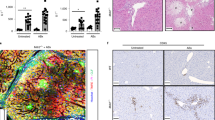
Abstract
Background and aims
Primary sclerosing cholangitis (PSC) is a chronic liver disease characterized by progressive biliary inflammation and bile duct injury. Berberine (BBR) is a bioactive isoquinoline alkaloid found in various herbs and has multiple beneficial effects on metabolic and inflammatory diseases, including liver diseases. This study aimed to examine the therapeutic effect of BBR on cholestatic liver injury in a PSC mouse model (Mdr2−/− mice) and elucidate the underlying mechanisms.
Methods
Mdr2−/−mice (12–14 weeks old, both sexes) received either BBR (50 mg/kg) or control solution daily for eight weeks via oral gavage. Histological and serum biochemical analyses were used to assess fibrotic liver injury severity. Total RNAseq and pathway analyses were used to identify the potential signaling pathways modulated by BBR in the liver. The expression levels of key genes involved in regulating hepatic fibrosis, bile duct proliferation, inflammation, and bile acid metabolism were validated by qRT-PCR or Western blot analysis. The bile acid composition and levels in the serum, liver, small intestine, and feces and tissue distribution of BBR were measured by LC–MS/MS. Intestinal inflammation and injury were assessed by gene expression profiling and histological analysis. The impact on the gut microbiome was assessed using 16S rRNA gene sequencing.
Results
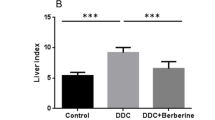
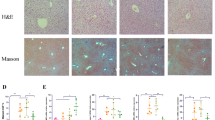
BBR treatment significantly ameliorated cholestatic liver injury, evidenced by decreased serum levels of AST, ALT, and ALP, and reduced bile duct proliferation and hepatic fibrosis, as shown by H&E, Picro-Sirius Red, and CK19 IHC staining. RNAseq and qRT-PCR analyses indicated a substantial inhibition of fibrotic and inflammatory gene expression. BBR also mitigated ER stress by downregulating Chop, Atf4 and Xbp-1 expression. In addition, BBR modulated bile acid metabolism by altering key gene expressions in the liver and small intestine, resulting in restored bile acid homeostasis characterized by reduced total bile acids in serum, liver, and small intestine and increased fecal excretion. Furthermore, BBR significantly improved intestinal barrier function and reduced bacterial translocation by modulating the gut microbiota.
Conclusion
BBR effectively attenuates cholestatic liver injury, suggesting its potential as a therapeutic agent for PSC and other cholestatic liver diseases.
Similar content being viewed by others
Background
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disorder characterized by inflammation and bile duct narrowing, which results in the accumulation of bile acids (BAs) in the liver, leading to hepatic damage, progressive liver fibrosis, cirrhosis, and even liver cancer [1, 2]. Over the last several decades, extensive efforts have been made to identify the mechanisms underlying cholestatic liver injury [3,4,5]. However, no effective therapy has been developed due to the complexity of disease pathogenesis. Liver transplantation remains the only life-extending treatment for end-stage PSC patients [4]. It has been well-accepted that dysregulation of BA homeostasis and inflammation are the major driving forces of the disease progression of PSC [6]. In addition, dysbiosis and intestinal barrier dysfunction also have been reported as key contributors to cholestatic liver injury [7,8,9]. Therefore, an effective therapeutic agent for PSC must be able to modulate bile acid metabolism, inflammatory response, and the gut microbiome.
Berberine (BBR), an isoquinoline alkaloid isolated from the rhizome of the herb Coptis chinensis and Berberis vulgaris, is one of the most commonly used plant medicines in China and Asia with various biological activities [10,1, 4]. Currently, there is no effective medication for improving transplant-free survival in these cases. Liver transplantation is the only definitive treatment for PSC, though it carries a high risk of disease recurrence [2, 29, 30]. Recent studies suggest that targeting pathways, such as BA synthesis and transport, hepatic inflammation, mitochondrial respiration, oxidative stress, intestinal inflammation, and gut microbiota, could provide new therapeutic strategies for cholestatic liver diseases [26, 27, 31,32,33,34,35].
BBR has long been used in Asia as an anti-bacterial medicine. Clinical and preclinical studies highlight its potential in treating metabolic diseases by modulating various molecular targets, including transcription factors, cell survival/proliferative proteins, enzymes, metastatic/invasion molecules, growth factors, platelet activation, inflammatory cytokines, apoptotic proteins, protein kinases, receptors, and the others [14, 36]. There are a considerable number of studies demonstrating that BBR has preventive or therapeutic effects on various liver diseases, such as hepatitis, MAFLD, and liver fibrosis [11, 37,38,39,40,41,42]. However, its impact on cholestatic liver injury remains unexplored. To elucidate the therapeutic effect and potential mechanisms of BBR on cholestatic liver injury, we conducted a series of analyses, including histological imaging, biochemical analysis, molecular biology, and RNA-seq transcriptome analysis. Using bioinformatic tools, we identified differentially expressed genes regulated by BBR followed by GO, KEGG pathway, and functional category analysis. The findings of this study strongly suggest that BBR is potentially effective in treating cholestatic liver injury. The major mechanisms underlying BBR's beneficial effects include reducing bile duct injury and hepatic fibrosis, alleviating hepatic inflammation and ER stress, restoring BA homeostasis, and improving intestinal barrier function as well as modulating gut microbiome.
PSC is an inflammatory liver disease often associated with severe cholestatic liver injury [43]. BBR is known for its potent anti-inflammatory activities in liver disease [16, 17]. Key pathways, such as NF-κB signaling pathway, MAPK pathways, and oxidative phosphorylation, are involved in inflammation-driven cholestatic liver injury [8, 23, 44, 45]. Our RNA-seq gene analysis and pathway profiling showed that BBR significantly reduced inflammation in Mdr2−/− mice. This reduction is achieved through BBR's ability to inhibit inflammatory macrophage infiltration in the liver, which is evident from the decreased expression of various chemokines, cytokines, and cell surface adhesion molecules (Fig. 4, Additional file 1: Fig. S6). Additionally, BBR modulates NF-κB signaling, the MAPK signaling pathway, and oxidative phosphorylation (Additional file 1: Fig. S7–S9).
CCL2/MCP-1 chemokines, produced by fibroblasts, activated cholangiocytes, resident macrophages, and endothelial cells, play a crucial role in the inflammatory response. A recent study suggests that targeting the CCR2/CCL2 axis can limit monocyte recruitment and reduce fibrosis and cholestasis, offering a potential treatment approach for PSC [46]. In line with these findings, our study demonstrates that BBR suppresses the hepatic expression of CCR2 and CCL2. Furthermore, the ER stress response, a key factor in inflammation and metabolic disorders, is significantly modulated by BBR [24, 47, 48]. Disruptions in ER homeostasis activate the UPR, leading to inflammation and cell injury. The IRE1, protein kinase RNA-like ER kinase (PERK), and ATF6 pathways are the three major branches of the UPR [24]. BBR has been previously shown to inhibit HIV protease inhibitor-induced ER stress in macrophages and inhibit free fatty acid and LPS-induced inflammation via modulating the PERK-ATF4-CHOP signaling pathway in macrophages and hepatocytes [17, 18]. Consistently, our current study found that BBR significantly reduced ER stress in Mdr2−/− mice, particularly inhibiting the PERK-ATF4-CHOP pathway (Fig. 5 and Additional file 1: Fig. S10).
BA homeostasis is crucial in managing cholestatic liver diseases [3, 49]. BAs are synthesized in hepatocytes and immediately secreted into bile through the bile duct. The majority of BAs are reabsorbed in the terminal ileum and transported back to the liver through portal vein. The enterohepatic circulation of BA is an important physiological process, making the synthesis and transport of BAs vital targets for cholestatic liver injury [50, 51]. Our studies show that BBR can restore BA homeostasis by modulating key enzymes, nuclear receptors, and hepatic transporters involved in BA synthesis and transport (Fig. 6 and Additional file 1: Fig. S11). Elevated serum BA levels, particularly total, primary, conjugated BAs, including TCA, were common in PSC patients [52,53,54]. The current study showed that BBR treatment in Mdr2−/− mice significantly reduced these BA levels in serum, liver, and small intestine while increasing fecal BA output without causing diarrhea (Fig. 7 and Additional file 1: Fig. S12). This suggests BBR's role as a differential BA transport inhibitor, indicated by reduced Ntcp and Asbt expression (Fig. 6). Furthermore, BBR has been shown to influence key regulators of BA homeostasis significantly. In our study, BBR increased the expression of Fxrα, a crucial regulator in BA homeostasis, and increased the expression of Shp, which represses Cyp7a1 by inhibiting LRH-1 activity [55, 56]. However, the RNA-seq data showed BBR had no significant effects on LRH-1, but upregulated both Cyp7a1 and Cyp27a1 levels in the liver (Fig. 6). These results suggest the potential compensatory mechanisms to counteract the inhibition of BA up taking in Mdr2−/− mice with BBR treatment. Although FXR agonists and ASBT inhibitors have been tested in clinical trials for various liver diseases, the potential to treat PSC remains uncertain. Our previous studies reported that increased primary conjugated BA is responsible for cholestatic liver injury and liver fibrosis via activating sphingosine-1 phosphate receptor 2 (S1PR2), which can upregulate lncRNA H19 in Mdr2−/− mice [57,58,59,60]. Our recent study showed that BBR reduced the expression of H19 in a MASH mouse model [16]. Consistently, in this study, our results showed that H19 was inhibited by BBR treatment in Mdr2−/− mice.
Recent clinical studies have established a link between disrupted intestinal barrier function, bacterial translocation, and the progression of cholestatic liver diseases, such as PSC and PBS [61]. Specifically, in Mdr2−/− mice, impairment in intestinal barrier function has been observed, including diminished tight junction protein expression, reduced mucus layers, increased permeability, and enhanced bacterial translocation [9]. Our previous study has reported that ER stress-induced activation of CHOP leads to disruption of intestinal barrier function, bacterial translocation, activation of inflammation, and eventually results in fibrosis in the liver [7]. In line with these findings, our current study demonstrates that BBR effectively decreased CHOP expression in both the liver and intestine (Fig. 5d and Additional file 1: Fig. S13b), suggesting its potential to mitigate these pathological processes. Moreover, recent studies reported that H19 exacerbates intestinal barrier dysfunction by inhibiting autophagy and impairing goblet and Paneth cell functions [62, 63]. Consistent with this, our results show that BBR significantly inhibits H19, correlating with restored epithelial barrier function as evidenced by increased expression of mucin-2 and ZO-1 (Figs. 8e-f & Additional file 1: Fig. S13b).
A previous study using hamsters found that orally administered BBR predominantly accumulates in the gut rather than in circulation, significantly affecting both gut and circulatory metabolites despite low serum levels [64]. Our study aligns with these findings, showing that BBR concentrations are highest in the stomach, intestine, and colon and relatively lower in the liver, kidney, heart, lung, brain, and spleen. This suggests that BBR mitigates cholestatic liver injury by modulating the gut-liver axis (Additional file 1: Fig. S14). It is well established that the gut microbiota regulates BA composition and levels, particularly in PSC. BBR has been reported to have antidiabetic effects by modulating the gut microbiome [10, 15]. Our current study further indicates that BBR alters the gut microbiota composition in Mdr2−/− mice. Specifically, BBR increased the relative abundance of Bacteroidetes and decreased that of Firmicutes (Additional file 1: Fig. S15), which is significant as bacteria in Firmicutes are known for high bile salt hydrolase (BSH) activity, promoting BA deconjugation and fecal excretion [65, 66]. This alteration in microbiota composition aligns with the interplay between intestinal microbiota and BAs, where each influence the other.
Conclusion
In summary, our study sheds light on the potential mechanisms by which BBR attenuates cholestatic liver injury in a PSC mouse model. As illustrated in Fig. 9, BBR can directly or indirectly target various liver cells, including hepatocytes, macrophages, stellate cells, and cholangiocytes, modulating multiple pathways related to bile duct injury, fibrosis, inflammation, ER stress, and BA metabolism and transport in the gut-liver-axis. Furthermore, BBR enhances intestinal barrier function and reduces bacterial translocation, while also restoring BA homeostasis and gut microbiota. These findings suggest that BBR has potential as a pharmacological treatment for cholestatic liver injury such as PSC.
Schematic Representation of BBR’s Potential Mechanisms in Alleviating Cholestatic Liver Injury. This diagram illustrates the proposed molecular and cellular mechanisms through which BBR mitigates cholestatic liver injury in a mouse model of sclerosing cholangitis. It visually summarizes the pathways and interactions influenced by BBR treatment, highlighting its multifaceted role in addressing liver disease pathology
Materials and methods
Reagents
Berberine chloride hydrate (BBR) was purchased from Sigma (St. Louis, MO, USA, Cat #14050). Common laboratory chemicals were purchased from Sigma Aldrich (St. Louis, MO, USA). All antibodies used in this study are listed in Additional file 2: Table S1.
Animal experiments
FVB Mdr2−/− mice (100 days old, both sexes, n = 9–12) were originally obtained from Dr. Gianfranco Alpini (Texas A&M HSC College of Medicine). Mdr2−/− mouse (C57/BL6 background) is a kind gift from Dr. Daniel Goldenberg at the Department of Pathology, Hadassah-Hebrew University Medical Center, Jerusalem, Israel. Mice were randomly divided into the vehicle control group and BBR group. Mice were treated with BBR (50 mg/kg) or vehicle (0.5% carboxyl methyl cellulose sodium solution) by intragastric administration once daily for 8 weeks. All mice were housed in a 12 h light/12 h dark cycle with a controlled room temperature between 21 and 23 °C and free access to water. All the experimental procedures were performed according to protocols approved by the Richmond VA Medical Center and Virginia Commonwealth University Institutional Animal Care and Use Committee. All animal experiments were performed in accordance with institutional guidelines for ethical animal studies. At the end of the experiment, mice were weighed and anesthetized by exposure to inhaled isoflurane. The blood was collected by cardiac puncture. The serum was collected and stored at − 80 °C for later analysis. After euthanasia, the liver and small intestine were collected for histological analysis, RNA profiling, and Western blot analysis. Fecal samples were collected for 16S rRNA gene sequencing to measure the gut microbiome.
RNA sequencing (RNAseq) and bioinformatic analysis
Total liver RNA was isolated using Chemagic Prepito®-D Nucleic Acid Extractor (PerkinElmer, Waltham, MA, USA) with a Prepito RNA kit (PerkinElmer, USA). The RNAseq with ribosomal RNA (rRNA) depletion was done by Genewiz Company using the Illumina Hiseq® X platform (Genewiz Co., South Plainfield, NJ, USA). Sequencing reads were trimmed and filtered using bbduk to remove adapters and low-quality reads. Reads from mouse samples were mapped to Ensembl GRCm38 transcripts annotation (release 82), using RSEM. Gene expression data normalization and differential expression analysis were performed using the R package edgeR. Significantly up- or downregulated genes were determined as fold change ≥ 2 and p-value < 0.05. Hierarchical clustering was performed to show distinguishable mRNA expression profiles among the samples (Heatmap was plotted by http://www.bioinformatics.com.cn, an online platform for data analysis and visualization). The volcano graph and heatmaps were created to visualize significantly dysregulated mRNAs using GraphPad Prism (version 8; GraphPad Software Inc., San Diego, CA, USA). Gene Ontology (GO) analysis was used to investigate three functionality domains: biological process (BP), cellular component (CC), and molecular function (MF) using DAVID (Database for Annotation, Visualization, and Integrated Discovery) v6.8 (https://david.ncifcrf.gov/). Pathway analysis was performed to functionally analyze and map genes to Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways (https://pathview.uncc.edu/).
Serum biochemical analysis and hepatic hydroxyproline content measurement
The serum levels of ALP, AST and ALT, total triglyceride (TG), total cholesterol (TC), very-low-density lipoprotein (VLDL), and ALB were determined using the Alfa Wassermann Vet ACE Axcel® System with commercially available assay kits (Alfa Wassermann diagnostic technologies, NJ, USA). To quantify liver fibrosis, hepatic hydroxyproline was measured using the Hydroxyproline Assay kit (Sigma Aldrich, MO, USA) according to the manufacturer's instructions.
Histological and immunohistochemical staining
Liver tissues were processed for hematoxylin and eosin (H&E) staining and immunohistochemistry (IHC) staining for CK-19 and Ki67 at the Mouse Model Core at the VCU Massey Cancer Center (Richmond, VA, USA). Picro Sirius Red Staining was performed using the commercial Kit (Abcam, USA) with the paraffin-embedded tissue sections according to the manufacturer's instructions. Small intestine tissues were processed for H&E staining. Alcian blue staining was performed using the Alcian blue Stain Kit (Abcam, USA). Immunofluorescence staining of ZO-1 was performed with the paraffin-embedded tissue sections according to the manufacturer's instructions. All the stained slides were scanned using a Vectra Polaris Automated Quantitative Pathology Imaging System (Akoya Biosciences, MA, USA), and the images were captured using Phenochart software (Akoya Biosciences, MA, USA).
Bile acid (BA) analysis
The serum, liver tissues, intestine contents, and colon feces were processed for BA analysis, as described previously [16]. The composition and levels of BAs in serum, liver, intestine and fecal samples were measured using a Shimadzu liquid chromatography/tandem mass spectrometric (LC–MS/MS) 8600 system as described previously [16]. Data were collected and processed using Lab Solutions software.
Tissue distribution of BBR
Mdr2−/− mice were treated with BBR (50 mg/kg) by intragastric administration after a 12-h fast. Blood, heart, lung, liver, kidney, brain, spleen, stomach, intestine, colon, and feces were collected after 3, 6, 9, and 12 h of BBR treatment, respectively. The contents of BBR in serum and tissues were analyzed using LC–MS/MS.
A reliable LC–MS/MS method was developed and validated to quantify BBR, using L-tetrahydropalmatine as the internal standard (IS). To quantify BBR in the serum, serum samples and IS were incubated with acetonitrile/methanol/water l (1/1, v/v) in a 1.5 mL vial. For quantification of BBR in the spleen, lung, kidney, heart, stomach contents, intestine contents, and feces, tissue samples were incubated with acetonitrile/methanol (1/1, v/v) in a 2 mL vial with beads. The homogenized samples and IS were mixed with acetonitrile/methanol/water (1/1, v/v) in a 1.5 mL vial. After centrifugation at 12,000 × g for 2 min at room temperature, the supernatant was filtered through 0.2 µm PTFE membrane, and 2 µL aliquots were injected into the LC–MS/MS system. The analyte was separated on a C18 reverse phase column and analyzed in the multiple reaction monitoring (MRM) mode using ESI with positive ionization, m/z 335.9 → 320.1 for BBR and m/z 355.9 → 192.2 for IS. Mobile phase A was 0.05% acetic acid in water, while mobile phase B was acetonitrile. The gradient was optimized at 30% to 75% B in 2 min and then maintained 75% B for 0.5 min. The column was equilibrated with 30% B for 1.5 min. Data were collected and processed using Lab Solutions software.
Quantitative RT-PCR
Total liver RNA was isolated using Chemagic Prepito®-D Nucleic Acid Extractor (PerkinElmer, USA) with Prepito RNA kit (PerkinElmer, USA). cDNA synthesis and Quantitative RT-PCR analysis of relative mRNA expression levels of target genes were previously described [16]. Primer sequences will be provided upon request.
Immunoblotting analysis
Total proteins were prepared using cold RIPA buffer. Nuclear proteins were isolated, as previously described. Protein concentration was measured using the Bio-Rad Protein Assay reagent. Proteins were resolved on 10% SDS-PAGE and transferred to nitrocellulose membranes (Thermo, Waltham, MA, USA). 5% milk was used to block the background. The target proteins were probed with the specific primary antibodies and detected using HRP-conjugated secondary antibodies and ECL reagents (Thermo, USA). Images were captured using the Bio-Rad Gel Doc XR + Imaging System (Hercules, CA, USA). The density of immunoblotted bands was analyzed using BioRad Image Lab computer software and normalized with histone 3 or β-Actin.
FITC-DEXTRAN permeability and bacterial translocation assay
FITC-Dextran solution (100 mg/mL) was prepared in PBS. FITC-Dextran was administered to mice by oral gavage (600 mg/kg) and blood samples were taken after 4 h. The serum concentration of FITC-dextran was measured using Victor Multilabel Plate Counter (PerkinElmer, Waltham, MA) with an excitation wavelength of 490 nm and an emission wavelength of 530 nm. Blood and mesenteric lymph nodes (MLNs) were harvested in sterile conditions. Blood and homogenized MLNs were diluted in series and plated on Blood Agar Plates. After 72 h incubation at 37 °C in aerobic conditions, colony-forming units (CFUs) were counted and calculated.
Microbiota analysis
Fecal samples of Mdr2−/− mice treated with BBR 50 mg/kg or 100 mg/kg for 8 weeks were collected for 16S rRNA gene sequencing. Extraction, library preparation, sequencing, and analysis were performed at Rutgers Center for Microbiome Analysis Core, New Jersey Institute for Food, Nutrition and Health. All DNA samples were quantified using the Qubit 1 × dsDNA HS assay kit (Thermo Fisher Scientific), which measured DNA concentration based on the fluorescence intensity of a fluorescent dye binding to double-stranded DNA. DNA integrity was assessed using agarose gel electrophoresis.
Statistical analysis
Data are expressed as the mean ± SEM from at least three independent experiments. The student's t-test was used to analyze the difference between the two groups by GraphPad Prism (version 8; GraphPad Software Inc., San Diego, CA). A p-value < 0.05 was considered statistically significant.
Availability of data and materials
Detailed methods and datasets generated and/or analyzed during the current study are available in Additional file.
Abbreviations
- HSC:
-
Hepatic stellate cells
- lncRNA:
-
Long non-coding RNA
- PBC:
-
Primary biliary cholangitis
- PSC:
-
Primary sclerosing cholangitis
- S1PR2:
-
Sphingosine-1 phosphate receptor 2
- SHP:
-
Short heterodimer partner
- TCA:
-
Taurocholate sodium
- WT:
-
Wild type
References
Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis—a comprehensive review. J Hepatol. 2017;67:1298–323.
Dyson JK, Beuers U, Jones DEJ, Lohse AW, Hudson M. Primary sclerosing cholangitis. The Lancet. 2018;391:2547–59.
Jansen PL, Ghallab A, Vartak N, Reif R, Schaap FG, Hampe J, Hengstler JG. The ascending pathophysiology of cholestatic liver disease. Hepatology. 2017;65:722–38.
Wagner M, Fickert P. Drug therapies for chronic cholestatic liver diseases. Annu Rev Pharmacol Toxicol. 2020;60:503–27.
Jungst C, Lammert F. Cholestatic liver disease. Dig Dis. 2013;31:152–4.
Trauner M, Fuchs CD, Halilbasic E, Paumgartner G. New therapeutic concepts in bile acid transport and signaling for management of cholestasis. Hepatology. 2017;65:1393–404.
Liu R, Li X, Huang Z, Zhao D, Ganesh BS, Lai G, Pandak WM, et al. C/EBP homologous protein-induced loss of intestinal epithelial stemness contributes to bile duct ligation-induced cholestatic liver injury in mice. Hepatology. 2018;67:1441–57.
Shearn CT, Orlicky DJ, Petersen DR. Dysregulation of antioxidant responses in patients diagnosed with concomitant Primary Sclerosing Cholangitis/Inflammatory Bowel Disease. Exp Mol Pathol. 2018;104:1–8.
Liao L, Schneider KM, Galvez EJ, Frissen M, Marschall H-U, Su H, Hatting M, et al. Intestinal dysbiosis augments liver disease progression via NLRP3 in a murine model of primary sclerosing cholangitis. Gut. 2019;68:1477–92.
Zhang Y, Gu Y, Ren H, Wang S, Zhong H, Zhao X, Ma J, et al. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study). Nat Commun. 2020;11:5015.
Xu X, Zhu XP, Bai JY, **a P, Li Y, Lu Y, Li XY, Gao X. Berberine alleviates nonalcoholic fatty liver induced by a high-fat diet in mice by activating SIRT3. FASEB J. 2019;33:7289–300.
Sun R, Yang N, Kong B, Cao B, Feng D, Yu X, Ge C, et al. Orally administered berberine modulates hepatic lipid metabolism by altering microbial bile acid metabolism and the intestinal fxr signaling pathway. Mol Pharmacol. 2017;91:110–22.
Kong Y, Li L, Zhao LG, Yu P, Li DD. A patent review of berberine and its derivatives with various pharmacological activities (2016–2020). Expert Opin Ther Pat 2021.
Xu X, Yi H, Wu J, Kuang T, Zhang J, Li Q, Du H, et al. Therapeutic effect of berberine on metabolic diseases: Both pharmacological data and clinical evidence. Biomed Pharmacother. 2021;133: 110984.
Wolf PG, Devendran S, Doden HL, Ly LK, Moore T, Takei H, Nittono H, et al. Berberine alters gut microbial function through modulation of bile acids. BMC Microbiol. 2021;21:1–15.
Wang Y, Tai YL, Zhao D, Zhang Y, Yan J, Kakiyama G, Wang X, et al. Berberine prevents disease progression of nonalcoholic steatohepatitis through modulating multiple pathways. Cells. 2021;10:1.
Wang Y, Zhou X, Zhao D, Wang X, Gurley EC, Liu R, Li X, et al. Berberine inhibits free fatty acid and LPS-induced inflammation via modulating ER stress response in macrophages and hepatocytes. PLoS ONE. 2020;15: e0232630.
Zha W, Liang G, **ao J, Studer EJ, Hylemon PB, Pandak WM Jr, Wang G, et al. Berberine inhibits HIV protease inhibitor-induced inflammatory response by modulating ER stress signaling pathways in murine macrophages. PLoS ONE. 2010;5: e9069.
Mauad TH, Van Nieuwkerk CM, Dingemans KP, Smit JJ, Schinkel AH, Notenboom RG, van den Bergh Weerman MA, et al. Mice with homozygous disruption of the mdr2 P-glycoprotein gene a novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am J Pathol. 1994;145:1237.
Popov Y, Patsenker E, Fickert P, Trauner M, Schuppan D. Mdr2 (Abcb4)-/- mice spontaneously develop severe biliary fibrosis via massive dysregulation of pro- and antifibrogenic genes. J Hepatol. 2005;43:1045–54.
Smit J, Schinkel AH, Elferink RO, Groen A, Wagenaar E, Van Deemter L, Mol C, et al. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993;75:451–62.
Eaton JE, Talwalkar JA, Lazaridis KN, Gores GJ, Lindor KD. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology. 2013;145:521–36.
Plkarsky E. NF-KB functions as a tumour promoter in inflammation-associated cancer. Nature (Lond). 2004;431:461–6.
Zhou H, Liu R. ER stress and hepatic lipid metabolism. Front Genet. 2014;5:112.
Tamaki N, Hatano E, Taura K, Tada M, Kodama Y, Nitta T, Iwaisako K, et al. CHOP deficiency attenuates cholestasis-induced liver fibrosis by reduction of hepatocyte injury. Am J Physiol Gastrointest Liver Physiol. 2008;294:G498-505.
Fuchs CD, Paumgartner G, Mlitz V, Kunczer V, Halilbasic E, Leditznig N, Wahlström A, et al. Colesevelam attenuates cholestatic liver and bile duct injury in Mdr2−/− mice by modulating composition, signalling and excretion of faecal bile acids. Gut. 2018;67:1683–91.
Baghdasaryan A, Fuchs CD, Osterreicher CH, Lemberger UJ, Halilbasic E, Pahlman I, Graffner H, et al. Inhibition of intestinal bile acid absorption improves cholestatic liver and bile duct injury in a mouse model of sclerosing cholangitis. J Hepatol. 2016;64:674–81.
Yang R, Zhao Q, Hu DD, **ao XR, Huang JF, Li F. Metabolomic analysis of cholestatic liver damage in mice. Food Chem Toxicol. 2018;120:253–60.
Mendes FD, Kim WR, Pedersen R, Therneau T, Lindor KD. Mortality attributable to cholestatic liver disease in the United States. Hepatology. 2008;47:1241–7.
Song J, Li Y, Bowlus CL, Yang G, Leung PSC, Gershwin ME. Cholangiocarcinoma in patients with primary sclerosing cholangitis (PSC): a comprehensive review. Clin Rev Allergy Immunol. 2020;58:134–49.
Li J, Zhu X, Zhang M, Zhang Y, Ye S, Leng Y, Yang T, et al. Limb expression 1-like (LIX1L) protein promotes cholestatic liver injury by regulating bile acid metabolism. J Hepatol. 2021;75(2):400–13.
Tardelli M, Bruschi FV, Fuchs CD, Claudel T, Auer N, Kunczer V, Baumgartner M, et al. Monoacylglycerol lipase inhibition protects from liver injury in mouse models of sclerosing cholangitis. Hepatology. 2020;71:1750–65.
Liu Y, Chen K, Li F, Gu Z, Liu Q, He L, Shao T, et al. Probiotic Lactobacillus rhamnosus GG prevents liver fibrosis through inhibiting hepatic bile acid synthesis and enhancing bile acid excretion in mice. Hepatology. 2020;71:2050–66.
Pradhan-Sundd T, Kosar K, Saggi H, Zhang R, Vats R, Cornuet P, Green S, et al. Wnt/beta-catenin signaling plays a protective role in the Mdr2 knockout murine model of cholestatic liver disease. Hepatology. 2020;71:1732–49.
Miethke AG, Zhang W, Simmons J, Taylor AE, Shi T, Shanmukhappa SK, Karns R, et al. Pharmacological inhibition of apical sodium-dependent bile acid transporter changes bile composition and blocks progression of sclerosing cholangitis in multidrug resistance 2 knockout mice. Hepatology. 2016;63:512–23.
Feng X, Sureda A, Jafari S, Memariani Z, Tewari D, Annunziata G, Barrea L, et al. Berberine in cardiovascular and metabolic diseases: from mechanisms to therapeutics. Theranostics. 2019;9:1923–51.
Eissa LA, Kenawy HI, El-Karef A, Elsherbiny NM, El-Mihi KA. Antioxidant and anti-inflammatory activities of berberine attenuate hepatic fibrosis induced by thioacetamide injection in rats. Chem Biol Interact. 2018;294:91–100.
Hung TC, Jassey A, Liu CH, Lin CJ, Lin CC, Wong SH, Wang JY, et al. Berberine inhibits hepatitis C virus entry by targeting the viral E2 glycoprotein. Phytomedicine. 2019;53:62–9.
Zhu X, Bian H, Wang L, Sun X, Xu X, Yan H, **a M, et al. Berberine attenuates nonalcoholic hepatic steatosis through the AMPK-SREBP-1c-SCD1 pathway. Free Radic Biol Med. 2019;141:192–204.
Chang X, Wang Z, Zhang J, Yan H, Bian H, **a M, Lin H, et al. Lipid profiling of the therapeutic effects of berberine in patients with nonalcoholic fatty liver disease. J Transl Med. 2016;14:266.
Wu L, **a M, Duan Y, Zhang L, Jiang H, Hu X, Yan H, et al. Berberine promotes the recruitment and activation of brown adipose tissue in mice and humans. Cell Death Dis. 2019;10:468.
Mai W, Xu Y, Xu J, Zhao D, Ye L, Yu G, Wang Z, et al. Berberine inhibits nod-like receptor family pyrin domain containing 3 inflammasome activation and pyroptosis in nonalcoholic steatohepatitis via the ROS/TXNIP Axis. Front Pharmacol. 2020;11:185.
Gulamhusein AF, Hirschfield GM. Primary biliary cholangitis: pathogenesis and therapeutic opportunities. Nat Rev Gastroenterol Hepatol. 2020;17:93–110.
Nemeth J, Stein I, Haag D, Riehl A, Longerich T, Horwitz E, Breuhahn K, et al. S100A8 and S100A9 are novel nuclear factor kappa B target genes during malignant progression of murine and human liver carcinogenesis. Hepatology. 2009;50:1251–62.
Li S, Wang R, Wu B, Wang Y, Song F, Gu Y, Yuan Y. Salvianolic acid B protects against ANIT-induced cholestatic liver injury through regulating bile acid transporters and enzymes, and NF-kappaB/IkappaB and MAPK pathways. Naunyn Schmiedebergs Arch Pharmacol. 2019;392:1169–80.
Guicciardi ME, Trussoni CE, Krishnan A, Bronk SF, Lorenzo Pisarello MJ, O’Hara SP, Splinter PL, et al. Macrophages contribute to the pathogenesis of sclerosing cholangitis in mice. J Hepatol. 2018;69:676–86.
Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–30.
Dara L, Ji C, Kaplowitz N. The contribution of endoplasmic reticulum stress to liver diseases. Hepatology. 2011;53:1752–63.
Fickert P, Wagner M. Biliary bile acids in hepatobiliary injury - What is the link? J Hepatol. 2017;67:619–31.
Wang Y, Gunewardena S, Li F, Matye DJ, Chen C, Chao X, Jung T, et al. An FGF15/19-TFEB regulatory loop controls hepatic cholesterol and bile acid homeostasis. Nat Commun. 2020;11:3612.
Chiang JY. Bile acid metabolism and signaling. Compr Physiol. 2013;3:1191–212.
Ghonem NS, Auclair AM, Hemme CL, Gallucci GM, de la Rosa RR, Boyer JL, Assis DN. Fenofibrate improves liver function and reduces the toxicity of the bile acid pool in patients with primary biliary cholangitis and primary sclerosing cholangitis who are partial responders to ursodiol. Clin Pharmacol Ther. 2020;108:1213–23.
Mousa OY, Juran BD, McCauley BM, Vesterhus MN, Folseraas T, Turgeon CT, Ali AH, et al. Bile acid profiles in primary sclerosing cholangitis and their ability to predict hepatic decompensation. Hepatology. 2020;74(1):281–95.
Sanyal AJ, Ling L, Beuers U, DePaoli AM, Lieu HD, Harrison SA, Hirschfield GM. Potent suppression of hydrophobic bile acids by aldafermin, an FGF19 analogue, across metabolic and cholestatic liver diseases. JHEP Rep. 2021;3: 100255.
Kuipers F, Claudel T, Sturm E, Staels B. The Farnesoid X Receptor (FXR) as modulator of bile acid metabolism. Rev Endocr Metab Disord. 2004;5:319–26.
Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–26.
Wang Y, Aoki H, Yang J, Peng K, Liu R, Li X, Qiang X, et al. The role of sphingosine 1-phosphate receptor 2 in bile-acid-induced cholangiocyte proliferation and cholestasis-induced liver injury in mice. Hepatology. 2017;65:2005–18.
Li X, Liu R, Huang Z, Gurley EC, Wang X, Wang J, He H, et al. Cholangiocyte-derived exosomal long noncoding RNA H19 promotes cholestatic liver injury in mouse and humans. Hepatology. 2018;68:599–615.
Li X, Liu R, Wang Y, Zhu W, Zhao D, Wang X, Yang H, et al. Cholangiocyte-derived exosomal lncRNA H19 promotes macrophage activation and hepatic inflammation under cholestatic conditions. Cells. 2020;9:1.
Liu R, Li X, Zhu W, Wang Y, Zhao D, Wang X, Gurley EC, et al. Cholangiocyte-derived exosomal long noncoding RNA H19 promotes hepatic stellate cell activation and cholestatic liver fibrosis. Hepatology. 2019;70:1317–35.
Chazouilleres O. Novel aspects in the management of cholestatic liver diseases. Dig Dis. 2016;34:340–6.
Zou T, Jaladanki SK, Liu L, **ao L, Chung HK, Wang JY, Xu Y, et al. H19 long noncoding RNA regulates intestinal epithelial barrier function via MicroRNA 675 by interacting with RNA-binding protein HuR. Mol Cell Biol. 2016;36:1332–41.
Yu TX, Chung HK, **ao L, Piao JJ, Lan S, Jaladanki SK, Turner DJ, et al. Long noncoding RNA H19 impairs the intestinal barrier by suppressing autophagy and lowering paneth and goblet cell function. Cell Mol Gastroenterol Hepatol. 2020;9:611–25.
Gu S, Cao B, Sun R, Tang Y, Paletta JL, Wu X, Liu L, et al. A metabolomic and pharmacokinetic study on the mechanism underlying the lipid-lowering effect of orally administered berberine. Mol Biosyst. 2015;11:463–74.
Jones BV, Begley M, Hill C, Gahan CG, Marchesi JR. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc Natl Acad Sci USA. 2008;105:13580–5.
Li Y, Tang R, Leung PSC, Gershwin ME, Ma X. Bile acids and intestinal microbiota in autoimmune cholestatic liver diseases. Autoimmun Rev. 2017;16:885–96.
Acknowledgements
We thank Mrs. Elaine Kennedy for proofreading and English editing. We thank the Cancer Mouse Models Core of VCU Massey Cancer Center for histological service and technical support. We thank Dr. Daniel Goldenberg at the Department of Pathology, Hadassah-Hebrew University Medical Center, Jerusalem, Israel for providing C57/BL6 Mdr2-/- mice. We thank Drs. Li** Zhao at Rutgers Center for Microbiome Analysis Core, New Jersey Institute for Food, Nutrition and Health for microbiome analysis.
Funding
This study was supported by VA Merit Award I01BX004033 and 5I01BX005730; ShEEP grant (1 IS1 BX004777-01); National Institutes of Health Grant R01 DK104893, DK115377, 2R56DK115377-05A1, NIH-NCI Cancer Center Support Grant P30 CA 016059. Dr. Zhou is the recipient of a Research Career Scientist Award from the Department of Veterans Affairs (IK6BX004477).
Author information
Authors and Affiliations
Contributions
YW, WC, XZ and HZ conceived the original ideas, designed the study, analyzed the data, and wrote the manuscript; YW, DZ, LS, YT, GWW, JZ, QY, YX, XW, and ECG carried out the experiments and data analysis. JL, JL, GWW, YX helped with RNAseq data analysis and process; XZ and PBH reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal experiments were performed following institutional guidelines for ethical animal studies and approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee, Virginia, USA.
Consent for publication
All authors reviewed and approved the final manuscript. All authors supported the publication of this manuscript.
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
Impact of BBR on body weight and serum albumin levels in FVB Mdr2-/- mice and cholestatic liver injury in C57/BL6 Mdr2-/- mice. Mdr2-/- mice with FVB background (Control) and Mdr2-/- mice with C57BL/6 background (Control BL) were treated with vehicle or BBR (50 mg/kg) via oral gavage once daily for 8 weeks, respectively. a Body weight change during the BBR treatment period of 8 weeks in FVB Mdr2-/- mice. b Serum albumin levels in FVB Mdr2-/- mice. c Liver functional enzyme levels in C57/BL6 Mdr2-/- mice. d Representative images of hematoxylin and eosin (H&E) staining of the liver slides (scale bar, 50 µm for 20x, 20 µm for 40× magnification) in Mdr2-/- BL mice. Data are expressed as the mean ± standard error of the mean (SEM). Statistical significance relative to Control BL: *p < 0.05 (n=9-12). Fig. S2. Comparative analysis of differentially expressed genes (DEGs) in experimental groups. a Hierarchical clustering heatmaps for DEGs in FVBWT, Mdr2-/- and Mdr2-/- mice treated with BBR. RNA-seq data were normalized using a Z-score for tag counts, with red and blue colors representing high and low gene expression, respectively. b Volcano plots for the Mdr2-/- vs. WT group comparison. Red dots represent upregulated genes, green dots represent downregulated genes, and black dots represent genes not differentially expressed. c Venn diagram illustrating the overlap of DEGs between the two comparisons: Mdr2-/- vs. WT and BBR-treated Mdr2-/- vs. Mdr2-/- Control. In Mdr2-/- vs. WT, there were a total of 1937 DEGs, including 1260 upregulated and 677 down-regulated genes. In BBR-treated Mdr2-/- vs. Mdr2-/- Control, there were a total of 587 DEGs, comprising 300 upregulated and 287 down-regulated genes. A total of 373 DEGs were common between the two comparisons. Fig. S3. Ingenuity pathway analysis (IPA) in experimental groups. The DEG data set with FC ≥2 and p-value <0.05 was used for IPA analysis. The top 10 activated pathways in Mdr2-/- control mice compared to WT mice and the top 10 inhibited pathways in BBR-treated Mdr2-/- mice compared to Mdr2-/- control mice are shown. Fig. S4. Impact of BBR on hepatic fibrosis. a Representative images of liver sections stained with Picro-Sirius Red and CK19 IHC (scale bar, 100 µm for 10× magnification) and processed images for quantification. b Hepatic hydroxyproline levels. Data are expressed as the mean ± SEM. Statistical significance relative to control: *p < 0.05 (n=9-12). Fig. S5. Impact of BBR on genes associated with hepatic fibrosis in Mdr2-/- mice. a Representative heatmap of key genes involved in hepatic fibrosis in the liver, comparing the BBR-treated group with the control group. The RNA-seq data were normalized using a Z-score for tag counts, with red and blue colors denoting up- and down-regulated gene expression, respectively. b Relative mRNA expression levels of fibrosis-related genes (Pai1,Col12a1, Sox9, Egr1, Egr2, Egr3, Hbegf, Cyr61, and P4ha1), normalized against HPRT1 as an internal control. Data are expressed as the mean ± SEM. Statistical significance relative to control: *p < 0.05, **p < 0.01, ***p < 0.001(n=9-12). Fig. S6. Impact of BBR on genes associated with inflammation in Mdr2-/- mice. Representative heatmap depicting the expression of key genes involved in hepatic inflammation, comparing the liver tissues of Mdr2-/- mice treated with BBR to the control group. The RNA-seq data were normalized using a Z-score, with red indicating upregulated gene expression and blue indicating downregulated gene expression. Fig. S7. Impact of BBR on NF-kB signaling pathway. KEGG pathway analysis was performed on RNA-seq data to analyze functionally and map genes involved in the NF-kB signaling pathway. a NF-kB signaling pathway in Mdr2-/- vs. WT. b NF-kB signaling pathway in Mdr2-/- treated with BBR vs. Mdr2-/- Control. Red and green colors indicate upregulated and downregulated gene expression, respectively. Fig. S8. Impact of BBR on MAPK signaling pathway. KEGG pathway analysis was performed on RNA-seq data to analyze functionally and map genes involved in the MAPK signaling pathway. a MAPK signaling pathway in Mdr2-/- vs. WT. b MAPK signaling pathway in Mdr2-/- treated with BBR vs. Mdr2-/- Control. Red and green colors indicate upregulated and downregulated gene expression, respectively. Fig. S9. Impact of BBR on Oxidative phosphorylation pathway. KEGG pathway analysis was performed on RNA-seq data to analyze functionally and map genes involved in the Oxidative phosphorylation pathway. a Oxidative phosphorylation pathway in Mdr2-/- vs. WT. b Oxidative phosphorylation pathway in Mdr2-/- treated with BBR vs. Mdr2-/- Control. Red and green colors indicate up- and down-regulated gene expression, respectively. Fig. S10. Impact of BBR on Protein processing in endoplasmic reticulum. RNA-seq data were performed to analyze functionally and map genes involved in the Protein processing in the endoplasmic reticulum pathway using KEGG. a Protein processing in endoplasmic reticulum pathway in Mdr2-/- Control vs. WT. b Protein processing in endoplasmic reticulum pathway in Mdr2-/- treated with BBR vs. Mdr2-/- Control. Red and green colors indicate up- and down-regulated gene expression, respectively. Fig. S11. Impact of BBR on BA Metabolism. Representative heatmap of key genes involved in bile acid metabolism in the liver of BBR-treated vs. Control Mdr2-/- mice. A Z-score is calculated for the RNA-seq data to normalize tag counts. Red and blue colors indicate up- and down-regulated gene expression, respectively. Fig. S12. Impact of BBR on bile acid homeostasis in Mdr2-/- mice. The small intestine and feces were processed for BA analysis using LC-MS/MS. a BA composition profile in the small intestine is expressed as a percentage of total BA. b Total BA, total primary BA, total conjugated BA, and TCA in the small intestine. c BA composition profile in the feces is expressed as a percentage of total BA. d Total BA, total secondary BA, TCA, and LCA in the feces. Data are expressed as the mean ± SEM. Statistical significance relative to control: *p < 0.05 (n=9-12). Fig. S13. Effect of BBR on inflammation and ER stress in the intestine of Mdr2-/- mice. Relative mRNA levels of key genes involved in inflammation and ER stress in the intestine were determined by real-time RT–PCR and normalized with HPRT1 as an internal control. a The relative mRNA levels of Mcp-1, Cd11b, Il-1β, Vcam-1, Il-1α, and Cxcl1. b Relative mRNA levels of Asbt, Chop and H19. Data are expressed as the mean ± SEM. Statistical significance relative to Control: *p < 0.05 (n=9-12). Fig. S14. Tissue distribution of BBR in Mdr2-/- mice. Mdr2-/- mice were treated with BBR (50 mg/kg, n = 3) by intragastric administration after a 12-h fast. Blood, spleen, brain, lung, heart, kidney, liver, stomach contents, intestine contents, and colon feces were collected at 3, 6, and 9 h post-treatment. The concentrations of BBR in the serum and various tissues were quantified using LC-MS/MS. a BBR concentration in the serum. b BBR concentration in the tissues. Data are expressed as the mean ± standard error of the mean (SEM). Fig. S15. Analysis of Fecal Microbiota Diversity in Mdr2-/- Mice Treated with BBR. Fecal samples of FVB Mdr2-/- mice treated with either 50 mg/kg or 100 mg/kg of BBR for 8 weeks were subjected to 16S rRNA gene sequencing to assess microbiota composition. a Alpha diversity of the fecal microbiota, presented through various metrics: Shannon Index (a), observed Amplicon Sequence Variants (ASVs) (b), Faith’s phylogenetic diversity (c), and evenness (d). b Beta diversity analysis using Principal Coordinates Analysis (PCoA) plots, which illustrate variations in microbial communities. These plots are based on different distance metrics: Bray-Curtis distance (a), Jaccard distance (b), Weighted UniFrac distance (c), and Unweighted UniFrac distance (d), with each plot depicting variations along two principal coordinates that account for most of the variation. Fig. S16. Influence of BBR on the Proportions of Firmicutes and Bacteroidetes in the Gut Microbiota of Mdr2-/- mice. The pie chart shows the relative percentages of the Firmicutes and Bacteroidetes phyla in the gut microbiota of Mdr2-/- mice. Comparative analysis is shown across three groups: control, BBR-treated at 50 mg/kg, and BBR-treated at 100 mg/kg. This visualization highlights the specific shifts in these major bacterial phyla due to BBR treatment.
Additional file 2.
Supplement tables with captions.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, Y., Zhao, D., Su, L. et al. Therapeutic potential of berberine in attenuating cholestatic liver injury: insights from a PSC mouse model. Cell Biosci 14, 14 (2024). https://doi.org/10.1186/s13578-024-01195-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13578-024-01195-8