from herbal medicines as immune checkpoints inhibitors: Hel** to fight against cancer via multiple targets. Med Res Rev. 2022;42(3):1246–79.
Saha D, Martuza RL, Rabkin SD. Macrophage polarization contributes to glioblastoma eradication by combination immunovirotherapy and immune checkpoint blockade. Cancer cell. 2017;32(2):253–67 e5.
Nicholas NS, Apollonio B, Ramsay AG. Tumor microenvironment (TME)-driven immune suppression in B cell malignancy. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 2016;1863(3):471–82.
Cassetta L, Kitamura T. Macrophage targeting: opening new possibilities for cancer immunotherapy. Immunology. 2018;155(3):285–93.
Marin-Acevedo JA, Kimbrough EO, Lou Y. Next generation of immune checkpoint inhibitors and beyond. J Hematol Oncol. 2021;14(1):1–29.
Chow A, Perica K, Klebanoff CA, Wolchok JD. Clinical implications of T cell exhaustion for cancer immunotherapy. Nat Rev Clin Oncol. 2022;19(12):775–90.
Zhao Y, Shao Q, Peng G. Exhaustion and senescence: two crucial dysfunctional states of T cells in the tumor microenvironment. Cell Mol Immunol. 2020;17(1):27–35.
Gonçalves-Lopes RM, Lima NF, Carvalho KI, Scopel KK, Kallás EG, Ferreira MU. Surface expression of inhibitory (CTLA-4) and stimulatory (OX40) receptors by CD4+ regulatory T cell subsets circulating in human malaria. Microbes Infect. 2016;18(10):639–48.
Peretz Y, He Z, Shi Y, Yassine-Diab B, Goulet J-P, Bordi R, et al. CD160 and PD-1 co-expression on HIV-specific CD8 T cells defines a subset with advanced dysfunction. 2012;8(8):e1002840.
Nebbia G, Peppa D, Schurich A, Khanna P, Singh HD, Cheng Y, et al. Upregulation of the Tim-3/galectin-9 pathway of T cell exhaustion in chronic hepatitis B virus infection. PLoS ONE. 2012;7(10): e47648.
Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, et al. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol. 2012;13(2):188–95.
Wykes MN, Lewin SR. Immune checkpoint blockade in infectious diseases. Nat Rev Immunol. 2018;18(2):91–104.
Chew GM, Fujita T, Webb GM, Burwitz BJ, Wu HL, Reed JS, et al. TIGIT marks exhausted T cells, correlates with disease progression, and serves as a target for immune restoration in HIV and SIV infection. PLoS Pathog. 2016;12(1): e1005349.
Hobo W, Hutten TJ, Schaap NP, Dolstra H. Immune checkpoint molecules in acute myeloid leukaemia: managing the double-edged sword. Br J Haematol. 2018;181(1):38–53.
Byun DJ, Wolchok JD, Rosenberg LM, Girotra M. Cancer immunotherapy—immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinol. 2017;13(4):195–207.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64.
Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol. 2020;20(1):25–39.
Kubli SP, Berger T, Araujo DV, Siu LL, Mak TW. Beyond immune checkpoint blockade: emerging immunological strategies. Nat Rev Drug Discovery. 2021;20(12):899–919.
Cha J-H, Yang W-H, **a W, Wei Y, Chan L-C, Lim S-O, et al. Metformin promotes antitumor immunity via endoplasmic-reticulum-associated degradation of PD-L1. Mol Cell. 2018;71(4):606–20 e7.
Lim S-O, Li C-W, **a W, Cha J-H, Chan L-C, Wu Y, et al. Deubiquitination and stabilization of PD-L1 by CSN5. Cancer Cell. 2016;30(6):925–39.
Hsu J-M, **a W, Hsu Y-H, Chan L-C, Yu W-H, Cha J-H, et al. STT3-dependent PD-L1 accumulation on cancer stem cells promotes immune evasion. Nat Commun. 2018;9(1):1908.
Zhang F, Wei H, Wang X, Bai Y, Wang P, Wu J, et al. Structural basis of a novel PD-L1 nanobody for immune checkpoint blockade. Cell discovery. 2017;3(1):17004.
Ma L, Gai J, Qiao P, Li Y, Li X, Zhu M, et al. A novel bispecific nanobody with PD-L1/TIGIT dual immune checkpoint blockade. Biochem Biophys Res Commun. 2020;531(2):144–51.
Wu Q, Jiang L, Li S-c, He Q-j, Yang B, Cao J. Small molecule inhibitors targeting the PD-1/PD-L1 signaling pathway. Acta Pharmacologica Sinica. 2021;42(1):1–9.
Huang C, Zhu H-X, Yao Y, Bian Z-H, Zheng Y-J, Li L, et al. Immune checkpoint molecules. Possible future therapeutic implications in autoimmune diseases. J Autoimmun. 2019;104:102333.
Zappasodi R, Serganova I, Cohen IJ, Maeda M, Shindo M, Senbabaoglu Y, et al. CTLA-4 blockade drives loss of Treg stability in glycolysis-low tumours. Nature. 2021;591(7851):652–8.
Verma N, Burns SO, Walker LS, Sansom DM. Immune deficiency and autoimmunity in patients with CTLA-4 (CD152) mutations. Clin Exp Immunol. 2017;190(1):1–7.
Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, et al. CTLA-4–Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3(11):1097–101.
Hoff H, Kolar P, Ambach A, Radbruch A, Brunner-Weinzierl MC. CTLA-4 (CD152) inhibits T cell function by activating the ubiquitin ligase Itch. Mol Immunol. 2010;47(10):1875–81.
Gaber T, Schönbeck K, Hoff H, Tran CL, Strehl C, Lang A, et al. CTLA-4 mediates inhibitory function of mesenchymal stem/stromal cells. Int J Mol Sci. 2018;19(8):2312.
Ueda H, Howson JM, Esposito L, Heward J, Snook, Chamberlain G, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423(6939):506–11.
Chenari A, Hazrati A, Hosseini AZ, Motiee M, Soudi S. The effect of mesenchymal stem cell-derived supernatant nasal administration on lung inflammation and immune response in BCG-vaccinated BALB/c mice. Life Sci. 2023;317: 121465.
Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182(2):459–65.
Glennie S, Soeiro I, Dyson PJ, Lam EW-F, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105(7):2821–7.
Patel SA, Dave MA, Bliss SA, Giec-Ujda AB, Bryan M, Pliner LF, et al. Treg/Th17 polarization by distinct subsets of breast cancer cells is dictated by the interaction with mesenchymal stem cells. Journal of cancer stem cell research. 2014;2014(2).
Akbari O, Stock P, Singh A, Lombardi V, Lee W, Freeman G, et al. PD-L1 and PD-L2 modulate airway inflammation and iNKT-cell-dependent airway hyperreactivity in opposing directions. Mucosal Immunol. 2010;3(1):81–91.
Augello A, Tasso R, Negrini SM, Amateis A, Indiveri F, Cancedda R, et al. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35(5):1482–90.
Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8(3):239–45.
Chen Z, Yao M-W, Shen Z-L, Li S-D, **ng W, Guo W, et al. Interferon-gamma and tumor necrosis factor-alpha synergistically enhance the immunosuppressive capacity of human umbilical-cord-derived mesenchymal stem cells by increasing PD-L1 expression. World Journal of Stem Cells. 2023;15(8):787.
Sheng H, Wang Y, ** Y, Zhang Q, Zhang Y, Wang L, et al. A critical role of IFNγ in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7–H1. Cell Res. 2008;18(8):846–57.
Kim JY, Park M, Kim YH, Ryu KH, Lee KH, Cho KA, et al. Tonsil-derived mesenchymal stem cells (T-MSCs) prevent Th17-mediated autoimmune response via regulation of the programmed death-1/programmed death ligand-1 (PD-1/PD-L1) pathway. J Tissue Eng Regen Med. 2018;12(2):e1022–33.
Motallebnezhad M, Hazrati A, Ghaleh HEG, Jonaidi-Jafari N, Abbaspour-Aghdam S, Malekpour K, et al. Exosomes from Adipose Tissue-derived Mesenchymal Stem Cells Induce Regulatory T Cells in COVID-19 Patients. Iran J Allergy Asthma Immunol. 2023;22(3):233–44.
Gao F, Cui D, Zuo D, Shou Z, Yang J, Yu T, et al. BMSCs improve TNBS-induced colitis in rats by inducing Treg differentiation by expressing PD-L1. Biotech Lett. 2022;44(11):1263–75.
Zhou K, Guo S, Tong S, Sun Q, Li F, Zhang X, et al. Immunosuppression of human adipose-derived stem cells on T cell subsets via the reduction of NF-kappaB activation mediated by PD-L1/PD-1 and Gal-9/TIM-3 pathways. Stem Cells and Development. 2018;27(17):1191–202.
Di Tinco R, Bertani G, Pisciotta A, Bertoni L, Pignatti E, Maccaferri M, et al. Role of PD-L1 in licensing immunoregulatory function of dental pulp mesenchymal stem cells. Stem Cell Res Ther. 2021;12:598.
Davies LC, Heldring N, Kadri N, Le Blanc K. Mesenchymal stromal cell secretion of programmed death-1 ligands regulates T cell mediated immunosuppression. Stem cells. 2017;35(3):766–76.
Pisciotta A, Bertani G, Bertoni L, Di Tinco R, De Biasi S, Vallarola A, et al. Modulation of cell death and promotion of chondrogenic differentiation by Fas/FasL in human dental pulp stem cells (hDPSCs). Frontiers in Cell and Developmental Biology. 2020;8:279.
Strauch V, Saul D, Berisha M, Mackensen A, Mougiakakos D, Jitschin R. N-glycosylation controls inflammatory licensing-triggered PD-L1 upregulation in human mesenchymal stromal cells. Stem cells. 2020;38(8):986–93.
Bai X, Chen T, Li Y, Ge X, Qiu C, Gou H, et al. PD-L1 expression levels in mesenchymal stromal cells predict their therapeutic values for autoimmune hepatitis. Stem Cell Res Ther. 2023;14(1):370.
Dunavin N, Li M, Atay S, Mitchell J, Soder R, Abhyankar S, et al. CRISPR/Cas9-Mediated Disruption of PD-L1 Reduces the T Cell Suppressive Effect of Wharton’s Jelly Mesenchymal Stromal Cells and Their Extracellular Vesicles. Blood. 2018;132:5095.
Silva-Carvalho AÉ, Sousa MRR, Alencar-Silva T, Carvalho JL, Saldanha-Araujo F. Mesenchymal stem cells immunomodulation: The road to IFN-γ licensing and the path ahead. Cytokine Growth Factor Rev. 2019;47:32–42.
Li M, Soder R, Abhyankar S, Abdelhakim H, Braun MW, Trinidad CV, et al. WJMSC-derived small extracellular vesicle enhance T cell suppression through PD-L1. Journal of extracellular vesicles. 2021;10(4): e12067.
Ding S, Sun Z, Jiang J, Chang X, Shen Y, Gu Y, et al. Inducible costimulator ligand (ICOSL) on CD19+ B cells is involved in immunopathological damage of rheumatoid arthritis (RA). Front Immunol. 2022;13:1015831.
Rottman JB, Smith T, Tonra JR, Ganley K, Bloom T, Silva R, et al. The costimulatory molecule ICOS plays an important role in the immunopathogenesis of EAE. Nat Immunol. 2001;2(7):605–11.
Martin-Orozco N, Li Y, Wang Y, Liu S, Hwu P, Liu Y-J, et al. Melanoma cells express ICOS ligand to promote the activation and expansion of T-regulatory cells. Can Res. 2010;70(23):9581–90.
Strauss L, Bergmann C, Szczepanski MJ, Lang S, Kirkwood JM, Whiteside TL. Expression of ICOS on human melanoma-infiltrating CD4+ CD25highFoxp3+ T regulatory cells: implications and impact on tumor-mediated immune suppression. J Immunol. 2008;180(5):2967–80.
Yi T, Lee D-S, Jeon M-S, Kwon SW, Song SU. Gene expression profile reveals that STAT2 is involved in the immunosuppressive function of human bone marrow-derived mesenchymal stem cells. Gene. 2012;497(2):131–9.
Lee H-J, Kim S-N, Jeon M-S, Yi T, Song SU. ICOSL expression in human bone marrow-derived mesenchymal stem cells promotes induction of regulatory T cells. Sci Rep. 2017;7(1):44486.
Sun Y-Q, Deng M-X, He J, Zeng Q-X, Wen W, Wong DS, et al. Human pluripotent stem cell-derived mesenchymal stem cells prevent allergic airway inflammation in mice. Stem cells. 2012;30(12):2692–9.
Fan X, Xu Z-B, Li C-L, Zhang H-Y, Peng Y-Q, He B-X, et al. Mesenchymal stem cells regulate type 2 innate lymphoid cells via regulatory T cells through ICOS-ICOSL interaction. Stem Cells. 2021;39(7):975–87.
Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, et al. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–82.
Matyash M, Zabiegalov O, Wendt S, Matyash V, Kettenmann H. The adenosine generating enzymes CD39/CD73 control microglial processes ramification in the mouse brain. PLoS ONE. 2017;12(4): e0175012.
Bagheri S, Saboury A, Haertlé T. Adenosine deaminase inhibition. Int J Biol Macromol. 2019;141:1246–57.
Ernst PB, Garrison JC, Thompson LF. Much ado about adenosine: adenosine synthesis and function in regulatory T cell biology. J Immunol. 2010;185(4):1993–8.
Saldanha-Araujo F, Ferreira FI, Palma PV, Araujo AG, Queiroz RH, Covas DT, et al. Mesenchymal stromal cells up-regulate CD39 and increase adenosine production to suppress activated T-lymphocytes. Stem cell research. 2011;7(1):66–74.
Mandapathil M, Hilldorfer B, Szczepanski MJ, Czystowska M, Szajnik M, Ren J, et al. Generation and accumulation of immunosuppressive adenosine by human CD4+ CD25highFOXP3+ regulatory T cells. J Biol Chem. 2010;285(10):7176–86.
Sattler C, Steinsdoerfer M, Offers M, Fischer E, Schierl R, Heseler K, et al. Inhibition of T-cell proliferation by murine multipotent mesenchymal stromal cells is mediated by CD39 expression and adenosine generation. Cell Transplant. 2011;20(8):1221–30.
Snijdewint F, Kaliński P, Wierenga E, Bos J, Kapsenberg M. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J Immunol (Baltimore, Md: 1950). 1993;150(12):5321–9.
Däubener W, Wanagat N, Pilz K, Seghrouchni S, Fischer HG, Hadding U. A new, simple, bioassay for human IFN-γ. J Immunol Methods. 1994;168(1):39–47.
El-Fattah A, Eslam E. IDO/kynurenine pathway in cancer: possible therapeutic approaches. J Transl Med. 2022;20(1):1–13.
Gao Y, Wang N, Jia D. H3K27 tri-demethylase JMJD3 inhibits macrophage apoptosis by promoting ADORA2A in lipopolysaccharide-induced acute lung injury. Cell Death Discovery. 2022;8(1):475.
Liu L, Hou Y, Deng C, Tao Z, Chen Z, Hu J, et al. Single cell sequencing reveals that CD39 inhibition mediates changes to the tumor microenvironment. Nat Commun. 2022;13(1):6740.
Lee JJ, Jeong HJ, Kim MK, Wee WR, Lee WW, Kim SU, et al. CD39-mediated effect of human bone marrow-derived mesenchymal stem cells on the human Th17 cell function. Purinergic Signalling. 2014;10:357–65.
Tan K, Zhu H, Zhang J, Ouyang W, Tang J, Zhang Y, et al. CD73 expression on mesenchymal stem cells dictates the reparative properties via its anti-inflammatory activity. Stem cells international. 2019;2019:8717694.
Wang X, Liu X, Zhang H, Nie L, Chen M, Ding Z. Reconstitute the damaged heart via the dual reparative roles of pericardial adipose-derived flk-1+ stem cells. Int J Cardiol. 2016;202:256–64.
Luo Y, Wu W, Gu J, Zhang X, Dang J, Wang J, et al. Human gingival tissue-derived MSC suppress osteoclastogenesis and bone erosion via CD39-adenosine signal pathway in autoimmune arthritis. EBioMedicine. 2019;43:620–31.
Barrow M. An Overview of the NF-kB mechanism of pathophysiology in rheumatoid arthritis, investigation of the NF-kB ligand RANKL and related nutritional interventions. Autoimmun Rev. 2021;20(2): 102741.
Moll G, Ignatowicz L, Catar R, Luecht C, Sadeghi B, Hamad O, et al. Different procoagulant activity of therapeutic mesenchymal stromal cells derived from bone marrow and placental decidua. Stem cells and development. 2015;24(19):2269–79.
Netsch P, Elvers-Hornung S, Uhlig S, Klüter H, Huck V, Kirschhöfer F, et al. Human mesenchymal stromal cells inhibit platelet activation and aggregation involving CD73-converted adenosine. Stem Cell Res Ther. 2018;9:184.
Gao Z-w, Wang X, Zhang H-z, Lin F, Liu C, Dong K. The roles of adenosine deaminase in autoimmune diseases. Autoimmun Rev. 2021;20(1):102709.
Grigorian A, Torossian S, Demetriou M. T-cell growth, cell surface organization, and the galectin–glycoprotein lattice. Immunol Rev. 2009;230(1):232–46.
Lv Y, Ma X, Ma Y, Du Y, Feng J. A new emerging target in cancer immunotherapy: Galectin-9 (LGALS9). Genes & Diseases. 2022;10(6):2366–82.
Seki M, Oomizu S, Sakata K-m, Sakata A, Arikawa T, Watanabe K, et al. Galectin-9 suppresses the generation of Th17, promotes the induction of regulatory T cells, and regulates experimental autoimmune arthritis. Clin Immunol. 2008;127(1):78–88.
Han G, Chen G, Shen B, Li Y. Tim-3: an activation marker and activation limiter of innate immune cells. Front Immunol. 2013;4:449.
Oomizu S, Arikawa T, Niki T, Kadowaki T, Ueno M, Nishi N, et al. Galectin-9 suppresses Th17 cell development in an IL-2-dependent but Tim-3-independent manner. Clin Immunol. 2012;143(1):51–8.
Gieseke F, Kruchen A, Tzaribachev N, Bentzien F, Dominici M, Müller I. Proinflammatory stimuli induce galectin-9 in human mesenchymal stromal cells to suppress T-cell proliferation. Eur J Immunol. 2013;43(10):2741–9.
Fan J, Tang X, Wang Q, Zhang Z, Wu S, Li W, et al. Mesenchymal stem cells alleviate experimental autoimmune cholangitis through immunosuppression and cytoprotective function mediated by galectin-9. Stem Cell Res Ther. 2018;9:237.
Zhao Y, Yu D, Wang H, ** W, Li X, Hu Y, et al. Galectin-9 Mediates the Therapeutic Effect of Mesenchymal Stem Cells on Experimental Endotoxemia. Frontiers in Cell and Developmental Biology. 2022;10: 700702.
Luo C, Luo F, Che L, Zhang H, Zhao L, Zhang W, et al. Mesenchymal stem cells protect against sepsis-associated acute kidney injury by inducing Gal-9/Tim-3 to remodel immune homeostasis. Ren Fail. 2023;45(1):2187229.
Zhang Y, Ge X-h, Guan S-b, Li X-m, Gu W, et al. Bone marrow mesenchymal stem cells inhibit the function of dendritic cells by secreting galectin-1. BioMed Res Int. 2017;2017:3248605.
Seo Y, Ahn J-S, Shin YY, Oh S-J, Song M-H, Kang M-J, et al. Mesenchymal stem cells target microglia via galectin-1 production to rescue aged mice from olfactory dysfunction. Biomed Pharmacother. 2022;153: 113347.
Gieseke F, Böhringer J, Bussolari R, Dominici M, Handgretinger R, Müller I. Human multipotent mesenchymal stromal cells use galectin-1 to inhibit immune effector cells. Blood, The Journal of the American Society of Hematology. 2010;116(19):3770–9.
Liu ZY, Deng L, Jia Y, Liu H, Ding K, Wang W, et al. CD155/TIGIT signalling plays a vital role in the regulation of bone marrow mesenchymal stem cell–induced natural killer–cell exhaustion in multiple myeloma. Clin Transl Med. 2022;12(7): e861.
Tomasec P, Wang EC, Davison AJ, Vojtesek B, Armstrong M, Griffin C, et al. Downregulation of natural killer cell–activating ligand CD155 by human cytomegalovirus UL141. Nat Immunol. 2005;6(2):181–8.
Tahara-Hanaoka S, Shibuya K, Onoda Y, Zhang H, Yamazaki S, Miyamoto A, et al. Functional characterization of DNAM-1 (CD226) interaction with its ligands PVR (CD155) and nectin-2 (PRR-2/CD112). Int Immunol. 2004;16(4):533–8.
Yu X, Harden K, C Gonzalez L, Francesco M, Chiang E, Irving B, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10(1):48–57.
Wagner AK, Kadri N, Snäll J, Brodin P, Gilfillan S, Colonna M, et al. Expression of CD226 is associated to but not required for NK cell education. Nat Commun. 2017;8(1):15627.
Chan CJ, Martinet L, Gilfillan S, Souza-Fonseca-Guimaraes F, Chow MT, Town L, et al. The receptors CD96 and CD226 oppose each other in the regulation of natural killer cell functions. Nat Immunol. 2014;15(5):431–8.
Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, Novik A, et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci. 2009;106(42):17858–63.
Greenberg PL, Young NS, Gattermann N. Myelodysplastic syndromes. ASH Education Program Book. 2002;2002(1):136–61.
Anderson RW, Volsky DJ, Greenberg B, Knox SJ, Bechtold T, Kuszynski C, et al. Lymphocyte abnormalities in preleukemia—I. Decreased NK activity, anomalous immunoregulatory cell subsets and deficient EBV receptors. Leukemia research. 1983;7(3):389–95.
Buyl K, Merimi M, Rodrigues RM, Moussa Agha D, Melki R, Vanhaecke T, et al. The impact of cell-expansion and inflammation on the immune-biology of human adipose tissue-derived mesenchymal stromal cells. J Clin Med. 2020;9(3):696.
Liu Z, Guo Y, Huang L, Jia Y, Liu H, Peng F, et al. Bone marrow mesenchymal stem cells regulate the dysfunction of NK cells via the T cell immunoglobulin and ITIM domain in patients with myelodysplastic syndromes. Cell Communication and Signaling. 2022;20(1):169.
Fu R, Guo Y, Liu Z, Shao Z. BMSCs Regulate the Function of NK Cell By CD155/Tigit/CD226 Pathway in MDS Patients. Blood. 2021;138:4651.
Lupo KB, Matosevic S. CD155 immunoregulation as a target for natural killer cell immunotherapy in glioblastoma. J Hematol Oncol. 2020;13:76.
Mu Y, Guan X. CD155-TIGIT Axis as a Therapeutic Target for Cancer Immunotherapy. Curr Med Chem. 2023;31(13):1634–45.
Han M-Z, Wang S, Zhao W-B, Ni S-L, Yang N, Kong Y, et al. Immune checkpoint molecule herpes virus entry mediator is overexpressed and associated with poor prognosis in human glioblastoma. EBioMedicine. 2019;43:159–70.
Qin S, Xu L, Yi M, Yu S, Wu K, Luo S. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol Cancer. 2019;18:155.
He X, Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020;30(8):660–9.
Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 2003;4(7):670–9.
Malissen N, Macagno N, Granjeaud S, Granier C, Moutardier V, Gaudy-Marqueste C, et al. HVEM has a broader expression than PD-L1 and constitutes a negative prognostic marker and potential treatment target for melanoma. Oncoimmunology. 2019;8(12): e1665976.
Fourcade J, Sun Z, Pagliano O, Guillaume P, Luescher IF, Sander C, et al. CD8+ T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Can Res. 2012;72(4):887–96.
Rong Z, Zhang F, Wang Z, He W, Dong S, Xu J, et al. Improved osteogenesis by HVEM-expressing allogenic bone marrow-derived mesenchymal stem cells in an immune activation condition and mouse femoral defect model. Tissue Eng Part A. 2018;24(15–16):1167–78.
Cheng T, Feng Y, Chen X, Zhou J, Song Y. Lung-resident mesenchymal stem cells regulated the inflammatory responses in innate and adaptive immune cells through HVEM-BTLA pathway during ARDS. Exp Cell Res. 2020;395(1): 112155.
Xuan X, Tian C, Zhao M, Sun Y, Huang C. Mesenchymal stem cells in cancer progression and anticancer therapeutic resistance. Cancer Cell Int. 2021;21(1):595.
Gan L, Shen H, Li X, Han Z, **g Y, Yang X, et al. Mesenchymal stem cells promote chemoresistance by activating autophagy in intrahepatic cholangiocarcinoma. Oncol Rep. 2021;45(1):107–18.
Wu WK, Zhang L, Chan MT. Autophagy, nafld and nafld-related hcc. Obesity, fatty liver and liver cancer. 2018:127–38.
Jang D-i, Lee A-H, Shin H-Y, Song H-R, Park J-H, Kang T-B, et al. The role of tumor necrosis factor alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics. Int J Mole Sci. 2021;22(5):2719.
van Loo G, Bertrand MJ. Death by TNF: a road to inflammation. Nat Rev Immunol. 2023;23(5):289–303.
Gough P, Myles IA. Tumor necrosis factor receptors: pleiotropic signaling complexes and their differential effects. Front Immunol. 2020;11: 585880.
MacEwan DJ. TNF ligands and receptors–a matter of life and death. Br J Pharmacol. 2002;135(4):855.
Wajant H, Siegmund D. TNFR1 and TNFR2 in the Control of the Life and Death Balance of Macrophages. Frontiers in cell and developmental biology. 2019;7:91.
Van Hauwermeiren F, Vandenbroucke RE, Libert C. Treatment of TNF mediated diseases by selective inhibition of soluble TNF or TNFR1. Cytokine Growth Factor Rev. 2011;22(5–6):311–9.
Ahmad S, Azid NA, Boer JC, Lim J, Chen X, Plebanski M, et al. The key role of TNF-TNFR2 interactions in the modulation of allergic inflammation: a review. Front Immunol. 2018;9:2572.
Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296(5573):1634–5.
Chen X, Subleski JJ, Kopf H, Howard O, Männel DN, Oppenheim JJ. Cutting edge: expression of TNFR2 defines a maximally suppressive subset of mouse CD4+ CD25+ FoxP3+ T regulatory cells: applicability to tumor-infiltrating T regulatory cells. J Immunol. 2008;180(10):6467–71.
Chen X, Hamano R, Subleski JJ, Hurwitz AA, Howard O, Oppenheim JJ. Expression of costimulatory TNFR2 induces resistance of CD4+ FoxP3− conventional T cells to suppression by CD4+ FoxP3+ regulatory T cells. J Immunol. 2010;185(1):174–82.
Chen X, Oppenheim JJ. Targeting TNFR2, an immune checkpoint stimulator and oncoprotein, is a promising treatment for cancer. Science signaling. 2017;10(462):eaal2328.
Chen AY, Wolchok JD, Bass AR. TNF in the era of immune checkpoint inhibitors: friend or foe? Nat Rev Rheumatol. 2021;17(4):213–23.
Tan J, Weil BR, Abarbanell AM, Wang Y, Herrmann JL, Dake ML, et al. Ablation of TNF-α receptors influences mesenchymal stem cell-mediated cardiac protection against ischemia. Shock. 2010;34(3):236–42.
Kelly ML, Wang M, Crisostomo PR, Abarbanell AM, Herrmann JL, Weil BR, et al. TNF receptor 2, not TNF receptor 1 enhances mesenchymal stem cell-mediated cardiac protection following acute ischemia. Shock (Augusta, Ga). 2010;33(6):602.
Beldi G, Khosravi M, Abdelgawad ME, Salomon BL, Uzan G, Haouas H, et al. TNFα/TNFR2 signaling pathway: an active immune checkpoint for mesenchymal stem cell immunoregulatory function. Stem Cell Res Ther. 2020;11:281.
Naserian S, Shamdani S, Arouche N, Uzan G. Regulatory T cell induction by mesenchymal stem cells depends on the expression of TNFR2 by T cells. Stem Cell Res Ther. 2020;11:534.
Beldi G, Bahiraii S, Lezin C, Nouri Barkestani M, Abdelgawad ME, Uzan G, et al. TNFR2 is a crucial hub controlling mesenchymal stem cell biological and functional properties. Frontiers in Cell and Developmental Biology. 2020;8: 596831.
Fujisawa K, Takami T, Okada S, Hara K, Matsumoto T, Yamamoto N, et al. Analysis of metabolomic changes in mesenchymal stem cells on treatment with desferrioxamine as a hypoxia mimetic compared with hypoxic conditions. Stem Cells. 2018;36(8):1226–36.
Liu X-B, Wang J-A, Ji X-Y, Yu SP, Wei L. Preconditioning of bone marrow mesenchymal stem cells by prolyl hydroxylase inhibition enhances cell survival and angiogenesis in vitro and after transplantation into the ischemic heart of rats. Stem Cell Res Ther. 2014;5(5):111.
Sun Y, Li Q-f, Yan J, Hu R, Jiang H. Isoflurane preconditioning promotes the survival and migration of bone marrow stromal cells. Cell Physiol Biochem. 2015;36(4):1331–45.
Takeda K, Ning F, Domenico J, Okamoto M, Ashino S, Kim S-H, et al. Activation of p70S6 kinase-1 in mesenchymal stem cells is essential to lung tissue repair. Stem Cells Transl Med. 2018;7(7):551–8.
Li D, Wang P, Li Y, **e Z, Wang L, Su H, et al. All-trans retinoic acid improves the effects of bone marrow-derived mesenchymal stem cells on the treatment of ankylosing spondylitis: an in vitro study. Stem cells international. 2015;2015: 484528.
Wang B, Lin Y, Hu Y, Shan W, Liu S, Xu Y, et al. mTOR inhibition improves the immunomodulatory properties of human bone marrow mesenchymal stem cells by inducing COX-2 and PGE2. Stem Cell Res Ther. 2017;8(1):292.
Zhang S, Jiang L, Hu H, Wang H, Wang X, Jiang J, et al. Pretreatment of exosomes derived from hUCMSCs with TNF-α ameliorates acute liver failure by inhibiting the activation of NLRP3 in macrophage. Life Sci. 2020;246: 117401.
Fan H, Zhao G, Liu L, Liu F, Gong W, Liu X, et al. Pre-treatment with IL-1β enhances the efficacy of MSC transplantation in DSS-induced colitis. Cell Mol Immunol. 2012;9(6):473–81.
Redondo-Castro E, Cunningham C, Miller J, Martuscelli L, Aoulad-Ali S, Rothwell NJ, et al. Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res Ther. 2017;8:79.
Shao M, Xu Q, Wu Z, Chen Y, Shu Y, Cao X, et al. Exosomes derived from human umbilical cord mesenchymal stem cells ameliorate IL-6-induced acute liver injury through miR-455-3p. Stem Cell Res Ther. 2020;11(1):37.
Takeuchi S, Tsuchiya A, Iwasawa T, Nojiri S, Watanabe T, Ogawa M, et al. Small extracellular vesicles derived from interferon-γ pre-conditioned mesenchymal stromal cells effectively treat liver fibrosis. NPJ Regenerative Medicine. 2021;6(1):19.
Li M, Jiang Y, Hou Q, Zhao Y, Zhong L, Fu X. Potential pre-activation strategies for improving therapeutic efficacy of mesenchymal stem cells: current status and future prospects. Stem Cell Res Ther. 2022;13(1):146.
Chen Y, Cao J, **ong M, Petersen AJ, Dong Y, Tao Y, et al. Engineering human stem cell lines with inducible gene knockout using CRISPR/Cas9. Cell Stem Cell. 2015;17(2):233–44.
Cheung MB, Sampayo-Escobar V, Green R, Moore ML, Mohapatra S, Mohapatra SS. Respiratory syncytial virus-infected mesenchymal stem cells regulate immunity via interferon beta and indoleamine-2, 3-dioxygenase. PLoS ONE. 2016;11(10): e0163709.
Yoshida Y, Yamanaka S. Recent stem cell advances: induced pluripotent stem cells for disease modeling and stem cell–based regeneration. Circulation. 2010;122(1):80–7.
Cho RJ, Kim Y-S, Kim J-Y, Oh Y-M. Human adipose-derived mesenchymal stem cell spheroids improve recovery in a mouse model of elastase-induced emphysema. BMB Rep. 2017;50(2):79.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
A.H. and K.M. Conceptualization, Investigation, Writing - original draft, Writing - review & editing, Validation, Supervision, and Final approval of the manuscript. H.K.A. Designing figures and Final approval of the manuscript. S.M.H. and S.R. Supervision and final approval of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hazrati, A., Malekpour, K., Khorramdelazad, H. et al. Therapeutic and immunomodulatory potentials of mesenchymal stromal/stem cells and immune checkpoints related molecules. Biomark Res 12, 35 (2024). https://doi.org/10.1186/s40364-024-00580-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40364-024-00580-2