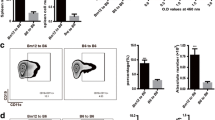
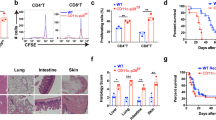
Abstract
Background
Chronic graft-versus-host disease (cGVHD) remains a major complication during the late phase of allogeneic hematopoietic stem cell transplantation (allo-HSCT). IL-39, a newly described pro-inflammatory cytokine belonging to the IL-12 family, plays a role in lupus development. Recently, IL-39 has been identified as a pathogenic factor in acute GVHD (aGVHD). However, the role of IL-39 in the pathogenesis of cGVHD remains unclear.
Methods
We constructed a recombinant IL-39 plasmid and established scleroderma and lupus-like cGVHD models. Quantitative PCR and enzyme-linked immunosorbent assay (ELISA) were used to detect IL-39 expression in mice and patients post transplantation, respectively. Hydrodynamic gene transfer (HGT) was performed to achieve IL-39 overexpression in vivo. Multiparameter flow cytometry, western blotting, and assays in vitro were performed to investigate the effect of IL-39 on cGVHD.
Results
The relative expression of IL-23p19 and EBi3 was significantly increased in the intestine of cGVHD mice on day 40 post allo-HSCT, and IL-39 levels were significantly elevated in the serum of patients following allo-HSCT. Overexpression of IL-39 significantly aggravated the severity of cGVHD. Increased IL-39 levels promoted T-cell activation and germinal center responses, and may exacerbate thymic damage. Consistently, blocking IL-39 markedly ameliorated immune dysregulation in the cGVHD mice. Furthermore, we found that IL-39 was produced by B cells, CD11b+ cells, and CD8+T cells after activation. Stimulation of IL-39 led to upregulation of the IL-39 receptor on CD4+T cells and further caused activation of the STAT1/STAT3 pathway, through which IL-39 may exert its pro-inflammatory effects.
Conclusion
Our study reveals a critical role for IL-39 in cGVHD pathogenesis and indicates that IL-39 may serve as a potential therapeutic target for cGVHD prevention.
Similar content being viewed by others
Background
Allo-HSCT is an effective method for the treatment of hematologic malignancies. However, cGVHD is a major complication of allo-HSCT, occurring in approximately 50% of patients [1,2,3,4]. Similar to aGVHD, donor T cells play a critical pathogenic role in initiating tissue injury and develo** cGVHD [5]. Depleting mature T cells from stem cell grafts markedly reduces the incidence of cGVHD [6,7,8,9]. In addition to T cell activation, autoreactive B cells have also been found to play a pathogenic role in cGVHD. Under the action of high-level B cell activation factor (BAFF), more donor B cells differentiate into autoantibody-secreting subsets upon sustained stimulation of receptor antigens, resulting in pathological organ damage [10,11,12]. Therefore, exploring key molecules that cause immune dysregulation in T and B cells is expected to provide new mechanisms and targets for cGVHD prevention and treatment.
The IL-12 family consists of IL-12 (IL-12p35/IL-12p40), IL-23(IL-23p19/IL-12p40), IL-27 (IL-27p28/EBi3), IL-Y (IL-27p28/ IL-12p40), and IL-35 (IL-12p35/EBi3) [13, 14]. IL-12 and IL-23 play crucial roles in inducing differentiation of Th1 and Th17 cells, respectively [15]. IL-Y promotes cGVHD by activating pathogenic T and B cells. In contrast, IL-27 and IL-35 suppress inflammatory responses by promoting the expansion of regulatory B and T cell subsets [13, 15,16,17]. Wang et al. first described a new IL-12 member composed of IL-23p19 and an EBi3 heterodimer, IL-39 [Mice 6–8-week-old female C57BL/6 (H-2b) and BALB/c (H-2d) mice were purchased from SLAC Animal Laboratory (Shanghai, China). 8–10-week-old female DBA/2 mice (H-2d) were purchased from Charles River Laboratories (Bei**g, China). The experimental animals were kept under specific pathogen-free (SPF) conditions. One week before setting up the experiments, recipient mice were fed gentamicin aqueous solution to prevent intestinal infections. For HGT, recipient mice (BALB/c) were injected i.v. with 120 μg of recombinant plasmid in a total of 2 ml PBS within 5 s at 3 days before transplantation. Recipient BALB/c mice were given total body irradiation (TBI) at 650 cGy using a RAD 320 X-ray Irradiator 6–8 h prior to transplantation. To establish a scleroderma-like cGVHD model, recipients were infused with 1 × 107 bone marrow cells and 1 × 106 splenocytes from the C57BL/6 mice. To establish a lupus-like cGVHD model, irradiated recipients (BALB/c mice) were infused with 5 × 106 bone marrow cells and 4 × 107 CD25− splenocytes from DBA/2 mice. To investigate the preventable effect of anti-IL-39 on cGVHD, 14 days after transplantation, mice received 100 μl (100 μg) of anti-IL-39 antibody (DETAIBIO, China) or 100 μl (100 μg) of non-specific IgG (BioXcell, BE0095) via intraperitoneal injection twice a week for 6 weeks. Representative samples of the lung, liver, small intestine, skin, and kidney were obtained from transplanted recipients, fixed in 4% formalin, and stained with H&E. The pathology score of the target organs was based on a previous scoring system [40, 41]. The antibodies used for flow cytometry are listed in Additional file 1: Table S3. For intracellular staining, cells were treated with brefeldin A (BFA, 10 μg/ml) (Biolegend, San Diego, CA), phorbol-12-myristate-13-acetate (PMA, 50 ng/ml) (Beyotime Biotechnology, China), and ionomycin (500 ng/ml) (Beyotime Biotechnology, China) in a 37 °C cell culture incubator containing 5% CO2. The cells were then fixed and permeabilized with FACS Permeabilizing Solution (BD Biosciences, San Diego, CA, USA). Foxp3 staining kit was purchased from eBioscience (San Diego, CA, USA). Data were acquired using FACS NovoCyte (ACEA Biosciences, San Diego, CA, USA) and analyzed using FlowJo software (FlowJo, Ashland, OR, USA). Fifty patients who underwent hematopoietic stem cell transplantation at the First Affiliated Hospital of Soochow University between April 2015 and August 2017 were enrolled. We grouped the patients into no cGVHD, mild cGVHD, and moderate/severe (M/S) cGVHD groups according to the NIH consensus criteria [42]. The characteristics of the patients are shown in Additional file 1: Table S2. Peripheral blood samples obtained from 8 patients without cGVHD after HSCT were used as controls. The levels of IL-39 (RapidBio, USA) and CXCL13 (R&D Systems, UK) in serum samples were detected using an enzyme-linked immunosorbent assay (ELISA). Murine CD4+T cells, CD8+T cells, and B cells were sorted from splenocytes of C57BL/6 mice, whereas human CD4+T cells, CD8+T cells, and B cells were sorted from PBMCs of healthy individuals using CD4+T, CD8+T, and B Cell Isolation Kits according to the manufacturer’s protocol (StemCell Technologies, Vancouver, Canada). Similarly, CD11b+ cells were sorted from splenocytes of C57BL/6 mice or human PBMCs using CD11b micromagnetic beads, according to the manufacturer’s protocol (Miltenyi Biotec, Germany). For T cell activation, plates were coated with 2 mg/ml anti-CD3 and 0.4 mg/ml anti-CD28 Abs (BioLegend, San Diego, CA) overnight. 2 × 105 CD4+T and CD8+T cells (2 × 105) were cultured with various concentrations of mouse rIL-39 for 72 h at 37 °C in a cell culture incubator containing 5% CO2. For B cell and CD11b+ activation, 2 × 105 B cells and CD11b+ cells were cultured with LPS (5 ng/ml) and various concentrations of rIL-39 protein for 72 h. Consistently, activated CD4+T, CD8+T, B, and CD11b+ cells isolated from the PBMCs of healthy individuals were cultured with various concentrations of human rIL-39 for 72 h. Total RNA was isolated using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions. cDNA was synthesized using reverse transcription, random hexamer primers, and 10 mM dNTP (Promega). The expression of EBi3 and IL-23p19 in the spleen at 10 days and in the liver, lung, and intestine at 30 days post-HSCT was regarded as control. The relative expression of genes in one organ at the remaining time point was calculated by 2−ΔΔCt according to the control. The primer sequences are listed in Table S1. Murine CD3+T cells were sorted from splenocytes of C57BL/6 mice, and human CD3+T cells were sorted from peripheral blood mononuclear cells (PBMCs) of healthy individuals using a T Cell Isolation Kit, according to the manufacturer’s protocol (StemCell Technologies, Vancouver, Canada). For T cell activation, plates were coated with 2 mg/ml anti-CD3 and 0.4 mg/ml anti-CD28 Abs (BioLegend, San Diego, CA) overnight. Activated T cells were cultured with various concentrations of mouse or human rIL-39 protein for 72 h. Equal amounts of protein were subjected to 10% SDS-PAGE and transferred to a polyvinylidene fluoride (PVDF) membrane. After blocking with 3% BSA, the membrane was incubated with primary antibodies, including STAT1 (D1K9Y), Phospho-STAT1 (58D6), STAT3 (D3Z2G), Phospho-STAT3 (D3A7), and GAPDH (D4C6R) (CST, USA) at 4 °C overnight, followed by incubation with secondary antibodies (Absin, China) at room temperature for 2 h. All results were normalized to GAPDH expression, which was used as the loading control. Data were presented using GraphPad Prism 7 software (GraphPad Software, San Diego, CA, USA). The nonparametric Mann–Whitney U test was used to analyze the body weight and clinical scores between the groups. Comparisons between two groups were performed using unpaired two-way Student’s t-tests. The diagnostic value of biomarkers was evaluated using ROC curves. Data are expressed as the mean ± SD or SEM. For all statistics, if P < 0.05, they were considered statistically significant (*), less than 0.01 or 0.001 were shown as ** or ***, respectively.Establishment of murine models of cGVHD
Histology
Flow cytometry
Patients and sample preparation
Real-time quantitative polymerase chain reaction (qPCR)
Western blotting analysis
Statistical analysis
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- aGVHD:
-
Acute graft-versus-host disease
- allo-HSCT:
-
Allogeneic hematopoietic stem cell transplantation
- ACS:
-
Acute coronary syndrome
- BAFF:
-
B cell activation factor
- BFA:
-
Brefeldin A
- cGVHD:
-
Chronic graft-versus-host disease
- DP:
-
Double positive
- ELISA:
-
Enzyme-linked immunosorbent assay
- GC:
-
Germinal center
- HSCT:
-
Hematopoietic stem cell transplantation
- HGT:
-
Hydrodynamic gene transfer
- PBMCs:
-
Peripheral blood mononuclear cells
- PMA:
-
Phorbol-12-myristate-13-acetate
- SP:
-
Single positive
- SPF:
-
Specific pathogen-free
- TBI:
-
Total body irradiation
- Tfh:
-
T follicular helper
References
Zeiser R, Blazar BR. Pathophysiology of chronic graft-versus-host disease and therapeutic targets. N Engl J Med. 2017;377(26):2565–79.
Pidala J, Kurland B, Chai X, Majhail N, Weisdorf DJ, Pavletic S, et al. Patient-reported quality of life is associated with severity of chronic graft-versus-host disease as measured by NIH criteria: report on baseline data from the Chronic GVHD Consortium. Blood. 2011;117(17):4651–7.
MacDonald KP, Hill GR, Blazar BR. Chronic graft-versus-host disease: biological insights from preclinical and clinical studies. Blood. 2017;129(1):13–21.
Socié G, Ritz J. Current issues in chronic graft-versus-host disease. Blood. 2014;124(3):374–84.
Forcade E, Kim HT, Cutler C, Wang K, Alho AC, Nikiforow S, et al. Circulating T follicular helper cells with increased function during chronic graft-versus-host disease. Blood. 2016;127(20):2489–97.
Devine SM, Carter S, Soiffer RJ, Pasquini MC, Hari PN, Stein A, et al. Low risk of chronic graft-versus-host disease and relapse associated with T cell-depleted peripheral blood stem cell transplantation for acute myelogenous leukemia in first remission: results of the blood and marrow transplant clinical trials network protocol 0303. Biol Blood Marrow Transplant. 2011;17(9):1343–51.
Soiffer RJ, Lerademacher J, Ho V, Kan F, Artz A, Champlin RE, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117(25):6963–70.
Sakoda Y, Hashimoto D, Asakura S, Takeuchi K, Harada M, Tanimoto M, et al. Donor-derived thymic-dependent T cells cause chronic graft-versus-host disease. Blood. 2007;109(4):1756–64.
Wu T, Young JS, Johnston H, Ni X, Deng R, Racine J, et al. Thymic damage, impaired negative selection, and development of chronic graft-versus-host disease caused by donor CD4+ and CD8+ T cells. J Immunol. 2013;191(1):488–99.
Sarantopoulos S, Stevenson KE, Kim HT, Cutler CS, Bhuiya NS, Schowalter M, et al. Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood. 2009;113(16):3865–74.
McManigle W, Youssef A, Sarantopoulos S. B cells in chronic graft-versus-host disease. Hum Immunol. 2019;80(6):393–9.
Flynn R, Du J, Veenstra RG, Reichenbach DK, Panoskaltsis-Mortari A, Taylor PA, et al. Increased T follicular helper cells and germinal center B cells are required for cGVHD and bronchiolitis obliterans. Blood. 2014;123(25):3988–98.
Wan L, ** Z, Hu B, Lv K, Lei L, Liu Y, et al. IL-Y aggravates murine chronic graft-versus-host disease by enhancing T and B cell responses. Front Immunol. 2020;11: 559740.
Sun L, He C, Nair L, Yeung J, Egwuagu CE. Interleukin 12 (IL-12) family cytokines: Role in immune pathogenesis and treatment of CNS autoimmune disease. Cytokine. 2015;75(2):249–55.
Bastian D, Wu Y, Betts BC, Yu XZ. The IL-12 cytokine and receptor family in graft-vs.-host disease. Front Immunol. 2019;10:988.
Wang RX, Yu CR, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV, et al. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat Med. 2014;20(6):633–41.
Shen P, Roch T, Lampropoulou V, O’Connor RA, Stervbo U, Hilgenberg E, et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature. 2014;507(7492):366–70.
Wang X, Wei Y, **ao H, Liu X, Zhang Y, Han G, et al. A novel IL-23p19/EBi3 (IL-39) cytokine mediates inflammation in Lupus-like mice. Eur J Immunol. 2016;46(6):1343–50.
Luo Y, Liu F, Liu H, Chen H, Cheng W, Dong S, et al. Elevated serum IL-39 in patients with ST-segment elevation myocardial infarction was related with left ventricular systolic dysfunction. Biomark Med. 2017;11(6):419–26.
Yang MG, Tian S, Zhang Q, Han J, Liu C, Zhou Y, et al. Elevated serum interleukin-39 levels in patients with neuromyelitis optica spectrum disorders correlated with disease severity. Mult Scler Relat Disord. 2020;46: 102430.
Bastian D, Sui X, Nguyen HD, Wu Y, Schutt S, Tian L, et al. Interleukin-23 receptor signaling by interleukin-39 potentiates T cell pathogenicity in acute graft-versus-host disease. Am J Transplant. 2021;21(11):3538–49.
Liu Y, Wu Y, Wang Y, Cai Y, Hu B, Bao G, et al. IL-35 mitigates murine acute graft-versus-host disease with retention of graft-versus-leukemia effects. Leukemia. 2015;29(4):939–46.
Chen X, Vodanovic-Jankovic S, Johnson B, Keller M, Komorowski R, Drobyski WR. Absence of regulatory T-cell control of TH1 and TH17 cells is responsible for the autoimmune-mediated pathology in chronic graft-versus-host disease. Blood. 2007;110(10):3804–13.
Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17(8):975–82.
McDonald-Hyman C, Flynn R, Panoskaltsis-Mortari A, Peterson N, MacDonald KP, Hill GR, et al. Therapeutic regulatory T-cell adoptive transfer ameliorates established murine chronic GVHD in a CXCR5-dependent manner. Blood. 2016;128(7):1013–7.
Festa ED, Hankiewicz K, Kim S, Skurnick J, Wolansky LJ, Cook SD, et al. Serum levels of CXCL13 are elevated in active multiple sclerosis. Mult Scler. 2009;15(11):1271–9.
Schiffer L, Kümpers P, Davalos-Misslitz AM, Haubitz M, Haller H, Anders HJ, et al. B-cell-attracting chemokine CXCL13 as a marker of disease activity and renal involvement in systemic lupus erythematosus (SLE). Nephrol Dial Transplant. 2009;24(12):3708–12.
Stockinger B. T lymphocyte tolerance: from thymic deletion to peripheral control mechanisms. Adv Immunol. 1999;71:229–65.
Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12(6):443–58.
Pidala J, Sarwal M, Roedder S, Lee SJ. Biologic markers of chronic GVHD. Bone Marrow Transplant. 2014;49(3):324–31.
Busca A, Locatelli F, Marmont F, Ceretto C, Falda M. Recombinant human soluble tumor necrosis factor receptor fusion protein as treatment for steroid refractory graft-versus-host disease following allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2007;82(1):45–52.
Wang X, Zhang Y, Wang Z, Liu X, Zhu G, Han G, et al. Anti-IL-39 (IL-23p19/EBi3) polyclonal antibodies ameliorate autoimmune symptoms in lupus-like mice. Mol Med Rep. 2018;17(1):1660–6.
Rieger K, Loddenkemper C, Maul J, Fietz T, Wolff D, Terpe H, et al. Mucosal FOXP3+ regulatory T cells are numerically deficient in acute and chronic GvHD. Blood. 2006;107(4):1717–23.
Sagoo P, Ratnasothy K, Tsang Y, Barber LD, Noble A, Lechler RI, et al. Alloantigen-specific regulatory T cells prevent experimental chronic graft-versus-host disease by simultaneous control of allo- and autoreactivity. Eur J Immunol. 2012;42(12):3322–33.
Young JS, Wu T, Chen Y, Zhao D, Liu H, Yi T, et al. Donor B cells in transplants augment clonal expansion and survival of pathogenic CD4+ T cells that mediate autoimmune-like chronic graft-versus-host disease. J Immunol. 2012;189(1):222–33.
Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41(4):529–42.
Shimabukuro-Vornhagen A, Hallek MJ, Storb RF, von Bergwelt-Baildon MS. The role of B cells in the pathogenesis of graft-versus-host disease. Blood. 2009;114(24):4919–27.
Larousserie F, Charlot P, Bardel E, Froger J, Kastelein RA, Devergne O. Differential effects of IL-27 on human B cell subsets. J Immunol. 2006;176(10):5890–7.
Charlot-Rabiega P, Bardel E, Dietrich C, Kastelein R, Devergne O. Signaling events involved in interleukin 27 (IL-27)-induced proliferation of human naive CD4+ T cells and B cells. J Biol Chem. 2011;286(31):27350–62.
Zhao D, Zhang C, Yi T, Lin CL, Todorov I, Kandeel F, et al. In vivo-activated CD103+CD4+ regulatory T cells ameliorate ongoing chronic graft-versus-host disease. Blood. 2008;112(5):2129–38.
Kaplan DH, Anderson BE, McNiff JM, Jain D, Shlomchik MJ, Shlomchik WD. Target antigens determine graft-versus-host disease phenotype. J Immunol. 2004;173(9):5467–75.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21(3):389–401.
Acknowledgements
Not applicable.
Funding
This work is strongly supported by National Key R&D Program of China (2019YFC0840604, 2017YFA0104502, 2017YFA0104500), National Natural Science Foundation of China (81730003, 81500146, 82070186), National Science and Technology Major Project (2017ZX09304021), Key R&D Program of Jiangsu Province (BE2019798), Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), Jiangsu Medical Outstanding Talents Project (JCRCA2016002), Jiangsu Provincial Key Medical Center (YXZXA2016002), Jiangsu Natural Science Foundation (BK20150356), Suzhou Science and Technology Program Project (SLT201911), Suzhou Science and Technology Development Project (SYS2019021), Translational Research Grant of NCRCH (2020WSC05), China Postdoctoral Science Foundation (2019M661938), Jiangsu Planned Projects for Postdoctoral Research Funds (2019K098), The Natural Science Foundation of the Jiangsu Higher Education Institutes of China (20KJD320001).
Author information
Authors and Affiliations
Contributions
HL, DW and YL designed the study. KL, BH, and MiX performed the research. LW, ZJ, YD, MiX, KM, LL, and HG contributed to experiments. QL collected clinical samples. KL and MiX analyzed the data. KL, BH, and YL wrote the manuscript. YX provided suggestions for the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All animal protocols were approved by the Institutional Animal Care and Use Committee of the Soochow University. Informed consent was obtained from all patients, and this study was approved by the Academic Advisory Board of Soochow University (approval number: [2021] 313).
Consent for publication
Not applicable.
Competing interests
We declare that no competing interests exists.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Table S1. Sequences of primers used. Table S2. Clinical characteristics of the patients. Table S3. Antibodies used in flow cytometry. Table S4. Primary data of CXCL13 and IL-39 concentrations in patients. Figure S1. Relative expression of IL-23p19 and EBi3 in spleen and target tissues of cGVHD mice. Irradiated BALB/c recipients were infused with 1×107 bone marrow cells and 1×106 splenocytes from the C57BL/6 mice. Mice infused with bone marrow cells were used as the controls. The expression of IL-39 in the lungs, liver, small intestine, and spleen of recipients was quantified by qPCR on days 30, 40, and 50 after transplantation (n=3, each group and time point). Data are representative of at least three independent experiments. Values are presented as mean ± SEM. **P< 0.01, ***P< 0.001. Figure S2: Survival of transduced flag-tagged IL-39 mice in scleroderma and lupus-like cGVHD models. Scleroderma-like (A) and lupus-like (B) cGVHD models were established. The survival of mice was observed for 56 days. Survival was assessed using the Kaplan-Meier method and compared using the log-rank test. Figure S3: IL-39 promotes the activation of T cells in the lupus-like cGVHD mice model. Irradiated BALB/c recipients were infused with 5×106 bone marrow cells and 4×107 CD25- splenocytes from the DBA/2 mice. Splenocytes (n=6 per group) were collected and stained for FACS analysis 8 weeks post transplantation. The percentages and numbers of CD4+T, CD69+CD4+T, CD8+T, and CD69+CD8+T cells in lymphocytes from the spleens of the recipients are shown (A). Lymphocytes were isolated from the spleens of recipients and treated with PMA, brefeldin A, and ionomycin for 4-6h. The percentage and number of TNF-α-(B), IL-4-(C) positive T cells and Tregs (D) in the spleens of recipients are shown. Values are presented as mean ± SD. *P< 0.05. Figure S4. Anti-IL-39 antibody suppressed secretion of the pro-inflammatory cytokines in vitro. CD3+T cells were isolated from the spleens of C57BL/6 mice using magnetic bead sorting. T cells were stimulated with anti-CD3 and anti-CD28 and then treated with PBS, rIL-39, or anti-IL-39 antibody for 72h. The percentage of cytokines in the CD3+T cells is shown. Data are representative of at least three independent experiments. Values are presented as mean ± SD. *P< 0.05. Figure S5: Effect of IL-39 blockade on scleroderma-like cGVHD development. Irradiated BALB/c recipients (n=4 each group) were infused with 1×107 bone marrow cells and 1×106 splenocytes from the C57BL/6 mice. Fourteen days after transplantation, each mouse in the antibody group received 100μl (100μg) of IL-39 antibody, while each mouse in the control group received 100μl (100μg) of isotype control antibody via intraperitoneal injection twice a week for 6 weeks. The overall survival (A), body weight (B) and GVHD scores (C) are shown. Values are presented as mean ± SEM. *P< 0.05; ***P< 0.001. Figure S6: Effect of IL-39 blockade on cGVHD development and immune cells in the target tissues. Irradiated BALB/c recipients (n=4 each group) were infused with 1×107 bone marrow cells and 1×106 splenocytes from C57BL/6 mice. Fourteen days after transplantation, each mouse in the antibody group received 100μl (100μg) of IL-39 antibody, while each mouse in the control group received 100μl (100μg) of isotype control antibody via intraperitoneal injection twice a week for 6 weeks. The percentage and number of CD8+TNF-α+cells and CD4+IL-4+ T cells in the intestine (A), the numbers of CD69+CD4+T cells and CD69+CD8+T cells in the liver (B) and the percentage and number and of CD8+T cells in the lungs (C) are shown. Values are presented as mean ± SEM. Figure S7: Phosphorylation of STAT1 and total STAT1 was detected by western blotting in sorted primary T cells from the mice. Plates were coated with 2 mg/ml anti-CD3 and 0.4 mg/ml anti-CD28 Abs overnight. T cells (2×105 T cells were cultured with various concentrations of recombinant mouse IL-39 proteins for 72h. The phosphorylation of STAT1 and total STAT1 detected by western blotting in sorted primary T cells from mice is shown. The data are representative of three independent experiments. Figure S8: Effects of IL-39 blockade on IFN-γ and IL-17A expression in CD4+ and CD8+ donor T cells in the spleens of cGVHD mice. Irradiated BALB/c recipients (n=4 in each group) were infused with 1×107 bone marrow cells and 1×106 splenocytes from the C57BL/6 mice. Fourteen days after transplantation, each mouse in the antibody group received 100μl (100μg) of IL-39 antibody, whereas each mouse in the control group received 100μl (100μg) of isotype control antibody via intraperitoneal injection twice a week for 6 weeks. The percentages and numbers of donor Th1(A), Tc1 (B), Th17 (C), and Tc17 (D) cells in the spleen on day 56 are shown. Values are presented as mean ± SD. Figure S9: Effects of IL-39 overexpression on donor CD4+ and CD8+T cells in the spleens of cGVHD mice four weeks post-transplantation. Irradiated BALB/c recipients were infused with 1×107 bone marrow cells and 1×106 splenocytes from the C57BL/6 mice. Splenocytes (n=4 in each group) were collected and stained for FACS analysis four weeks after transplantation. The percentages and numbers of CD4+T, CD69+CD4+T, CD8+T, and CD69+CD8+T cells in CD3+ cells from the spleens of the recipients are shown (A). Lymphocytes were isolated from the spleens of recipients and treated with PMA, brefeldin A, and ionomycin for 4-6h. The percentage and number of TNF-α (B) and IL-4 (C) positive T cells in the spleens of recipients are shown.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lv, K., Hu, B., Xu, M. et al. IL-39 promotes chronic graft-versus-host disease by increasing T and B Cell pathogenicity. Exp Hematol Oncol 11, 34 (2022). https://doi.org/10.1186/s40164-022-00286-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40164-022-00286-x