Abstract
The interaction between programmed cell death ligand 1 (PD-L1), which is expressed on the surface of tumor cells, and programmed cell death 1 (PD-1), which is expressed on T cells, impedes the effective activation of tumor antigen-specific T cells, resulting in the evasion of tumor cells from immune-mediated killing. Blocking the PD-1/PD-L1 signaling pathway has been shown to be effective in preventing tumor immune evasion. PD-1/PD-L1 blocking antibodies have garnered significant attention in recent years within the field of tumor treatments, given the aforementioned mechanism. Furthermore, clinical research has substantiated the efficacy and safety of this immunotherapy across various tumors, offering renewed optimism for patients. However, challenges persist in anti-PD-1/PD-L1 therapies, marked by limited indications and the emergence of drug resistance. Consequently, identifying additional regulatory pathways and molecules associated with PD-1/PD-L1 and implementing judicious combined treatments are imperative for addressing the intricacies of tumor immune mechanisms. This review briefly outlines the structure of the PD-1/PD-L1 molecule, emphasizing the posttranslational modification regulatory mechanisms and related targets. Additionally, a comprehensive overview on the clinical research landscape concerning PD-1/PD-L1 post-translational modifications combined with PD-1/PD-L1 blocking antibodies to enhance outcomes for a broader spectrum of patients is presented based on foundational research.
Similar content being viewed by others
Introduction
Programmed cell death 1 (PD-1) and its ligand PD-L1 have become pivotal in advancing tumor treatment by effectively modulating immune responses [1]. PD-L1 is expressed across various tumors, while PD-1 is primarily expressed on T cells within tumor tissues [2]. PD-L1 engages with PD-1, creating a molecular barrier that inhibits the cytotoxic actions of immune cells [3]. Overcoming this inhibition is possible through blocking antibodies or recombinant proteins that target signaling pathways, reactivating immune responses. Monoclonal antibodies against PD-1 and PD-L1 have demonstrated significant therapeutic success, indicating that immune checkpoint blockade therapy is a potent antitumor treatment. However, its current use mainly as a second-line treatment for advanced tumors and the emergence of drug resistance highlight ongoing challenges [4]. These factors underscore the necessity for continued research to potentially expand its use earlier in treatment protocols.
Exploring new biomarkers and develo** combination drug therapies are essential for combating these challenges. Research has shown that PD-1 transcription can be increased by activating B-cell CLL/lymphoma 6 (BCL6), and various elements, such as cytokines, hypoxia, bromodomain-containing protein 4 (BRD4), and noncoding RNA, can elevate PD-L1 expression by influencing transcription [5, 90].
Glycosylation of PD-L1 affects clinical immunohistochemistry
The glycosylation of PD-L1 can interfere with its detection by immunohistochemical antibodies, potentially causing false-negative results in tests that assess PD-L1 expression in cancer patients. This issue arises when glycosyl structures on the PD-L1 protein prevent antibody binding [90]. To address this issue, researchers have developed a method of removing these sugars—called deglycosylation—before testing. This technique significantly improves the accuracy of PD-L1 detection and correlates better with patients’ responses to anti-PD-1/PD-L1 therapies [182].
Etoposide can inhibit the enzyme STT3, which is involved in N-glycosylation, through its anti-EMT effects, reducing PD-L1 levels and increasing the effectiveness of anti-Tim3 therapy [96]. Clinical trials of etoposide combined with anti-PD-1/PD-L1 immunotherapy are currently underway [183,184,185,186,187,188,189] (Table 2). In a phase III trial for extensive small cell lung cancer, compared with placebo, pembrolizumab combined with etoposide and platinum significantly improved 12-month PFS (13.6% vs. 3.1%, P = 0.0023), enhancing patient quality of life [183, 184]. Another study revealed that atezolizumab combined with carboplatin and etoposide increased overall survival (OS) to 12.3 months from 10.3 months with chemotherapy alone (P = 0.0154) and was well tolerated [185, 186]. Similarly, tislelizumab or serplulimab with the same regimen in different trials extended OS and PFS [188, 189].
Targeting the PD-L1 dimer inhibits PD-L1 function
PD-L1 can form homodimers and tetramers, and its complex glycosylation is linked to the homodimeric structure of its intracellular domain [190]. Natural compounds such as capsaicin, 6-gingerol, and curcumin may block the PD-1/PD-L1 interaction by targeting PD-L1 dimerization, enhancing anticancer immunity [191]. The small molecule BMS-202, with modified carbonyl to hydroxyl groups, produces two enantiomers, MS and MR, both of which disrupt PD-L1 function by targeting its dimerization [192]. Furthermore, compounds such as α-mangostin and ethanol extracts can inhibit PD-L1 glycosylation and promote its degradation by binding within the pocket of the PD-L1 dimer [193]. These findings from preclinical studies highlight the potential of designing inhibitors that target PD-L1 dimers to enhance immunotherapy efficacy.
Treatment prospects and clinical transformation of PD-L1/PD-1 palmitoylation
Palmitoylation of PD-L1 stabilizes the protein, and targeting this modification enhances PD-L1 immunotherapy efficacy. Porcupine, a membrane-bound o-acyltransferase, is targeted by inhibitors such as LGK974, ETC-1,922,159, CGX1321, and RXC004 and is now in phase I trials [194]. These inhibitors have also been tested in combination with anti-PD-1/PD-L1 antibodies in clinical trials (Table 2). Research shows that chloroquine derivatives improve anti-PD-1 therapy in melanoma by targeting palmitoyl protein thioesterase 1 (PPT1) [195]. Combining PPT1 inhibitors with anti-PD-1 antibodies activates T cells, enhancing tumor immunity [196]. Innovative therapies include HHAT and APT1/2 inhibitors and 2-bromopalmitate (2-BP) in polymer-lipid hybrid nanoparticles (2-BP/CPT-PLNs) that replace anti-PD-L1 antibodies in immune checkpoint blockade, showing potent antitumor effects and improved survival in melanoma models [197,198,199]. Additionally, a novel peptide (CPP-S1) that inhibits PD-L1 palmitoylation and promotes its degradation offers another strategy to enhance immunotherapy efficacy [21].
Dai et al. demonstrated that targeting PD-L1 palmitoylation was more effective than direct targeting [200]. Additionally, Shi et al. created a PROTAC (SP-PROTAC) using an anastomotic peptide targeting the palmitoyl transferase ZDHHC3, which significantly reduced PD-L1 expression in a human cervical cancer cell line [201].
ZDHHC9 palmitoylates cGAS at Cys 404/405, enhancing its activation, while depalmitoylation byLYPLAL1 impairs cGAS function. TargetingLYPLAL1-mediated cGAS depalmitoylation could boost cGAS activation and improve antitumor immunotherapy efficacy [202]. As ZDHHC9 also affects PD-L1 palmitoylation, inhibitingLYPLAL1 might enhance overall immunotherapy outcomes.
Therapeutic promise of other PD-L1/PD-1 posttranslational modifications
Several preclinical treatments targeting PD-1/PD-L1 posttranslational modifications are being developed. HDAC2 inhibitors combined with PD-1 antibodies have been shown to significantly delay tumor growth and improve survival in syngeneic MC38 mouse models [22]. JQ-1, which reduces PD-L1 expression through acetylation, shows potential for treating pancreatic cancer [127]. Pevonedistat, a NEDDylation inhibitor, is undergoing clinical trials for various cancers and may upregulate PD-L1 expression, although its effectiveness is still under study [203, 204]. Zhou et al. discovered a UFSP2 inhibitor that enhances UFMyation, decreases PD-L1 expression, and supports PD-1 blockade [59]. CK2 inhibitors trigger PD-L1 autophagic degradation and enhance antitumor immunotherapy when combined with PD-1 antibodies [61]. Additionally, paclitaxel, which increases MMP-13 in certain cancer cells, shows promise for head and neck cancer treatment when used with anti-PD-1 therapy [65]. These methods represent promising strategies for cancer immunotherapy.
Summary and prospective
In this review, we summarize the PTMs of PD-1/PD-L1 and their regulatory mechanisms and propose new targets for biomarkers and combination therapies to enhance PD-1/PD-L1 blockade in immunotherapy. Despite these advances, many aspects of PD-1/PD-L1 PTMs remain elusive. For diagnosis, PD-L1 glycosylation can obscure antibody binding sites, causing false negatives [177]. Additionally, the degradation of the glycan region of the PD-L1 epitope may lead to a loss of staining on immunohistochemistry [205]. The absolute and effective glycosylation levels may also vary significantly [206]. In treatment contexts, PD-L1 PTMs can contribute to tumor progression. In addition to PD-1/PD-L1 blockade, PTMs are vital for antigen presentation, CAR-T-cell therapy, and vaccine development [207]. Innovations such as multifluorescence resonance energy transfer (multi-FRET) are enhancing PTM research, offering new avenues for advancing tumor immunotherapy [208].
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- 2F‒Fuc:
-
2-fluoro-L-fucose
- ADCC:
-
Antibody-dependent cytotoxicity
- ADPR:
-
ADP-ribose
- ARF9:
-
ADP-ribosylation factor-6
- BC:
-
Breast cancer
- BCL:
-
B-cell lymphoma
- BL:
-
Burkitt lymphoma
- CISH:
-
Cytokine-inducible SH2 domain-containing protein
- CPT:
-
Camptothecin
- CR:
-
Complete response
- CRC:
-
Colorectal cancer
- CSCC:
-
Cutaneous squamous cell carcinoma
- CTLs:
-
Cytotoxic T lymphocytes
- CxCa:
-
Cervical cancer
- Cys or C:
-
Cysteine
- DHHC:
-
Aspartic acid-histidine-histidine-cysteine
- DOR:
-
Duration of response
- ESCC:
-
Esophageal squamous cell carcinoma
- FASN:
-
Fatty acid synthase
- GBM:
-
Glioblastoma
- GC:
-
Gastric cancer
- Gly:
-
Glycine
- HCC:
-
Hepatocellular carcinoma
- HNSCC:
-
Head and neck squamous cell carcinoma
- HRD:
-
Hyperprogressive disease
- ICB:
-
Immune checkpoint blockade
- ICD:
-
Immunogenic cell death
- KATs:
-
Lysine acetylases
- LUAD:
-
Lung adenocarcinoma
- MD:
-
Molecular dynamics
- MHC:
-
Major histocompatibility complex
- MULTI-FRET:
-
Multifluorescence resonance energy transfer
- NPC:
-
Nasopharyngeal carcinoma
- NSCLC:
-
Non-small cell lung cancer
- NSQ:
-
Nonsquamous
- OC:
-
Ovarian cancer
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- OSCC:
-
Oral squamous cell carcinoma
- PC:
-
Prostate cancer
- PCA:
-
pancreatic cancer
- PD-1:
-
Programmed cell death protein 1
- PD-L1:
-
Programmed death-ligand 1
- PDAC:
-
Pancreatic ductal adenocarcinoma
- PFS:
-
Progression-free survival
- PROTACs:
-
PROteolysis-Targeting Chimeras
- PTM:
-
Posttranslational modification
- SCLC:
-
Small cell lung cancer
- SD:
-
Stable disease
- Ser or S:
-
Seronine
- SQ:
-
Squamous
- TCR:
-
T-cell antigen receptor
- Thr or T:
-
Threonine
- TNBC:
-
Triple-negative breast cancer
- TRAE:
-
Treatment-related adverse events
- Tyr or Y:
-
Tyrosine
- ZAP70:
-
ζchain-related tyrosine kinase 70
References
Weber EW, Maus MV, Mackall CL. The Emerging Landscape of Immune Cell therapies. Cell. 2020;181(1):46–62.
Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48(3):434–52.
Feng C, Zhang L, Chang X, Qin D, Zhang T. Regulation of post-translational modification of PD-L1 and advances in tumor immunotherapy. Front Immunol. 2023;14:1230135.
Yang Y, Zhou T, Chen X, Li J, Pan J, He X, et al. Efficacy, safety, and biomarker analysis of Camrelizumab in previously treated recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN study). J Immunother Cancer. 2021;9(12):e003790.
Zhu H, Bengsch F, Svoronos N, Rutkowski MR, Bitler BG, Allegrezza MJ, et al. BET bromodomain inhibition promotes anti-tumor immunity by suppressing PD-L1 expression. Cell Rep. 2016;16(11):2829–37.
Wang J, Ge J, Wang Y, **ong F, Guo J, Jiang X, et al. EBV miRNAs BART11 and BART17-3p promote immune escape through the enhancer-mediated transcription of PD-L1. Nat Commun. 2022;13(1):866.
Hsu JM, Li CW, Lai YJ, Hung MC. Posttranslational modifications of PD-L1 and their applications in Cancer Therapy. Cancer Res. 2018;78(22):6349–53.
Ikemizu S, Gilbert RJC, Fennelly JA, Collins AV, Harlos K, Jones EY, et al. Structure and dimerization of a soluble form of B7-1. Immunity. 2000;12(1):51–60.
Zhang X, Schwartz JC, Almo SC, Nathenson SG. Crystal structure of the receptor-binding domain of human B7-2: insights into organization and signaling. Proc Natl Acad Sci U S A. 2003;100(5):2586–91.
Zhang X, Schwartz JC, Guo X, Bhatia S, Cao E, Chen L, et al. Structural and functional analysis of the costimulatory receptor programmed death-1. Immunity. 2004;20(3):337–47.
Sun L, Li CW, Chung EM, Yang R, Kim YS, Park AH, et al. Targeting glycosylated PD-1 induces potent Antitumor immunity. Cancer Res. 2020;80(11):2298–310.
Liu J, Wei L, Hu N, Wang D, Ni J, Zhang S, et al. FBW7-mediated ubiquitination and destruction of PD-1 protein primes sensitivity to anti-PD-1 immunotherapy in non-small cell lung cancer. J Immunother Cancer. 2022;10(9):e005116.
Meng X, Liu X, Guo X, Jiang S, Chen T, Hu Z, et al. FBXO38 mediates PD-1 ubiquitination and regulates anti-tumour immunity of T cells. Nature. 2018;564(7734):130–35.
Patsoukis N, Wang Q, Strauss L, Boussiotis VA. Revisiting the PD-1 pathway. Sci Adv. 2020;6(38):eabd2712.
Tit-oon P, Wonglangka A, Boonkanta K, Ruchirawat M, Fuangthong M, Khongmanee A, et al. Intact mass analysis reveals the novel O-linked glycosylation on the stalk region of PD-1 protein. Sci Rep. 2023;13(1):9631.
Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–34.
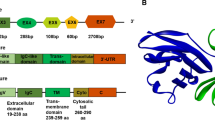
Cheng X, Veverka V, Radhakrishnan A, Waters LC, Muskett FW, Morgan SH, et al. Structure and interactions of the human programmed cell death 1 receptor. J Biol Chem. 2013;288(17):11771–85.
Benicky J, Sanda M, Brnakova Kennedy Z, Grant OC, Woods RJ, Zwart A, et al. PD-L1 glycosylation and its impact on binding to clinical antibodies. J Proteome Res. 2021;20(1):485–97.
Cha JH, Chan LC, Li CW, Hsu JL, Hung MC. Mechanisms Controlling PD-L1 expression in Cancer. Mol Cell. 2019;76(3):359–70.
Sun K, Zhang X, Lao M, He L, Wang S, Yang H, et al. Targeting leucine-rich repeat serine/threonine-protein kinase 2 sensitizes pancreatic ductal adenocarcinoma to anti-PD-L1 immunotherapy. Mol Ther. 2023;31(10):2929–47.
Yao H, Lan J, Li C, Shi H, Brosseau JP, Wang H, et al. Inhibiting PD-L1 palmitoylation enhances T-cell immune responses against tumours. Nat Biomed Eng. 2019;3(4):306–17.
Gao Y, Nihira NT, Bu X, Chu C, Zhang J, Kolodziejczyk A, et al. Acetylation-dependent regulation of PD-L1 nuclear translocation dictates the efficacy of anti-PD-1 immunotherapy. Nat Cell Biol. 2020;22(9):1064–75.
Huang C, Ren S, Chen Y, Liu A, Wu Q, Jiang T, et al. PD-L1 methylation restricts PD-L1/PD-1 interactions to control cancer immune surveillance. Sci Adv. 2023;9(21):eade4186.
Tu X, Qin B, Zhang Y, Zhang C, Kahila M, Nowsheen S, et al. PD-L1 (B7-H1) competes with the RNA exosome to regulate the DNA damage response and can be targeted to sensitize to Radiation or Chemotherapy. Mol Cell. 2019;74(6):1215–e264.
Singh V, Ram M, Kumar R, Prasad R, Roy BK, Singh KK. Phosphorylation: implications in Cancer. Protein J. 2017;36(1):1–6.
Chan LC, Li CW, **a W, Hsu JM, Lee HH, Cha JH, et al. IL-6/JAK1 pathway drives PD-L1 Y112 phosphorylation to promote cancer immune evasion. J Clin Invest. 2019;129(8):3324–38.
Li CW, Lim SO, **a W, Lee HH, Chan LC, Kuo CW, et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat Commun. 2016;7:12632.
Zhang R, Yang Y, Dong W, Lin M, He J, Zhang X, et al. D-mannose facilitates immunotherapy and radiotherapy of triple-negative breast cancer via degradation of PD-L1. Proc Natl Acad Sci U S A. 2022;119(8):e2114851119.
Park HB, Baek KH. E3 ligases and deubiquitinating enzymes regulating the MAPK signaling pathway in cancers. Biochim Biophys Acta Rev Cancer. 2022;1877(3):188736.
Rape M. Ubiquitylation at the crossroads of development and disease. Nat Rev Mol Cell Biol. 2018;19(1):59–70.
Popovic D, Vucic D, Dikic I. Ubiquitination in disease pathogenesis and treatment. Nat Med. 2014;20(11):1242–53.
Chen N, Fang W, Zhan J, Hong S, Tang Y, Kang S, et al. Upregulation of PD-L1 by EGFR activation mediates the Immune escape in EGFR-Driven NSCLC: implication for Optional Immune targeted therapy for NSCLC patients with EGFR Mutation. J Thorac Oncol. 2015;10(6):910–23.
Horita H, Law A, Hong S, Middleton K. Identifying Regulatory Posttranslational modifications of PD-L1: a focus on Monoubiquitinaton. Neoplasia. 2017;19(4):346–53.
Wang S, Xu L, Che X, Li C, Xu L, Hou K, et al. E3 ubiquitin ligases Cbl-b and c-Cbl downregulate PD-L1 in EGFR wild-type non-small cell lung cancer. FEBS Lett. 2018;592(4):621–30.
Zhang J, Bu X, Wang H, Zhu Y, Geng Y, Nihira NT, et al. Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance. Nature. 2018;553(7686):91–5.
Gao Y, Zou T, Xu P, Wang Y, Jiang Y, Chen YX, et al. Fusobacterium nucleatum stimulates cell proliferation and promotes PD-L1 expression via IFIT1-related signal in colorectal cancer. Neoplasia. 2023;35:100850.
Zhang Y, Zeng L, Wang M, Yang Z, Zhang H, Gao L, et al. RIG-I promotes immune evasion of colon cancer by modulating PD-L1 ubiquitination. J Immunother Cancer. 2023;11(9):e007313.
Jiang C, He L, **ao S, Wu W, Zhao Q, Liu F. E3 ubiquitin ligase RNF125 suppresses Immune escape in Head and Neck squamous cell carcinoma by regulating PD-L1 expression. Mol Biotechnol. 2023;65(6):891–903.
Shi C, Wang Y, Wu M, Chen Y, Liu F, Shen Z, et al. Promoting anti-tumor immunity by targeting TMUB1 to modulate PD-L1 polyubiquitination and glycosylation. Nat Commun. 2022;13(1):6951.
Yi J, Tavana O, Li H, Wang D, Baer RJ, Gu W. Targeting USP2 regulation of VPRBP-mediated degradation of p53 and PD-L1 for cancer therapy. Nat Commun. 2023;14(1):1941.
Zhang H, Chen H, Yin S, Fan L, ** C, Zhao C, et al. Docosahexaenoic acid reverses PD-L1-mediated immune suppression by accelerating its ubiquitin-proteasome degradation. J Nutr Biochem. 2023;112:109186.
Lin CY, Huang KY, Kao SH, Lin MS, Lin CC, Yang SC, et al. Small-molecule PIK-93 modulates the tumor microenvironment to improve immune checkpoint blockade response. Sci Adv. 2023;9(14):eade9944.
Zou J, **a H, Zhang C, Xu H, Tang Q, Zhu G, et al. Casp8 acts through A20 to inhibit PD-L1 expression: the mechanism and its implication in immunotherapy. Cancer Sci. 2021;112(7):2664–78.
Guo W, Ma J, Guo S, Wang H, Wang S, Shi Q, et al. A20 regulates the therapeutic effect of anti-PD-1 immunotherapy in melanoma. J Immunother Cancer. 2020;8(2):e001866.
Mezzadra R, Sun C, Jae LT, Gomez-Eerland R, de Vries E, Wu W, et al. Identification of CMTM6 and CMTM4 as PD-L1 protein regulators. Nature. 2017;549(7670):106–10.
Burr ML, Sparbier CE, Chan Y-C, Williamson JC, Woods K, Beavis PA, et al. CMTM6 maintains the expression of PD-L1 and regulates anti-tumour immunity. Nature. 2017;549(7670):101–05.
Wang H, Yao H, Li C, Shi H, Lan J, Li Z, et al. HIP1R targets PD-L1 to lysosomal degradation to alter T cell–mediated cytotoxicity. Nat Chem Biol. 2019;15:42–50.
Ding L, Chen X, Zhang W, Dai X, Guo H, Pan X, et al. Canagliflozin primes antitumor immunity by triggering PD-L1 degradation in endocytic recycling. J Clin Invest. 2023;133(1):e154754.
Yu X, Li W, Liu H, Wang X, Coarfa C, Cheng C, et al. PD-L1 translocation to the plasma membrane enables tumor immune evasion through MIB2 ubiquitination. J Clin Invest. 2023;133(3):e160456.
Dong LF, Chen FF, Fan YF, Zhang K, Chen HH. circ-0000512 inhibits PD-L1 ubiquitination through sponging miR-622/CMTM6 axis to promote triple-negative breast cancer and immune escape. J Immunother Cancer. 2023;11(6):e005461.
Li J, Dong X, Kong X, Wang Y, Li Y, Tong Y, et al. Circular RNA hsa_circ_0067842 facilitates tumor metastasis and immune escape in breast cancer through HuR/CMTM6/PD-L1 axis. Biol Direct. 2023;18(1):48.
Le NT, Xue M, Castelnoble LA, Jackson CJ. The dual personalities of matrix metalloproteinases in inflammation. Front Biosci. 2007;12:1475–87.
Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2(3):161–74.
Dezutter-Dambuyant C, Durand I, Alberti L, Bendriss-Vermare N, Valladeau-Guilemond J, Duc A, et al. A novel regulation of PD-1 ligands on mesenchymal stromal cells through MMP-mediated proteolytic cleavage. Oncoimmunology. 2015;5(3):e1091146.
Hira-Miyazawa M, Nakamura H, Hirai M, Kobayashi Y, Kitahara H, Bou-Gharios G, et al. Regulation of programmed-death ligand in the human head and neck squamous cell carcinoma microenvironment is mediated through matrix metalloproteinase-mediated proteolytic cleavage. Int J Oncol. 2018;52(2):379–88.
Rowswell-Turner RB, Singh RK, Urh A, Yano N, Kim KK, Khazan N, et al. HE4 overexpression by Ovarian Cancer promotes a suppressive Tumor Immune Microenvironment and enhanced tumor and macrophage PD-L1 expression. J Immunol. 2021;206(10):2478–88.
Raposo AE, Piller SC. Protein arginine methylation: an emerging regulator of the cell cycle. Cell Div. 2018;13:3.
Dai X, Ren T, Zhang Y, Nan N. Methylation multiplicity and its clinical values in cancer. Expert Rev Mol Med. 2021;23:e2.
Zhou J, Ma X, He X, Chen B, Yuan J, ** Z, et al. Dysregulation of PD-L1 by UFMylation imparts tumor immune evasion and identified as a potential therapeutic target. Proc Natl Acad Sci U S A. 2023;120(11):e2215732120.
Tatsumi K, Sou Y, Tada N, Nakamura E, Iemura S, Natsume T, et al. A novel type of E3 ligase for the Ufm1 conjugation system. J Biol Chem. 2010;285(8):5417–27.
Malakhov MP, Kim KI, Malakhova OA, Jacobs BS, Borden EC, Zhang DE. High-throughput immunoblotting. Ubiquitiin-like protein ISG15 modifies key regulators of signal transduction. J Biol Chem. 2003;278(19):16608–13.
Qu T, Zhang W, Yan C, Ren D, Wang Y, Guo Y, et al. ISG15 targets glycosylated PD-L1 and promotes its degradation to enhance antitumor immune effects in lung adenocarcinoma. J Transl Med. 2023;21(1):341.
Enchev RI, Schulman BA, Peter M. Protein neddylation: beyond cullin-RING ligases. Nat Rev Mol Cell Biol. 2015;16(1):30–44.
Wang X, Chen C, Vuong D, Rodriguez-Rodriguez S, Lam V, Roleder C, et al. Pharmacologic targeting of Nedd8-activating enzyme reinvigorates T-cell responses in lymphoid neoplasia. Leukemia. 2023;37(6):1324–35.
Zhou S, Zhao X, Yang Z, Yang R, Chen C, Zhao K, et al. Neddylation inhibition upregulates PD-L1 expression and enhances the efficacy of immune checkpoint blockade in glioblastoma. Int J Cancer. 2019;145(3):763–74.
Zhang S, You X, Xu T, Chen Q, Li H, Dou L, et al. PD-L1 induction via the MEK-JNK-AP1 axis by a neddylation inhibitor promotes cancer-associated immunosuppression. Cell Death Dis. 2022;13(10):844.
Byun JK, Park M, Lee S, Yun JW, Lee J, Kim JS, et al. Inhibition of glutamine utilization synergizes with Immune checkpoint inhibitor to promote Antitumor Immunity. Mol Cell. 2020;80(4):592–e6068.
Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147(4):728–41.
Gou Q, Chen H, Chen M, Shi J, ** J, Liu Q et al. Inhibition of CK2/ING4 pathway facilitates Non-small Cell Lung Cancer Immunotherapy. Adv Sci (Weinh). 2023;e2304068.
Antao AM, Tyagi A, Kim KS, Ramakrishna S. Advances in deubiquitinating enzyme inhibition and applications in Cancer therapeutics. Cancers (Basel). 2020;12(6):1579.
Mennerich D, Kubaichuk K, Kietzmann T. DUBs, Hypoxia, and Cancer. Trends Cancer. 2019;5(10):632–53.
Jumpertz S, Hennes T, Asare Y, Schütz AK, Bernhagen J. CSN5/JAB1 suppresses the WNT inhibitor DKK1 in colorectal cancer cells. Cell Signal. 2017;34:38–46.
Lim SO, Li CW, **a W, Cha JH, Chan LC, Wu Y, et al. Deubiquitination and stabilization of PD-L1 by CSN5. Cancer Cell. 2016;30(6):925–39.
Wang Y, Lu H, Fang C, Xu J. Palmitoylation as a Signal for Delivery. Adv Exp Med Biol. 2020;1248:399–424.
Ma Y, **a P, Wang Z, Xu J, Zhang L, Jiang Y. PDIA6 promotes pancreatic cancer progression and immune escape through CSN5-mediated deubiquitination of β-catenin and PD-L1. Neoplasia. 2021;23(9):912–28.
Huang X, Zhang Q, Lou Y, Wang J, Zhao X, Wang L, et al. USP22 Deubiquitinates CD274 to suppress anticancer immunity. Cancer Immunol Res. 2019;7(10):1580–90.
Wang Y, Sun Q, Mu N, Sun X, Wang Y, Fan S, et al. The deubiquitinase USP22 regulates PD-L1 degradation in human cancer cells. Cell Commun Signal. 2020;18(1):112.
Wu J, Guo W, Wen D, Hou G, Zhou A, Wu W. Deubiquitination and stabilization of programmed cell death ligand 1 by ubiquitin-specific peptidase 9, X-linked in oral squamous cell carcinoma. Cancer Med. 2018;7(8):4004–11.
Kuang Z, Liu X, Zhang N, Dong J, Sun C, Yin, et al. USP2 promotes tumor immune evasion via deubiquitination and stabilization of PD-L1. Cell Death Differ. 2023;30(10):2249–64.
Li J, **ao X, Ou Y, Cao L, Guo M, Qi C, et al. USP51/PD-L1/ITGB1-deployed juxtacrine interaction plays a cell-intrinsic role in promoting chemoresistant phenotypes in non-small cell lung cancer. Cancer Commun (Lond). 2023;43(7):765–87.
Yang H, Zhang X, Lao M, Sun K, He L, Xu J, et al. Targeting ubiquitin-specific protease 8 sensitizes anti-programmed death-ligand 1 immunotherapy of pancreatic cancer. Cell Death Differ. 2023;30(2):560–75.
Yu ZZ, Liu YY, Zhu W, **ao D, Huang W, Lu SS, et al. ANXA1-derived peptide for targeting PD-L1 degradation inhibits tumor immune evasion in multiple cancers. J Immunother Cancer. 2023;11(3):e006345.
Mao R, Tan X, **ao Y, Wang X, Wei Z, Wang J, et al. Ubiquitin C-terminal hydrolase L1 promotes expression of programmed cell death-ligand 1 in non-small-cell lung cancer cells. Cancer Sci. 2020;111(9):3174–83.
Zhu D, Xu R, Huang X, Tang Z, Tian Y, Zhang J, et al. Deubiquitinating enzyme OTUB1 promotes cancer cell immunosuppression via preventing ER-associated degradation of immune checkpoint protein PD-L1. Cell Death Differ. 2021;28(6):1773–89.
Wang Q, Li G, Ma X, Liu L, Liu J, Yin Y, et al. LncRNA TINCR impairs the efficacy of immunotherapy against breast cancer by recruiting DNMT1 and downregulating MiR-199a-5p via the STAT1-TINCR-USP20-PD-L1 axis. Cell Death Dis. 2023;14(2):76.
Zheng R, Gao F, Mao Z, **ao Y, Yuan L, Huang Z, et al. LncRNA BCCE4 genetically enhances the PD-L1/PD-1 Interaction in Smoking-related bladder Cancer by modulating mir-328-3p-USP18 signaling. Adv Sci (Weinh). 2023;10(30):e2303473.
Sahin U, de Thé H, Lallemand-Breitenbach V. Sumoylation in Physiology, Pathology and Therapy. Cells. 2022;11(5):814.
Ma X, Jia S, Wang G, Liang M, Guo T, Du H, et al. TRIM28 promotes the escape of gastric cancer cells from immune surveillance by increasing PD-L1 abundance. Signal Transduct Target Ther. 2023;8(1):246.
Wang J, Wang Y, Jiang X, Xu M, Wang M, Wang R, et al. Unleashing the power of immune checkpoints: post-translational modification of novel molecules and clinical applications. Cancer Lett Published Online Febr. 2024;22. https://doi.org/10.1016/j.canlet.2024.216758.
Li CW, Lim SO, Chung EM, Kim YS, Park AH, Yao J, et al. Eradication of Triple-negative breast Cancer cells by targeting glycosylated PD-L1. Cancer Cell. 2018;33(2):187–e20110.
Maher CM, Thomas JD, Haas DA, Longen CG, Oyer HM, Tong JY, et al. Small-molecule Sigma1 modulator induces autophagic degradation of PD-L1. Mol Cancer Res. 2018;16(2):243–55.
D’Arrigo P, Russo M, Rea A, Tufano M, Guadagno E, De Del Basso ML, et al. A regulatory role for the co-chaperone FKBP51s in PD-L1 expression in glioma. Oncotarget. 2017;8(40):68291–304.
Liu X, Zhang Y, Han Y, Lu W, Yang J, Tian J, et al. Overexpression of GLT1D1 induces immunosuppression through glycosylation of PD-L1 and predicts poor prognosis in B-cell lymphoma. Mol Oncol. 2020;14(5):1028–44.
Zhang Y, Zhang N, Song W, Yousuf S, Li W. Ablation of the GDP-fucose transporter suppresses lung cancer cell proliferation and migration by reducing expression of PD-L1. J Cancer. 2023;14(17):3295–308.
Cui Y, Li J, Zhang P, Yin D, Wang Z, Dai J, et al. B4GALT1 promotes immune escape by regulating the expression of PD-L1 at multiple levels in lung adenocarcinoma. J Exp Clin Cancer Res. 2023;42(1):146.
Hsu JM, **a W, Hsu YH, Chan LC, Yu WH, Cha JH, et al. STT3-dependent PD-L1 accumulation on cancer stem cells promotes immune evasion. Nat Commun. 2018;9(1):1908. https://doi.org/10.1038/s41467-018-04313-6. Published 2018 May 15.
Paul S, Das K, Ghosh A, Chatterjee A, Bhoumick A, Basu A et al. Coagulation factor VIIa enhances programmed death-ligand 1 expression and its stability in breast cancer cells to promote breast cancer immune evasion. J Thromb Haemost. 2023;S1538-7836(23)00632-3.
Ma XM, Luo YF, Zeng FF, Su C, Liu X, Li XP, et al. TGF-β1-Mediated PD-L1 glycosylation contributes to Immune escape via c-Jun/STT3A pathway in nasopharyngeal carcinoma. Front Oncol. 2022;12:815437.
Wang D, Wu S, He J, Sun L, Zhu H, Zhang Y, et al. FAT4 overexpression promotes antitumor immunity by regulating the β-catenin/STT3/PD-L1 axis in cervical cancer. J Exp Clin Cancer Res. 2023;42(1):222.
Zeng K, Zeng Y, Zhan H, Zhan Z, Wang L, **e Y, et al. SEC61G assists EGFR-amplified glioblastoma to evade immune elimination. Proc Natl Acad Sci U S A. 2023;120(32):e2303400120.
Duan X, **e Y, Yu J, Hu X, Liu Z, Li N, et al. MCT4/Lactate promotes PD-L1 glycosylation in Triple-negative breast Cancer cells. J Oncol. 2022;2022:3659714.
Erlichman N, Meshel T, Baram T, Abu Raiya A, Horvitz T, Ben-Yaakov H, et al. The Cell-Autonomous Pro-metastatic activities of PD-L1 in breast Cancer are regulated by N-Linked glycosylation-dependent activation of STAT3 and STAT1. Cells. 2023;12(19):2338.
Gong B, Kiyotani K, Sakata S, Nagano S, Kumehara S, Baba S, et al. Secreted PD-L1 variants mediate resistance to PD-L1 blockade therapy in non-small cell lung cancer. J Exp Med. 2019;216(4):982–1000.
Sagawa R, Sakata S, Gong B, Seto Y, Takemoto A, Takagi S, et al. Soluble PD-L1 works as a decoy in lung cancer immunotherapy via alternative polyadenylation. JCI Insight. 2022;7(1):e153323.
Yu Y, Wang Z, Zheng Q, Li J. GALNT2/14 overexpression correlate with prognosis and methylation: potential therapeutic targets for lung adenocarcinoma. Gene. 2021;790:145689.
Chen W, Saxton B, Tessema M, Belinsky SA. Inhibition of GFAT1 in lung cancer cells destabilizes PD-L1 protein. Carcinogenesis. 2021;42(9):1171–78.
Hetz C, Zhang K, Kaufman RJ. Mechanisms, regulation and functions of the unfolded protein response. Nat Rev Mol Cell Biol. 2020;21(8):421–38.
Tanaka T, Kutomi G, Kajiwara T, Kukita K, Kochin V, Kanaseki T, et al. Cancer-associated oxidoreductase ERO1-α promotes immune escape through up-regulation of PD-L1 in human breast cancer. Oncotarget. 2017;8(15):24706–18.
Chen Y, He J, Chen R, Wang Z, Dai Z, Liang X, et al. Pan-cancer Analysis of the immunological role of PDIA5: a potential target for Immunotherapy. Front Immunol. 2022;13:881722.
Ko PJ, Dixon SJ. Protein palmitoylation and cancer. EMBO Rep. 2018;19(10):e46666.
Das T, Yount JS, Hang HC. Protein S-palmitoylation in immunity. Open Biol. 2021;11(3):200411.
Runkle KB, Kharbanda A, Stypulkowski E, Cao XJ, Wang W, Garcia BA et al. Inhibition of DHHC20-Mediated EGFR Palmitoylation creates a dependence on EGFR Signaling. Mol Cell 2016,62(3):385–96.
Yang Y, Hsu JM, Sun L, Chan LC, Li CW, Hsu JL, et al. Palmitoylation stabilizes PD-L1 to promote breast tumor growth. Cell Res. 2019;29(1):83–6.
Li Z, Jiang D, Liu F, Li Y. Involvement of ZDHHC9 in lung adenocarcinoma: regulation of PD-L1 stability via palmitoylation. Vitro Cell Dev Biol Anim. 2023;59(3):193–203.
Lin Z, Huang K, Guo H, Jia M, Sun Q, Chen X, et al. Targeting ZDHHC9 potentiates anti-programmed death-ligand 1 immunotherapy of pancreatic cancer by modifying the tumor microenvironment. Biomed Pharmacother. 2023;161:114567.
Lin Z, Lv Z, Liu X, Huang K. Palmitoyl transferases act as novel drug targets for pancreatic cancer. J Transl Med. 2023;21(1):249.
Shahid M, Kim M, ** P, Zhou B, Wang Y, Yang W, et al. S-Palmitoylation as a Functional Regulator of Proteins Associated with Cisplatin Resistance in bladder Cancer. Int J Biol Sci. 2020;16(14):2490–505.
Zhang Z, Chen Y, Fang L, Zhao J, Deng S. The involvement of high succinylation modification in the development of prostate cancer. Front Oncol. 2022;12:1034605.
Yang Y, Gibson GE. Succinylation Links metabolism to protein functions. Neurochem Res. 2019;44(10):2346–59.
Mu R, Ma Z, Lu C, Wang H, Cheng X, Tuo B, et al. Role of succinylation modification in thyroid cancer and breast cancer. Am J Cancer Res. 2021;11(10):4683–99.
Dai X, Zhou Y, Han F, Li J. Succinylation and redox status in cancer cells. Front Oncol. 2022;12:1081712.
Yang H, Zou X, Yang S, Zhang A, Li N, Ma Z. Identification of lactylation related model to predict prognostic, tumor infiltrating immunocytes and response of immunotherapy in gastric cancer. Front Immunol. 2023;14:1149989.
Huang ZW, Zhang XN, Zhang L, Liu LL, Zhang JW, Sun YX, et al. STAT5 promotes PD-L1 expression by facilitating histone lactylation to drive immunosuppression in acute myeloid leukemia. Signal Transduct Target Ther. 2023;8(1):391.
Shvedunova M, Akhtar A. Modulation of cellular processes by histone and non-histone protein acetylation. Nat Rev Mol Cell Biol. 2022;23(5):329–49.
Narita T, Weinert BT, Choudhary C. Functions and mechanisms of non-histone protein acetylation. Nat Rev Mol Cell Biol. 2019;20(3):156–74.
Yu J, Zhuang A, Gu X, Hua Y, Yang L, Ge S, et al. Nuclear PD-L1 promotes EGR1-mediated angiogenesis and accelerates tumorigenesis. Cell Discov. 2023;9(1):33.
Romeo MA, Gilardini Montani MS, Santarelli R, Benedetti R, Arena A, Cirone M. Acetylation increases expression, interaction with TRAPPC4 and surface localization of PD-L1. Discov Oncol. 2023;14(1):152.
Cohen MS, Chang P. Insights into the biogenesis, function, and regulation of ADP-ribosylation. Nat Chem Biol. 2018;14(3):236–43.
Hashimoto S, Furukawa S, Hashimoto A, Tsutaho A, Fukao A, Sakamura Y, et al. ARF6 and AMAP1 are major targets of KRAS and TP53 mutations to promote invasion, PD-L1 dynamics, and immune evasion of pancreatic cancer. Proc Natl Acad Sci U S A. 2019;116(35):17450–59.
Di Girolamo M, Fabrizio G, Scarpa ES, Di Paola S. NAD+-dependent enzymes at the endoplasmic reticulum. Curr Top Med Chem. 2013;13(23):3001–10.
Nickel W, Huber LA, Kahn RA, Kipper N, Barthel A, Fasshauer D, et al. ADP ribosylation factor and a 14-kD polypeptide are associated with heparan sulfate-carrying post-trans-golgi network secretory vesicles in rat hepatocytes. J Cell Biol. 1994;125(4):721–32.
D’Souza-Schorey C, van Donselaar E, Hsu VW, Yang C, Stahl PD, Peters PJ. ARF6 targets recycling vesicles to the plasma membrane: insights from an ultrastructural investigation. J Cell Biol. 1998;140(3):603–16.
Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science. 2017;355(6332):1428–33.
Celis-Gutierrez J, Blattmann P, Zhai Y, Jarmuzynski N, Ruminski K, Grégoire C, et al. Quantitative interactomics in primary T cells provides a rationale for concomitant PD-1 and BTLA Coinhibitor Blockade in Cancer Immunotherapy. Cell Rep. 2019;27(11):3315–e307.
**ao X, Shi J, He C, Bu X, Sun Y, Gao M, et al. ERK and USP5 govern PD-1 homeostasis via deubiquitination to modulate tumor immunotherapy. Nat Commun. 2023;14(1):2859.
Lv J, Qin L, Zhao R, Wu D, Wu Z, Zheng D, et al. Disruption of CISH promotes the antitumor activity of human T cells and decreases PD-1 expression levels. Mol Ther Oncolytics. 2022;28:46–58.
Lyle C, Richards S, Yasuda K, Napoleon MA, Walker J, Arinze N, et al. c-Cbl targets PD-1 in immune cells for proteasomal degradation and modulates colorectal tumor growth. Sci Rep. 2019;9(1):20257.
Wu Z, Cao Z, Yao H, Yan X, Xu W, Zhang M, et al. Coupled deglycosylation-ubiquitination cascade in regulating PD-1 degradation by MDM2. Cell Rep. 2023;42(7):112693.
Lee TA, Tsai EY, Liu SH, Hsu Hung SD, Chang SJ, et al. Post-translational modification of PD-1: potential targets for Cancer Immunotherapy. Cancer Res. 2024;84(6):800–7.
Okada M, Chikuma S, Kondo T, Hibino S, Machiyama H, Yokosuka T, et al. Blockage of Core Fucosylation reduces cell-surface expression of PD-1 and promotes Anti-tumor Immune responses of T cells. Cell Rep. 2017;20(5):1017–28.
Yao H, Li C, He F, Song T, Brosseau JP, Wang H, et al. A peptidic inhibitor for PD-1 palmitoylation targets its expression and functions. RSC Chem Biol. 2020;2(1):192–205.
Sugiyama E, Togashi Y, Takeuchi Y, Shinya S, Tada Y, Kataoka K, et al. Blockade of EGFR improves responsiveness to PD-1 blockade in EGFR-mutated non-small cell lung cancer. Sci Immunol. 2020;5(43):eaav3937.
Oxnard GR, Yang JC-H, Yu H, Kim S-W, Saka H, Horn L, et al. TATTON: a multi-arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann Oncol. 2020;31(4):507–16.
Yang JC, Gadgeel SM, Sequist LV, Wu CL, Papadimitrakopoulou VA, Su WC, et al. Pembrolizumab in Combination with Erlotinib or Gefitinib as First-Line Therapy for Advanced NSCLC with sensitizing EGFR Mutation. J Thorac Oncol. 2019;14(3):553–59.
Sacco AG, Chen R, Worden FP, Wong DJL, Adkins D, Swiecicki P, et al. Pembrolizumab plus Cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: an open-label, multi-arm, non-randomised, multicentre, phase 2 trial. Lancet Oncol. 2021;22(6):883–92.
Bonomo P, Desideri I, Loi M, Mangoni M, Sottili M, Marrazzo L, et al. Anti PD-L1 DUrvalumab combined with Cetuximab and RadiOtherapy in locally advanced squamous cell carcinoma of the head and neck: a phase I/II study (DUCRO). Clin Transl Radiat Oncol. 2018;9:42–7.
Gillison ML, Ferris RL, Harris J, Colevas AD, Mell LK, Kong C, et al. Safety of Nivolumab added to Chemoradiation Therapy platforms for Intermediate and High-Risk Locoregionally Advanced Head and Neck Squamous Cell Carcinoma: RTOG Foundation 3504. Int J Radiat Oncol Biol Phys. 2023;115(4):847–60.
Jiao S, **a W, Yamaguchi H, Wei Y, Chen MK, Hsu JM, et al. PARP inhibitor upregulates PD-L1 expression and enhances Cancer-Associated Immunosuppression. Clin Cancer Res. 2017;23(14):3711–20.
Yarchoan M, Powderly JD, Bastos BR, Karasic TB, Crysler OV, Munster PN, et al. A phase 1 study of TPST-1120 as a single agent and in combination with nivolumab in subjects with advanced solid tumors. J Clin Oncol. 2022;40(16suppl):3005–3005.
Tempest Therapeutics. TPST-1120 Randomized Data in First-Line HCC. Tempest Therapeutics. Published online October 11. 2023. https://ir.tempesttx.com/static-files/c2c921e9-1435-4f8a-b853-793da79f6765.
Yap TA, Bardia A, Dvorkin M, Galsky MD, Beck JT, Wise DR, et al. Avelumab Plus Talazoparib in patients with Advanced Solid tumors: the JAVELIN PARP Medley Nonrandomized Controlled Trial. JAMA Oncol. 2023;9(1):40–50.
Konstantinopoulos PA, Waggoner S, Vidal GA, Mita M, Moroney JW, Holloway R, et al. Single-arm phases 1 and 2 trial of Niraparib in Combination with Pembrolizumab in patients with recurrent platinum-resistant ovarian carcinoma. JAMA Oncol. 2019;5(8):1141–49.
Post CCB, Westermann AM, Boere IA, Witteveen PO, Ottevanger PB, Sonke GS, et al. Efficacy and safety of durvalumab with olaparib in metastatic or recurrent endometrial cancer (phase II DOMEC trial). Gynecol Oncol. 2022;165(2):223–29.
Domchek SM, Postel-Vinay S, Im S-A, Park YH, Delord J-P, Italiano A, et al. Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLA): an open-label, multicentre, phase 1/2, basket study. Lancet Oncol. 2020;21(9):1155–64.
Drew Y, Kim JW, Penson RT, O’Malley DM, Parkinson C, Roxburgh P, et al. Olaparib plus Durvalumab, with or without Bevacizumab, as treatment in PARP Inhibitor-Naïve platinum-sensitive relapsed ovarian Cancer: a phase II Multi-cohort Study. Clin Cancer Res. 2024;30(1):50–62.
Friedlander M, Meniawy T, Markman B, Mileshkin L, Harnett P, Millward M, et al. Pamiparib in combination with tislelizumab in patients with advanced solid tumours: results from the dose-escalation stage of a multicentre, open-label, phase 1a/b trial. Lancet Oncol. 2019;20(9):1306–15.
Liu J, Gaillard S, Hendrickson AW, Moroney J, Yeku O, Diver E, et al. An open-label phase II study of dostarlimab (TSR-042), bevacizumab (bev), and niraparib combination in patients (pts) with platinum-resistant ovarian cancer (PROC): cohort a of the OPAL trial. Gynecol Oncol. 2021;162(S1):S17–8.
Cha JH, Yang WH, **a W, Wei Y, Chan LC, Lim SO, et al. Metformin promotes Antitumor Immunity via Endoplasmic-Reticulum-Associated degradation of PD-L1. Mol Cell. 2018;71(4):606–. – 20.e7.
Zandberg DP, Menk AV, Velez M, Normolle D, DePeaux K, Liu A, et al. Tumor hypoxia is associated with resistance to PD-1 blockade in squamous cell carcinoma of the head and neck. J Immunother Cancer. 2021;9(5):e002088.
Békés M, Langley DR, Crews CM. PROTAC targeted protein degraders: the past is prologue. Nat Rev Drug Discov. 2022;21(3):181–200.
Paiva SL, Crews CM. Targeted protein degradation: elements of PROTAC design. Curr Opin Chem Biol. 2019;50:111–19.
Wang Y, Zhou Y, Cao S, Sun Y, Dong Z, Li C, et al. In vitro and in vivo degradation of programmed cell death ligand 1 (PD-L1) by a proteolysis targeting chimera (PROTAC). Bioorg Chem. 2021;111:104833.
Cheng B, Ren Y, Cao H, Chen J. Discovery of novel resorcinol diphenyl ether-based PROTAC-like molecules as dual inhibitors and degraders of PD-L1. Eur J Med Chem. 2020;199:112377.
Cotton AD, Nguyen DP, Gramespacher JA, Seiple IB, Wells JA. Development of antibody-based PROTACs for the degradation of the cell-surface Immune checkpoint protein PD-L1. J Am Chem Soc. 2021;143(2):593–98.
Su W, Tan M, Wang Z, Zhang J, Huang W, Song H, et al. Targeted degradation of PD-L1 and activation of the STING pathway by Carbon-dot-based PROTACs for Cancer Immunotherapy. Angew Chem Int Ed Engl. 2023;62(11):e202218128.
Sun R, Meng Z, Lee H, Offringa R, Niehrs C. ROTACs leverage signaling-incompetent R-spondin for targeted protein degradation. Cell Chem Biol. 2023;30(7):739–e528.
Li S, Chen T, Liu J, Zhang H, Li J, Wang Z, et al. PROTACs: novel tools for improving immunotherapy in cancer. Cancer Lett. 2023;560:216128.
Solomon B, Callejo A, Bar J, Berchem G, Bazhenova L, Saintigny P, et al. A WIN Consortium phase I study exploring avelumab, palbociclib, and axitinib in advanced non-small cell lung cancer. Cancer Med. 2022;11(14):2790–800.
Mayer EL, Ren Y, Wagle N, Mahtani R, Ma C, DeMichele A, et al. PACE: a randomized phase II study of Fulvestrant, Palbociclib, and Avelumab after Progression on cyclin-dependent kinase 4/6 inhibitor and aromatase inhibitor for hormone Receptor-Positive/Human epidermal growth factor receptor-negative metastatic breast Cancer. J Clin Oncol Published Online March. 2024;21. https://doi.org/10.1200/JCO.23.01940.
Zhang Y, Song J, Zhou Y, Jia H, Zhou T, Sun Y, et al. Discovery of selective and potent USP22 inhibitors via structure-based virtual screening and bioassays exerting anti-tumor activity. Bioorg Chem. 2023;141:106842.
Liu K, Tan S, ** W, Guan J, Wang Q, Sun H, et al. N-glycosylation of PD-1 promotes binding of camrelizumab. EMBO Rep. 2020;21(12):e51444.
Jiang M, Liu M, Liu G, Ma J, Zhang L, Wang S. Advances in the structural characterization of complexes of therapeutic antibodies with PD-1 or PD-L1. MAbs. 2023;15(1):2236740.
Chen D, Tan S, Zhang H, Wang H, He W, Shi R, et al. The FG Loop of PD-1 serves as a Hotspot for therapeutic monoclonal antibodies in Tumor Immune Checkpoint Therapy. iScience. 2019;14:113–24.
Lu D, Xu Z, Zhang D, Jiang M, Liu K, He J, et al. PD-1 N58-Glycosylation-dependent binding of monoclonal antibody cemiplimab for Immune Checkpoint Therapy. Front Immunol. 2022;13:826045.
Tan S, Zhang H, Chai Y, Song H, Tong Z, Wang Q, et al. An unexpected N-terminal loop in PD-1 dominates binding by Nivolumab. Nat Commun. 2017;8:14369.
Ha JY, Chun K-J, Ko S, Lee HW, Hwang OK, Lim CS, et al. Glycan-controlled human PD-1 variants displaying broad-spectrum high binding to PD-1 Ligands Potentiate T cell. Mol Pharm. 2023;20(4):2170–80.
Lee HH, Wang YN, **a W, Chen CH, Rau KM, Ye L, et al. Removal of N-Linked glycosylation enhances PD-L1 detection and predicts Anti-PD-1/PD-L1 therapeutic efficacy. Cancer Cell. 2019;36(2):168–e784.
Do KT, Chow LQM, Reckamp K, Sanborn RE, Burris H, Robert F, et al. First-In-Human, First-In-Class, Phase I trial of the Fucosylation inhibitor SGN-2FF in patients with Advanced Solid tumors. Oncologist. 2021;26(11):925–e1918.
Pijnenborg JFA, Visser EA, Noga M, et al. Cellular Fucosylation inhibitors based on Fluorinated Fucose-1-phosphates*. Chemistry. 2021;27(12):4022–27.
Pijnenborg JFA, Visser EA, Noga M, Rossing E, Veizaj R, Lefeber DJ, et al. Fluorinated rhamnosides inhibit cellular fucosylation. Nat Commun. 2021;12(1):7024.
Ren X, Cheng Z, He J, Yao X, Liu Y, Cai K, et al. Inhibition of glycolysis-driven immunosuppression with a nano-assembly enhances response to immune checkpoint blockade therapy in triple negative breast cancer. Nat Commun. 2023;14(1):7021.
Mao C, Li J, Feng L, Gao W. Beyond antibody fucosylation: α-(1,6)-fucosyltransferase (Fut8) as a potential new therapeutic target for cancer immunotherapy. Antib Ther. 2023;6(2):87–96.
Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csőszi T, et al. Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line therapy for extensive-stage small-cell Lung Cancer: Randomized, Double-Blind, phase III KEYNOTE-604 study. J Clin Oncol. 2020;38(21):2369–79.
Kim HR, Awad MM, Navarro A, Gottfried M, Peters S, Csőszi T, et al. Patient-reported Health-Related Quality of Life in KEYNOTE-604: Pembrolizumab or Placebo added to Etoposide and Platinum as First-Line therapy for extensive-stage SCLC. JTO Clin Res Rep. 2023;4(11):100572.
Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell Lung Cancer. N Engl J Med. 2018;379(23):2220–29.
Liu SV, Reck M, Mansfield AS, Mok T, Scherpereel A, Reinmuth N, et al. Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung Cancer treated with atezolizumab, carboplatin, and Etoposide (IMpower133). J Clin Oncol. 2021;39(6):619–30.
Bria E, Morgillo F, Garassino MC, Ciardiello F, Ardizzoni A, Stefani A, et al. Atezolizumab Plus Carboplatin and Etoposide in patients with untreated extensive-stage small-cell Lung Cancer: interim results of the MAURIS phase IIIb trial. Oncologist. Published Online Febr. 2024;20. https://doi.org/10.1093/oncolo/oyad342.
Wang Z, Zhao J, Ma Z, Cui J, Shu Y, Liu Z, et al. A phase 2 study of Tislelizumab in Combination with Platinum-based chemotherapy as first-line treatment for Advanced Lung Cancer in Chinese patients. Lung Cancer. 2020;147:259–68.
Cheng Y, Han L, Wu L, Chen J, Sun H, Wen G, et al. Effect of First-Line Serplulimab vs Placebo added to Chemotherapy on Survival in patients with extensive-stage small cell Lung Cancer: the ASTRUM-005 Randomized Clinical Trial. JAMA. 2022;328(12):1223–32.
Zhou L, Chai F, He Y, Zhou Z, Guo S, Li P, et al. Homodimerized cytoplasmic domain of PD-L1 regulates its complex glycosylation in living cells. Commun Biol. 2022;5(1):887.
Wu X, Wang N, Liang J, Wang B, ** Y, Liu B, et al. Is the triggering of PD-L1 dimerization a potential mechanism for Food-Derived Small molecules in Cancer Immunotherapy? A study by Molecular Dynamics. Int J Mol Sci. 2023;24(2):1413.
Liang J, Wang B, Yang Y, Liu B, ** Y. Approaching the dimerization mechanism of small molecule inhibitors targeting PD-L1 with Molecular Simulation. Int J Mol Sci. 2023;24(2):1280.
Naing S, Sandech N, Maiuthed A, Chongruchiroj S, Pratuangdejkul J, Lomarat P. Garcinia mangostana L. Pericarp Extract and its active compound α-Mangostin as potential inhibitors of Immune Checkpoint programmed death Ligand-1. Molecules. 2023;28(19):6991.
Shah K, Panchal S, Patel B. Porcupine inhibitors: Novel and emerging anti-cancer therapeutics targeting the wnt signaling pathway. Pharmacol Res. 2021;167:105532.
Sharma G, Ojha R, Noguera-Ortega E, Rebecca VW, Attanasio J, Liu S, et al. PPT1 inhibition enhances the antitumor activity of anti-PD-1 antibody in melanoma. JCI Insight. 2020;5(17):e133225.
Weng J, Liu S, Zhou Q, Xu W, Xu M, Gao D, et al. Intratumoral PPT1-positive macrophages determine immunosuppressive contexture and immunotherapy response in hepatocellular carcinoma. J Immunother Cancer. 2023;11(6):e006655.
Lin DT, Conibear E. ABHD17 proteins are novel protein depalmitoylases that regulate N-Ras palmitate turnover and subcellular localization. Elife. 2015;4:e11306.
Cortes JE, Gutzmer R, Kieran MW, Solomon JA. Hedgehog signaling inhibitors in solid and hematological cancers. Cancer Treat Rev. 2019;76:41–50.
Tan X, Wang C, Zhou H, Zhang S, Liu X, Yang X, et al. Bioactive fatty acid analog-derived hybrid nanoparticles confer antibody-independent chemo-immunotherapy against carcinoma. J Nanobiotechnol. 2023;21(1):183.
Dai MY, Shi YY, Wang AJ, Liu XL, Liu M, Cai HB. High-potency PD-1/PD-L1 degradation induced by Peptide-PROTAC in human cancer cells. Cell Death Dis. 2022;13(11):924.
Shi YY, Dong DR, Fan G, Dai MY, Liu M. A cyclic peptide-based PROTAC induces intracellular degradation of palmitoyltransferase and potently decreases PD-L1 expression in human cervical cancer cells. Front Immunol. 2023;14:1237964.
Fan Y, Gao Y, Nie L, Ma J, Wei W, Li L, et al. Targeting LYPLAL1-mediated cGAS depalmitoylation enhances the response to anti-tumor immunotherapy. Mol Cell. 2023;83(19):3520–e35327.
Lockhart AC, Bauer TM, Aggarwal C, Lee CB, Harvey RD, Cohen RB, et al. Phase ib study of pevonedistat, a NEDD8-activating enzyme inhibitor, in combination with docetaxel, carboplatin and paclitaxel, or gemcitabine, in patients with advanced solid tumors. Invest New Drugs. 2019;37(1):87–97.
Swords RT, Coutre S, Maris MB, Zeidner JF, Foran JM, Cruz J, et al. Pevonedistat, a first-in-class NEDD8-activating enzyme inhibitor, combined with azacitidine in patients with AML. Blood. 2018;131(13):1415–24.
Fernandez AI, Gaule P, Rimm DL. Tissue age affects antigenicity and scoring for the 22C3 immunohistochemistry Companion Diagnostic Test. Mod Pathol. 2023;36(7):100159.
Chongsaritsinsuk J, Steigmeyer AD, Mahoney KE, Rosenfeld MA, Lucas TM, Ince D, et al. Glycoproteomic landscape and structural dynamics of TIM family immune checkpoints enabled by mucinase SmE. Nat Commun. 2023;14(1):6169.
Long J, Wang Y, Jiang X, Ge J, Chen M, Zheng B, et al. Nanomaterials boost CAR-T therapy for solid tumors. Adv Healthc Mater Published Online March. 2024;14. https://doi.org/10.1002/adhm.202304615.
Fu Y, Qian H, Yang Y, Li J, **e G. Enhanced imaging of protein-specific palmitoylation with HCR-based cis-membrane multi-FRET. Talanta. 2024;266(Pt 1):124972.
Acknowledgements
Figures were created by PowerPoint (Microsoft Office 2016) and some materials of the figures were provided by the Servier Medical Art (https://smart.servier.com/) under the CC BY 4.0 license.
Funding
This work was supported partially by grants from the National Natural Science Foundation of China (82303069 [J.W.], 52102276 [J.L.], 82303126 [Y.W.]), the Natural Science Foundation of Fujian Province (2023J05038 [J.W.]), the Fujian Provincial Project of Education and Science for Young and Middle-aged Teachers (JAT220076 [J.W.]), the Startup Foundation of Fujian Medical University (2022QH1003 [J.W.], 2022QH1783 [M.C.]), and the Natural Science Foundation of Hunan Province (2024JJ6325 [Y.W.]).
Author information
Authors and Affiliations
Contributions
R.W. and S.H. collected the related paper and finished the manuscript and figures, R.W. and S.H. contributed equally to this work. J.W. and J.L. gave constructive guidance and made critical revisions. Y.W., X.J., M.C. participated in the design of this review. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, R., He, S., Long, J. et al. Emerging therapeutic frontiers in cancer: insights into posttranslational modifications of PD-1/PD-L1 and regulatory pathways. Exp Hematol Oncol 13, 46 (2024). https://doi.org/10.1186/s40164-024-00515-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40164-024-00515-5