Abstract
Background
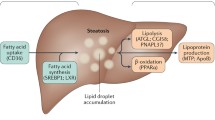
In the past 30 years, incidences of non-alcoholic fatty liver disease (NAFLD) has risen by 30%. However, there is still no clear mechanism or accurate method of anticipating liver failure. Here we reveal the phase transitions of liquid crystalline qualities in hepatic lipid droplets (HLDs) as a novel method of anticipating prognosis.
Methods
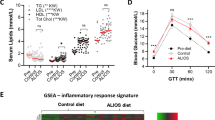
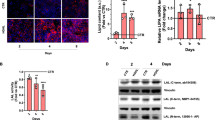
NAFLD was induced by feeding C57BL/6J mice on a high-fat (HiF) diet. These NAFLD livers were then evaluated under polarized microscopy, X-ray diffraction and small-angle scattering, lipid component chromatography analysis and protein expression analysis. Optically active HLDs from mouse model and patient samples were both then confirmed to have liquid crystal characteristics. Liver MAP1LC3A expression was then evaluated to determine the role of autophagy in liquid crystal HLD (LC-HLD) formation.
Results
Unlike the normal diet cohort, HiF diet mice developed NAFLD livers containing HLDs exhibiting Maltese cross birefringence, phase transition, and fluidity signature to liquid crystals. These LC-HLDs transitioned to anisotropic crystal at 0 °C and remain crystalline. Temperature increase to 42 °C causes both liquid crystal and crystal HLDs to convert to isotropic droplet form. These isotropic HLDs successfully transition to anisotropic LC with fast temperature decrease and anisotropic crystal with slow temperature decrease. These findings were duplicated in patient liver. Patient LC-HLDs with no inner optical activity were discovered, hinting at lipid saturation as the mechanism through which HLD acquire LC characteristics. Downregulation of MAP1LC3A in conjunction with increased LC-HLD also implicated autophagy in NAFLD LC-HLD formation.
Conclusions
Increasing concentrations of amphiphilic lipids in HLDs favors organization into alternating hydrophilic and hydrophobic layers, which present as LC-HLDs. Thus, evaluating the extent of liquid crystallization with phase transition in HLDs of NAFLD patients may reveal disease severity and predict impending liver damage.
Similar content being viewed by others
Background
Non-alcoholic fatty liver disease (NAFLD) is a disorder of excessive fat accumulation (steatosis) in the liver of those who consume little to no alcohol [1, 2]. With the increase of rich foods in the diets of Western counties and develo** countries around the world, NAFLD has become a rising cause of hepatic steatosis since its first reported in 1983 [3,4,5]. Up to 25% of adults in the United States has NAFLD, with more than 80% of the obese population affected by the disease. Because there are often no initial symptoms for patients with NAFLD and the disease can only be treated by addressing the underlying condition, over time NAFLD patients often progress to severe liver fibrosis and cirrhosis [6, 7]. Because of this difficulty in treatment, liver disease is becoming one of the top leading causes of death in societies where rich diets are common. Liver disease is becoming an increasing problem in develo** countries as well. According to the Asia–Pacific Working Party guidelines on NAFLD, over-nutrition has increased the prevalence of NAFLD in the Asia Pacific regions from 23.3 to 31.9% in the past 30 years [8]. Due to increasing childhood obesity, NAFLD has also become the primary form of liver disease in both children and adolescents [5, 9, 10].
Only a few genetic variations and environmental factors have been contributed to the development of the complex metabolic associated syndrome that is NAFLD. Two variants of the triacylglycerol lipase gene PNPLA3 have been associated liver disease, one linked to severity of hepatic steatosis while the other affects hepatic triglyceride content by association with TM6SF2, a regulator of hepatic fat metabolism [14]. We have also previously reported a novel association of NG37 with high fat diet induced NAFLD [15]. Our data demonstrated that overexpression of NG37 in a transgenic mouse model results in liver enlargement and cardiac dysfunction in a high-fat diet dependent matter. This model provided a direct link between genetic predisposition and nutritional factors in hepatic lipid deposition [15].
Until now, the specific genetic causes of NAFLD were unclear, making it difficult to identify patients at high risk for severe NAFLD that is likely to progress to steatohepatitis, fibrosis, and cirrhosis. Recently, some researches have claimed that hepatic cholesterol crystals and crown-like structures distinguish Nonalcoholic steatohepatitis (NASH), an advanced subtype of NAFLD, from simple steatosis in humans [16]. These cholesterol crystal structures can also be detected in the fatty livers of thyroidectomized chickens and a murine NASH model [17, 18]. In our NG37 murine model, we occasionally found lipid droplets (LD) as optically active anisotropic liquid crystals (LC) in the hepatocytes of NAFLD mice. These anisotropic liquid crystal hepatic lipid droplets (LC-HLD) can transform into isotropic LD under the right condition. Both these LC-HLDs and isotropic HLDs could also transition into crystal structures. Based on increasing investigations on unveiling molecular mechanism of diseases with phase transition and separation [19,20,21], it is certain that the liquid crystalline could be important clue of NAFLD genesis. This phenomenon of LC to crystal transition in vitro indicates that the buildup of lipids inside hepatocytes from overnutrition could be being stored as liquid crystals. Thus we hypothesized that the crystal and crown-like structures previously identified in human hepatocytes of NASH patients are likely the final form of LC-HLD after further over-accumulation of cholesterols [16, 17, 21].
Materials and methods
Human subjects and animals maintenance
All animal care and experiment procedures were conducted in accordance with protocol approved by the Animal Care and Use Committee of Shaanxi Normal University. All human studies were conducted according to the principles of the Declaration of Helsinki, and the study protocol were approved by the Institutional Ethics Committee of the Shaanxi Normal University with written informed consents from Alenabio (** steatohepatitis, fibrosis, and cirrhosis.
In lipid-rich conditions, the apo-CIII protein is thought to function as a promotor of hepatocellular triglyceride uptake and triglyceride-rich VLDL assembly [42,43,44]. We hypothesize that during HiF diet over-nutrition, apo-CIII are distributed on the surface of liquid crystal HLD as they are on the surface of VLDL particles. From these surface locations, apo-CIII could collect and assimilates cholesterol and cholesterol derivatives into the HLD on which it resides. This gives the gene a critical role in lipid accumulation within LC-HLDs. And supports the strong association of promoter region polymorphism in apolipoprotein C3 (APOC3) (rs2854117 [− 482C>T] and rs2854116 [− 455T > C]) found to be strongly associated with NAFLD and its associated diseases, hypertriglyceridemia, metabolic syndrome, and coronary artery disease [45,46,47,48]. Thus, polymorphisms in the APOC3 gene resulting in greater than normal incorporation of cholesteric lipids into LC-HLDs would put the individual at greater risk of over saturating the HLDs liquid crystal buffering system and transitioning the HLD into its damaging crystalline form.
In addition to the APOC3 polymorphism described above, variations of PNPLA3 and TM6SF2 have also been linked to severe hepatic steatosis. Although the results are inconclusive, these genes are thought to contribute the disease by affecting hepatic triglyceride metabolism [11, 12]. Our previous study showing that liver-specific overexpression of NG37 induced fatty liver disease in a high fat diet dependent manner also put this new member of the von Willebrand A (vWA) super family on the map of metabolic diseases [15]. These high fat diet liver-specific overexpression of NG37 mice developed rapid hepatocellular liquid crystalline lipid accumulation that was greater than both high fat-diet wild type litter mates and normal diet NG37 mutant mice. In addition to liver enlargement and steatosis, these mice also developed cardiac arrhythmias commonly seen in NAFLD patients. This evidence clearly points at the importance of NG37 in hepatocellular lipid metabolism and NAFLD. Further studies are currently investigating the links between metabolism syndromes, cardiac function, and NG37. In this study, we found that MAP1LC3A a marker of lipophagy, lipid specific autophagy, is dramatically down-regulated in over-nutrition induced NAFLD liquid crystal hepatic lipid droplet accumulation. As exercise can improve lipid over accumulation by increasing lipophagy [49, 50], the next step is to investigate whether exercise can reactivate MAP1LC3A associated autophagy. Understanding the relationship between liquid crystal hepatic lipid droplets and the MAP1LC3A autophagy could provide a unique prospective towards understanding, preventing, and treating NAFLD.
Conclusions
Characterization of liquid crystal hepatic lipid droplets in mouse and patient NAFLD samples is a novel mechanism based on phase transition for evaluating the level of hepatic steatosis. Our discovery of hepatic lipid droplets with no core optical birefringence hints at the role cholesterol saturation plays in liquid crystal hepatic lipid droplet formation. By the laws of physics, as concentration of amphiphilic molecules increase it become energetically sensible to shift from a chaotic core of amphiphilic molecules protected by a layer of external facing hydrophilic molecules into a solid sphere of alternating hydrophilic and hydrophobic layers. This finding indicates that studying the degree of birefringence in patient hepatic lipid droplets may reveal disease severity long before patients become symptomatic. The greater the cholesterol content, the more thorough the droplet birefringence, and the closer a droplet comes to reaching full lipid saturation, at which point the liquid crystal droplet becomes crystalline. These less malleable droplets then cause hepatic damage, leading to steatohepatitis, fibrosis, and cirrhosis. Thus, identifying the degree of hepatic lipid droplet birefringence could be a novel diagnostic for NAFLD patients.
Availability of data and materials
The data set supporting the conclusions of this article are included within the article.
Abbreviations
- ani-CRSYT:
-
Anisotropic crystal
- ani-LC:
-
Anisotropic liquid crystal
- APOC3:
-
Apolipoprotein C3
- FBA:
-
Full birefringent activity
- FEDXT:
-
Formaldehyde fixation, ethanol gradient dehydration, and xylene for transparent
- GL:
-
Glycophospholipid or glycosphingolipid
- H&E:
-
Hematoxylin and Eosin
- HiF:
-
High fat
- HIFU:
-
High intensity focused ultrasound
- HLD:
-
Hepatic lipid droplets
- INA:
-
Inner optical activity
- iso-HLD:
-
Isotropic lipid droplet
- LC-HLD:
-
Liquid crystal hepatic lipid droplets
- LD:
-
Lipid droplets
- MAP1LC3A:
-
Microtubule-associated protein light chain 3 A
- MRE:
-
Magnetic resonance elastography
- NAFLD:
-
Non-alcoholic fatty liver disease
- NASH:
-
Nonalcoholic steatohepatitis
- P&R:
-
Pressure-and-release procedure
- PNPLA3:
-
Patatin like phospholipase domain containing 3
- TLC:
-
Thin-layer chromatography
- TM6SF2:
-
Transmembrane 6 superfamily 2
- vWA:
-
von Willebrand A
- XRD:
-
X-ray diffraction
References
Shaker M, Tabbaa A, Albeldawi M, Alkhouri N. Liver transplantation for nonalcoholic fatty liver disease: new challenges and new opportunities. World J Gastroenterol. 2014;20(18):5320–30. https://doi.org/10.3748/wjg.v20.i18.5320.
Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313(22):2263–73. https://doi.org/10.1001/jama.2015.5370.
Yen CH, Wang KT, Lee PY, Liu CC, Hsieh YC, Kuo JY, et al. Gender-differences in the associations between circulating creatine kinase, blood pressure, body mass and non-alcoholic fatty liver disease in asymptomatic asians. PLoS One. 2017;12(6):e0179898. https://doi.org/10.1371/journal.pone.0179898.
Zhang Q, Wong CKH, Kung K, Chan JCY, Sy BTW, Lam M, et al. Development and validation study of a non-alcoholic fatty liver disease risk scoring model among adults in China. Fam Pract. 2017;34(6):667–72. https://doi.org/10.1093/fampra/cmx049.
Moran JR, Ghishan FK, Halter SA, Greene HL. Steatohepatitis in obese children: a cause of chronic liver dysfunction. Am J Gastroenterol. 1983;78(6):374–7.
Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55(6):2005–23. https://doi.org/10.1002/hep.25762.
Clark JM, Diehl AM. Nonalcoholic fatty liver disease: an underrecognized cause of cryptogenic cirrhosis. JAMA. 2003;289(22):3000–4. https://doi.org/10.1001/jama.289.22.3000.
Wang F-S, Fan J-G, Zhang Z, Gao B, Wang H-Y. The global burden of liver disease: the major impact of China. Hepatology. 2014;60(6):2099–108. https://doi.org/10.1002/hep.27406.
Wong VW-S, Chan W-K, Chitturi S, Chawla Y, Dan YY, Duseja A, et al. The Asia-Pacific working party on nonalcoholic fatty liver disease guidelines 2017 part 1: definition, risk factors and assessment. J Gastroenterol Hepatol. 2018;33(1):70–85. https://doi.org/10.1111/jgh.13857.
Anderson EL, Howe LD, Jones HE, Higgins JPT, Lawlor DA, Fraser A. The prevalence of non-alcoholic fatty liver disease in children and adolescents: a systematic review and meta-analysis. PLoS One. 2015;10(10):e0140908. https://doi.org/10.1371/journal.pone.0140908.
Romeo S, Kozlitina J, **ng C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40(12):1461–5. https://doi.org/10.1038/ng.257.
Kozlitina J, Smagris E, Stender S, Nordestgaard BG, Zhou HH, Tybjaerg-Hansen A, et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2014;46(4):352–6. https://doi.org/10.1038/ng.2901.
Petersen KF, Dufour S, Hariri A, Nelson-Williams C, Foo JN, Zhang XM, et al. Apolipoprotein C3 gene variants in nonalcoholic fatty liver disease. N Engl J Med. 2010;362(12):1082–9. https://doi.org/10.1056/NEJMoa0907295.
Zhang H, Chen L, ** non-alcoholic fatty liver disease: a meta-analysis. Hepatitis Mon. 2014;14(10):e23100. https://doi.org/10.5812/hepatmon.23100.
Zhou X, Xu M, Wang L, Mu Y, Feng R, Dong Z, et al. Liver-specific NG37 overexpression leads to diet-dependent fatty liver disease accompanied by cardiac dysfunction. Genes Nutr. 2016;11:1–10. https://doi.org/10.1186/s12263-016-0529-z.
Ioannou GN, Haigh WG, Thorning D, Savard C. Hepatic cholesterol crystals and crown-like structures distinguish NASH from simple steatosis. J Lipid Res. 2013;54(5):1326–34. https://doi.org/10.1194/jlr.M034876.
Fukuda Y, Sone T, Sakuraba H, Araki T, Ohshima T, Shibata T, et al. A novel NAD(P)H-dependent carbonyl reductase specifically expressed in the thyroidectomized chicken fatty liver: catalytic properties and crystal structure. FEBS J. 2015;282(20):3918–28. https://doi.org/10.1111/febs.13385.
Li P, Banjade S, Cheng HC, Kim S, Chen B, Guo L, et al. Phase transitions in the assembly of multivalent signalling proteins. Nature. 2012;483(7389):336–40. https://doi.org/10.1038/nature10879.
Beutel O, Maraspini R, Pombo-Garcia K, Martin-Lemaitre C, Honigmann A. Phase separation of zonula occludens proteins drives formation of tight junctions. Cell. 2019;179(4):923–936.e11. https://doi.org/10.1016/j.cell.2019.10.011.
Hofweber M, Hutten S, Bourgeois B, Spreitzer E, Niedner-Boblenz A, Schifferer M, et al. Phase separation of FUS is suppressed by its nuclear import receptor and arginine methylation. Cell. 2018;173(3):706–719.e13. https://doi.org/10.1016/j.cell.2018.03.004.
Ioannou GN, Subramanian S, Chait A, Haigh WG, Yeh MM, Farrell GC, et al. Cholesterol crystallization within hepatocyte lipid droplets and its role in murine NASH. J Lipid Res. 2017;58(6):1067–79. https://doi.org/10.1194/jlr.M072454.
Sundaresan S, Vijayagopal P, Mills N, Imrhan V, Prasad C. A mouse model for nonalcoholic steatohepatitis. J Nutr Biochem. 2011;22(10):979–84. https://doi.org/10.1016/j.jnutbio.2010.08.011.
Ling G, Wang L, Rui F, Li Z, Wang J, Ren K, et al. Transportation of liquid crystal and CaCO3 vaterite crystal in chicken embryo and early postnatal development. Mol Cryst Liq Cryst. 2017;647(1):373–84. https://doi.org/10.1080/15421406.2017.1289652.
Xu M, Xu X. Liquid-crystal in embryogenesis and pathogenesis of human diseases. In: Sato K, editor. Embryogenesis. Rijeka: InTech; 2012. p. 637–52.
Xu X, Dong C, Vogel BE. Hemicentins assemble on diverse epithelia in the mouse. J Histochem Cytochem. 2007;55(2):119–26. https://doi.org/10.1369/jhc.6A6975.2006.
Xu XH, Xu MM, Jones OD, Chen XZ, Li YF, Yan GF, et al. Liquid crystal in lung development and chicken embryogenesis. Mol Cryst Liq Cryst. 2011;547:164–72. https://doi.org/10.1080/15421406.2011.572042.
Small DM. George Lyman Duff memorial lecture. Progression and regression of atherosclerotic lesions. Insights from lipid physical biochemistry. Arteriosclerosis. 1988;8(2):103–29. https://doi.org/10.1161/01.atv.8.2.103.
Goldstein JL, Brown MS. The clinical investigator: bewitched, bothered, and bewildered—but still beloved. J Clin Invest. 1997;99(12):2803–12. https://doi.org/10.1172/JCI119470.
Xu MM, Jones OD, Wang L, Zhou X, Davis HG, Bryant JL, et al. Characterization of tubular liquid crystal structure in embryonic stem cell derived embryoid bodies. Cell Biosci. 2017;7:3. https://doi.org/10.1186/s13578-016-0130-6.
Liu K, Czaja MJ. Regulation of lipid stores and metabolism by lipophagy. Cell Death Differ. 2013;20(1):3–11. https://doi.org/10.1038/cdd.2012.63.
Xu MM, Xu XH, Cao GL, Pan YX, Jones O, Bryant JL, et al. The liquid crystalline in normal renal development amplifies the comprehension for anderson-fabry disease. Mol Cryst Liq Cryst. 2009;508:52–66. https://doi.org/10.1080/15421400903058437.
Haimovici R, Gantz DL, Rumelt S, Freddo TF, Small DM. The lipid composition of drusen, Bruch’s membrane, and sclera by hot stage polarizing light microscopy. Invest Ophthalmol Vis Sci. 2001;42(7):1592–9.
Lang PD, Insull W Jr. Lipid droplets in atherosclerotic fatty streaks of human aorta. J Clin Invest. 1970;49(8):1479–88. https://doi.org/10.1172/JCI106365.
Kruth HS. Lipoprotein cholesterol and atherosclerosis. Curr Mol Med. 2001;1(6):633–53. https://doi.org/10.2174/1566524013363212.
Brown MS, Faust JR, Goldstein JL. Role of the low density lipoprotein receptor in regulating the content of free and esterified cholesterol in human fibroblasts. J Clin Invest. 1975;55(4):783–93. https://doi.org/10.1172/JCI107989.
Goldstein JL, Anderson RG, Brown MS. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979;279(5715):679–85. https://doi.org/10.1038/279679a0.
Ballestri S, Romagnoli D, Nascimbeni F, Francica G, Lonardo A. Role of ultrasound in the diagnosis and treatment of nonalcoholic fatty liver disease and its complications. Expert Rev Gastroenterol Hepatol. 2015;9(5):603–27. https://doi.org/10.1586/17474124.2015.1007955.
Schwimmer JB, Behling C, Angeles JE, Paiz M, Durelle J, Africa J, et al. Magnetic resonance elastography measured shear stiffness as a biomarker of fibrosis in pediatric nonalcoholic fatty liver disease. Hepatology. 2017;66(5):1474–85. https://doi.org/10.1002/hep.29241.
Singh S, Muir AJ, Dieterich DT, Falck-Ytter YT. American Gastroenterological Association Institute Technical Review on the Role of Elastography in Chronic Liver Diseases. Gastroenterology. 2017;152(6):1544–77. https://doi.org/10.1053/j.gastro.2017.03.016.
Papandreou D, Rousso I, Mavromichalis I. Update on non-alcoholic fatty liver disease in children. Clin Nutr. 2007;26(4):409–15. https://doi.org/10.1016/j.clnu.2007.02.002.
Mansoor S, Collyer E, Alkhouri N. A comprehensive review of noninvasive liver fibrosis tests in pediatric nonalcoholic Fatty liver disease. Curr Gastroenterol Rep. 2015;17(6):23. https://doi.org/10.1007/s11894-015-0447-z.
Mendivil CO, Zheng C, Furtado J, Lel J, Sacks FM. Metabolism of very-low-density lipoprotein and low-density lipoprotein containing apolipoprotein C-III and not other small apolipoproteins. Arterioscler Thromb Vasc Biol. 2010;30(2):239. https://doi.org/10.1161/ATVBAHA.109.197830.
Sundaram M, Zhong S, Bou Khalil M, Links PH, Zhao Y, Iqbal J, et al. Expression of apolipoprotein C-III in McA-RH7777 cells enhances VLDL assembly and secretion under lipid-rich conditions. J Lipid Res. 2010;51(1):150–61. https://doi.org/10.1194/M900346-JLR200.
Qin W, Sundaram M, Wang Y, Zhou H, Zhong S, Chang CC, et al. Missense mutation in APOC3 within the C-terminal lipid binding domain of human ApoC-III results in impaired assembly and secretion of triacylglycerol-rich very low density lipoproteins: evidence that ApoC-III plays a major role in the formation of lipid precursors within the microsomal lumen. J Biol Chem. 2011;286(31):27769–80. https://doi.org/10.1074/jbc.M110.203679.
Cao Y, Kole A, Lan L, Wang P, Hui J, Sturek M, et al. Spectral analysis assisted photoacoustic imaging for lipid composition differentiation. Photoacoustics. 2017;7:12-9. https://doi.org/10.1016/j.pacs.2017.05.002.
Miller M, Rhyne J, Chen H, Beach V, Ericson R, Luthra K, et al. APOC3 promoter polymorphisms C-482T and T-455C are associated with the metabolic syndrome. Arch Med Res. 2007;38(4):444–51. https://doi.org/10.1016/j.arcmed.2006.10.013.
Petersen KF, Dufour S, Feng J, Befroy D, Dziura J, Dalla Man C, et al. Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian-Indian men. Proc Natl Acad Sci USA. 2006;103(48):18273–7. https://doi.org/10.1073/pnas.0608537103.
Kypreos KE. ABCA1 promotes the de novo biogenesis of apolipoprotein CIII-containing HDL particles in vivo and modulates the severity of apolipoprotein CIII-induced hypertriglyceridemia. Biochemistry. 2008;47(39):10491–502. https://doi.org/10.1021/bi801249c.
Flores-Toro JA, Go KL, Leeuwenburgh C, Kim JS. Autophagy in the liver: cell's cannibalism and beyond. Arch Pharm Res. 2016;39(8):1050–61. https://doi.org/10.1007/s12272-016-0807-8.
Chun SK, Lee S, Yang MJ, Leeuwenburgh C, Kim JS. Exercise-Induced Autophagy in Fatty Liver Disease. Exerc Sport Sci Rev. 2017;45(3):181–6. https://doi.org/10.1249/JES.0000000000000116.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. #31771377/31571273/31371256), the Foreign Distinguished Scientist Program (Grant No. MS2014SXSF038), the National Department of Education Central Universities Research Fund (Grant No. GK20130100 and 201701005), US Maryland Stem Cell Research Fund (2009MSCRFE008300), Advanced Cell Biology for Graduated study (Grant No. #GERP-17-45/2019TS079/2019TS082).
Author information
Authors and Affiliations
Contributions
XHX conceived of the study. LYW, MMX and ZGL developed protocols and collected all data and analyzed the data. ZGL, OJ, YL, QY, JLL, YW, XL, BH, HY, LX, RZ, WZ and XZ and partially involved in collection and analysis of the data. XXH, LYW, MMX, OJ, JB, YL and JM prepared the manuscript and all authors edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All experiments of human samples described in this study were approved by the Medical Ethics Committee of Shaanxi Normal University (**’an, Shaanxi Province, China) and was carried out in accordance with the Helsinki Declaration. The informed consent of patients were obtained according to standard procedure prior to study. Information of patients were guaranteed adequately in this study.
Consent for publication
Not applicable.
Competing interests
The submitted work were currently applying for patents relating to the content of the manuscript. The authors declare no other competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, L., Xu, M., Jones, O.D. et al. Nonalcoholic fatty liver disease experiences accumulation of hepatic liquid crystal associated with increasing lipophagy. Cell Biosci 10, 55 (2020). https://doi.org/10.1186/s13578-020-00414-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13578-020-00414-2