Abstract
Introduction
Epilepsy is a common neurological disorder that presents with challenging mechanisms and treatment strategies. This study investigated the neuroprotective effects of quinpirole on lithium chloride pilocarpine-induced epileptic rats and explored its potential mechanisms.
Methods
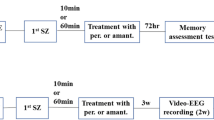
Lithium chloride pilocarpine was used to induce an epileptic model in rats, and the effects of quinpirole on seizure symptoms and cognitive function were evaluated. The Racine scoring method, electroencephalography, and Morris water maze test were used to assess seizure severity and learning and memory functions in rats in the epileptic group. Additionally, immunohistochemistry and Western blot techniques were used to analyze the protein expression levels and morphological changes in glutamate receptor 2 (GluR2; GRIA2), BAX, and BCL2 in the hippocampi of rats in the epileptic group.
Results
First, it was confirmed that the symptoms in rats in the epileptic group were consistent with features of epilepsy. Furthermore, these rats demonstrated decreased learning and memory function in the Morris water maze test. Additionally, gene and protein levels of GluR2 in the hippocampi of rats in the epileptic group were significantly reduced.
Quinpirole treatment significantly delayed seizure onset and decreased the mortality rate after the induction of a seizure. Furthermore, electroencephalography showed a significant decrease in the frequency of the spike waves. In the Morris water maze test, rats from the quinpirole treatment group demonstrated a shorter latency period to reach the platform and an increased number of crossings through the target quadrant. Network pharmacology analysis revealed a close association between quinpirole and GluR2 as well as its involvement in the cAMP signaling pathway, cocaine addiction, and dopaminergic synapses.
Furthermore, immunohistochemistry and Western blot analysis showed that quinpirole treatment resulted in a denser arrangement and a more regular morphology of the granule cells in the hippocampi of rats in the epileptic group. Additionally, quinpirole treatment decreased the protein expression of BAX and increased the protein expression of BCL2.
Conclusion
The current study demonstrated that quinpirole exerted neuroprotective effects in the epileptic rat model induced by lithium chloride pilocarpine. Additionally, it was found that the treatment not only alleviated the rats' seizure symptoms, but also improved their learning and memory abilities. This improvement was linked to the modulation of protein expression levels of GLUR2, BAX, and BCL2. These findings provided clues that would be important for further investigation of the therapeutic potential of quinpirole and its underlying mechanisms for epilepsy treatment.
Similar content being viewed by others
Introduction
Epilepsy is a common neurological disorder characterized by recurrent seizures and cognitive impairments. However, significant challenges persist in understanding its underlying mechanisms and develo** effective treatment strategies. Despite extensive research on the aforementioned topic, an optimal therapeutic approach for epilepsy remains elusive [1]. Therefore, it is critical to investigate new potential treatments that can provide neuroprotective effects and improve seizure symptoms and cognitive function.
In the present study, the researchers investigated the effects of quinpirole, which is a compound known for its potential neuroprotective properties, in an epileptic rat model induced by lithium chloride pilocarpine [2]. Understanding the mechanisms underlying the neuroprotective effects of quinpirole is essential for the development of improved therapeutic strategies for epilepsy.
To evaluate the effects of quinpirole on the symptoms of seizures and cognitive function, an epileptic rat model induced by lithium chloride pilocarpine was utilized. Manifestation of seizures was assessed using the Racine scoring method, and learning and memory functions were evaluated using the Morris water maze test [3]. In addition, electroencephalography (EEG) and immunohistochemistry techniques were used to analyze the protein expression levels of GluR2, BAX, and BCL2 and morphological changes in the hippocampi, which is a key brain region involved in epilepsy [4].
The results of the current study confirmed that the rat model displayed features that were consistent with those of epilepsy. Furthermore, the rats exhibited impaired learning and memory functions, as indicated by the Morris water maze test. The hippocampi of rats in the epileptic group exhibited significantly reduced gene and protein levels of GluR2, suggesting its involvement in the pathogenesis of epilepsy [5].
Quinpirole treatment demonstrated promising results in alleviating symptoms of seizures and improving cognitive function. Notably, quinpirole delayed the onset of seizure and resulted in a decreased frequency of spike waves, as observed by EEG. Moreover, the rats in the quinpirole treatment group exhibited improved performance in the Morris water maze test, with a shorter latency period to reach the platform and an increased number of crossings through the target quadrant.
Furthermore, network pharmacology analysis revealed an intriguing association between quinpirole and GluR2, suggesting its involvement in various signaling pathways associated with epilepsy, such as the cAMP signaling pathway and those related to cocaine addiction and dopaminergic synapses [6,7,8].
Immunohistochemistry and Western blot analysis further corroborated the neuroprotective effects of quinpirole. Notably, quinpirole treatment resulted in a denser arrangement and more regular morphology of granule cells in the hippocampus, indicating potential structural improvements [9]. Additionally, quinpirole treatment decreased the protein expression of pro-apoptotic BAX and increased the protein expression of anti-apoptotic BCL2 [10.11].
In conclusion, this study demonstrated the neuroprotective effects of quinpirole in a rat model of epilepsy. Improvements were observed in symptoms of seizures and cognitive function, which were associated with the modulation of the protein expression levels of GluR2, BAX, and BCL2. These findings provided valuable insights into the potential therapeutic role of quinpirole in epilepsy treatment and established a foundation for further investigation into the underlying mechanisms. Ultimately, the findings of the current research may contribute to the development of novel treatment strategies for epilepsy.
Methods
Animals
Twenty-one-day-old healthy male Wistar rats (weight: 55 ± 3 g) were purchased from the Experimental Animal Center of Shandong University. The rats were randomly divided into the following groups: the control group, the epileptic group, and the quinpirole group. The rats were housed in the animal facility of the Research Centre Laboratory, Tai'an Central Hospital, with a 12-h light/dark cycle and free access to food and water. All the rats were euthanized through an overdose of sodium pentobarbital. Thereafter, they underwent cardiac perfusion with physiological saline for biochemical analysis and were perfused with 4% paraformaldehyde for histological analysis. All the experimental procedures were approved by the Animal Care and Use Committee of the Tai'an Central Hospital and conducted in accordance with the guidelines of the institution. Ethics-related documents are presented in Supplement 1. Lithium chloride (30 mg/kg, Sigma-Aldrich, St. Louis, Missouri, USA) was injected intraperitoneally into the rats, and pilocarpine (30 mg/kg, Sigma-Aldrich) was injected in a similar manner after 24 h. In the control group, an equivalent volume of normal saline was administered as a substitute for pilocarpine. Quinpirole was injected into the lateral ventricle of the rats 60 min before injecting pilocarpine in the quinpirole group. Meanwhile, an equivalent volume of normal saline was injected as a substitute for quinpirole in the epileptic group. After the injection was completed, the syringe was kept at the site of injection for 5 min and then carefully withdrawn. The injection site was 0.7 mm behind the fontanel, 1.3 mm outside the midline, and 3.0 mm in depth [25]. However, rats treated with quinpirole showed improved cognitive performance, as indicated by a reduced latency period to reach the platform and an increased number of crossings of the target quadrant. These results suggested that quinpirole treatment not only reduced seizures, but also preserved cognitive function in rats with epilepsy induced by lithium chloride pilocarpine. Frequent and sustained seizures may cause changes in the variety of amino acids and neurotransmitters in the brain, further resulting in neuronal necrosis in the responding regions [26]. To further understand the underlying mechanisms of the neuroprotective effects of quinpirole, the protein expression levels of GluR2, BAX, and BCL2 in the hippocampus were investigated. GluR2 is a subunit of the AMPA receptor, which plays a critical role in excitatory synaptic transmission [27]. In the current study, a significant decrease in GluR2 expression was observed in the hippocampi of rats with epilepsy. This reduction in GluR2 expression may contribute to the increased permeability for calcium ions and apoptosis of neuronal cells observed in epilepsy [28]. Interestingly, the quinpirole treatment reversed the decrease in GluR2 expression, suggesting that it may regulate glutamate signaling and restore the balance between excitatory and inhibitory neurotransmission [10, 29, 30]. This was consistent with the findings of previous studies, which demonstrated the neuroprotective effects of dopamine receptor agonists on glutamatergic neurotransmission [31].
Moreover, network pharmacology analysis revealed a close relationship between quinpirole and GluR2, further supporting the involvement of glutamate signaling in the therapeutic effects of quinpirole. The decreased expression of GluR2 in a variety of neurological diseases increases the inward flow of calcium ions, which can activate protease, phospholipase, and ATPase, ultimately leading to cellular swelling and apoptosis of neurocytes. Additionally, KEGG pathway analysis suggested the potential involvement of the cAMP signaling pathway and other pathways related to cocaine addiction and dopaminergic synapses [32,33,34]. Furthermore, the protein expression levels of BAX and BCL2 were investigated, which are involved in regulating apoptosis. In the epileptic group, an increase in BAX expression and a decrease in BCL2 expression were observed, indicating an imbalance between pro-apoptotic and anti-apoptotic factors. However, quinpirole treatment reversed these changes, suggesting its potential role in inhibiting apoptosis and promoting neuronal survival [35, 36].
In conclusion, the study highlights promising neuroprotective effects of quinpirole in an epileptic model induced by lithium chloride pilocarpine. Further, quinpirole treatment effectively reduced seizure activity, improved cognitive function, and regulated the protein expression of GLUR2, BAX, and BCL2 in the hippocampus. These findings provided important insights into the potential therapeutic benefits of quinpirole and its underlying mechanisms in the treatment of epilepsy. Further studies are warranted to explore the clinical potential of quinpirole and its optimal dosage and treatment regimen for epilepsy.
Availability of data and materials
Not applicable.
References
Riva A, Golda A, Balagura G, Amadori E, Vari M, Piccolo G, et al. New trends and most promising therapeutic strategies for epilepsy treatment. Front Neurol. 2021;12:753753.
Koller W, Herbster G, Anderson D, Wack R, Gordon J. Quinpirole hydrochloride, a potential anti-parkinsonism drug. Neuropharmacology. 1987;26(8):1031–6.
Van Erum J, Van Dam D, De Deyn P. PTZ-induced seizures in mice require a revised Racine scale. Epilepsy Behavior E&B. 2019;95:51–5.
Gall C, Lauterborn J, Isackson P, White J. Seizures, neuropeptide regulation, and mRNA expression in the hippocampus. Prog Brain Res. 1990;83:371–90.
Friedman L, Velísková J, Kaur J, Magrys B, Liu H. GluR2(B) knockdown accelerates CA3 injury after kainate seizures. J Neuropathol Exp Neurol. 2003;62(7):733–50.
Huang Y, Chen C, Wang T, Qiu Y, Peng Y. Dopamine receptors modulate T lymphocytes via inhibition of cAMP-CREB signaling pathway. Neuro Endocrinol Lett. 2016;37(7):491–500.
De Vries T, Schoffelmeer A, Binnekade R, Raasø H, Vanderschuren L. Relapse to cocaine- and heroin-seeking behavior mediated by dopamine D2 receptors is time-dependent and associated with behavioral sensitization. Neuropsychopharmacology. 2002;26(1):18–26.
Escobar A, González M, Meza R, Noches V, Henny P, Gysling K, et al. Mechanisms of kappa opioid receptor potentiation of dopamine D2 Receptor Function in Quinpirole-Induced Locomotor Sensitization in Rats. Int J Neuropsychopharmacol. 2017;20(8):660–9.
Brozka H, Alexova D, Radostova D, Janikova M, Krajcovic B, Kubík Š, et al. Plasticity-related activity in the hippocampus, anterior cingulate, orbitofrontal, and prefrontal cortex following a repeated treatment with D/D Agonist Quinpirole. Biomolecules. 2021. https://doi.org/10.3390/biom11010084.
Alam S, Jo M, Park T, Ullah R, Ahmad S, Rehman S, et al. Quinpirole-mediated regulation of dopamine d2 receptors inhibits glial cell-induced neuroinflammation in cortex and striatum after brain injury. Biomedicines. 2021. https://doi.org/10.3390/biomedicines9010047.
Zhang W, Ye F, **ong J, He F, Yang L, Yin F, et al. Silencing of miR-132-3p protects against neuronal injury following status epilepticus by inhibiting IL-1β-induced reactive astrocyte (A1) polarization. FASEB J. 2022;36(10):e22554.
Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370:322–33.
Achilly NP, Wang W, Zoghbi HY. Presymptomatic training mitigates functional deficits in a mouse model of Rett syndrome. Nature. 2021;592(7855):596–600.
Fisher R, Acevedo C, Arzimanoglou A, Bogacz A, Cross J, Elger C, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–82.
Mohammad N, Nazli R, Zafar H, Fatima S. Effects of lipid based multiple micronutrients supplement on the birth outcome of underweight pre-eclamptic women: a randomized clinical trial. Pakistan J Med Sci. 2022;38(1):219–26.
Fattorusso A, Matricardi S, Mencaroni E, Dell’Isola G, Di Cara G, Striano P, et al. The pharmacoresistant epilepsy: an overview on existent and new emerging therapies. Front Neurol. 2021;12:674483.
Chen W, Li C, Liang W, Li Y, Zou Z, **e Y, et al. The roles of optogenetics and technology in neurobiology: a review. Front Aging Neurosci. 2022;14:867863.
Wengrovitz A, Ivantsova E, Crespo N, Patel M, Souders C, Martyniuk C. Differential effects of dopamine receptor agonists ropinirole and quinpirole on locomotor and anxiolytic behaviors in larval zebrafish (Danio rerio): a role for the GABAergic and glutamate system? Neurotoxicol Teratol. 2023;98:107183.
Monti J, Jantos H, Fernández M. Effects of the selective dopamine D-2 receptor agonist, quinpirole on sleep and wakefulness in the rat. Eur J Pharmacol. 1989;169(1):61–6.
Tang T, Bi M, Diao M, Zhang X, Chen L, **ao X, et al. Quinpirole ameliorates nigral dopaminergic neuron damage in Parkinson’s disease mouse model through activating GHS-R1a/DR heterodimers. Acta Pharmacol Sin. 2023;44(8):1564–75.
Zhang Y, Chen Y, Wu J, Manaenko A, Yang P, Tang J, et al. Activation of dopamine D2 receptor suppresses neuroinflammation through αB-crystalline by inhibition of NF-κB nuclear translocation in experimental ICH mice model. Stroke. 2015;46(9):2637–46.
Burke K, Chandler C, Starr B, Starr M. Seizure promotion and protection by D-1 and D-2 dopaminergic drugs in the mouse. Pharmacol Biochem Behav. 1990;36(4):729–33.
Al-Tajir G, Starr M. Anticonvulsant effect of striatal dopamine D2 receptor stimulation: dependence on cortical circuits? Neuroscience. 1991;43(1):51–7.
Wang L, Shi H, Kang Y, Guofeng W. Hippocampal low-frequency stimulation improves cognitive function in pharmacoresistant epileptic rats. Epilepsy Res. 2020;168:106194.
Cop** N, Adhikari A, Petkova S, Silverman J. Genetic backgrounds have unique seizure response profiles and behavioral outcomes following convulsant administration. Epilepsy Behavior E&B. 2019;101:106547.
Alford EL, Wheless JW, Phelps SJ. Treatment of generalized convulsive status epilepticus in pediatric patients. J Pediatric Pharmacol Therapeut. 2015;20(4):260–89.
Ekici M, Keim A, Rössler O, Hohl M, Thiel G. Chromatin structure and expression of the AMPA receptor subunit Glur2 in human glioma cells: major regulatory role of REST and Sp1. J Cell Biochem. 2012;113(2):528–43.
Van Damme P, Bogaert E, Dewil M, Hersmus N, Kiraly D, Scheveneels W, et al. Astrocytes regulate GluR2 expression in motor neurons and their vulnerability to excitotoxicity. Proc Natl Acad Sci USA. 2007;104(37):14825–30.
van den Pol A, Cao V, Belousov A. Dopamine enhancement and depression of glutamate-regulated calcium and electrical activity in hypothalamic neurons. J Neurophysiol. 1996;76(6):3934–48.
Shen K, Johnson S. Presynaptic dopamine D2 and muscarine M3 receptors inhibit excitatory and inhibitory transmission to rat subthalamic neurones in vitro. J Physiol. 2000. https://doi.org/10.1111/j.1469-7793.2000.00331.x.
Liu P, Qin D, Lv H, Fan W, Tao Z, Xu Y. Neuroprotective effects of dopamine D2 receptor agonist on neuroinflammatory injury in olfactory bulb neurons in vitro and in vivo in a mouse model of allergic rhinitis. Neurotoxicology. 2021;87:174–81.
Robinson S, Sohal V. Dopamine D2 receptors modulate pyramidal neurons in mouse medial prefrontal cortex through a stimulatory G-protein pathway. J Neurosci. 2017;37(42):10063–73.
Xue Y, Steketee J, Rebec G, Sun W. Activation of D2-like receptors in rat ventral tegmental area inhibits cocaine-reinstated drug-seeking behavior. Eur J Neurosci. 2011;33(7):1291–8.
Sañudo-Peña M, Force M, Tsou K, Miller A, Walker J. Effects of intrastriatal cannabinoids on rotational behavior in rats: interactions with the dopaminergic system. Synapse. 1998;30(2):221–6.
Griffiths M, Cooper A, Barber D, Mitchell I. Pharmacological mechanisms mediating phencyclidine-induced apoptosis of striatopallidal neurons: the roles of glutamate, dopamine, acetylcholine and corticosteroids. Brain Res. 2000;855(1):1–10.
Bono F, Savoia P, Guglielmi A, Gennarelli M, Piovani G, Sigala S, et al. Role of dopamine D2/D3 receptors in development, plasticity, and neuroprotection in human iPSC-derived midbrain dopaminergic neurons. Mol Neurobiol. 2018;55(2):1054–67.
Acknowledgements
Not applicable.
Funding
This project was supported by the Tai’an City Science and Technology Innovation Development Project (2018NS0221). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
WH, ZY, and ZD conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft. LJ and YK conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft. LB and YJ conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, H., Zhao, Y., Zhang, D. et al. Neuroprotective effects of quinpirole on lithium chloride pilocarpine-induced epilepsy in rats and its underlying mechanisms. Eur J Med Res 29, 121 (2024). https://doi.org/10.1186/s40001-024-01694-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-024-01694-x