Abstract
Background
Ball mill is an effective, and green method for the synthesis of heterocyclic compounds in very good yields. This method is a simple, economical, and environmentally friendly process. In this work, an efficient approach for the synthesis of pyranopyrazoles (PPzs) using ball milling and metal-free nano-catalyst (Nano-silica/aminoethylpiperazine), under solvent-free conditions was reported.
Results
The new nano-catalyst silica/aminoethylpiperazine was prepared by immobilization of 1-(2-aminoethyl)piperazine on nano-silica chloride. The structure of the prepared nano-catalyst was identified by FT-IR, FESEM, TGA, EDX, EDS-map, XRD, and pH techniques. This novel nano-catalyst was used for the synthesis of dihydropyrano[2,3-c]pyrazole derivatives under ball milling and solvent-free conditions.
Conclusions
Unlike other pyranopyrazoles synthesis reactions, this method has advantages including short reaction time (5–20 min), room temperature, and relatively high efficiency, which makes this protocol very attractive for the synthesis of pyranopyrazoles derivatives.
Similar content being viewed by others
Introduction
Recent advances in chemistry have led to its increasing use of it in various fields of life and industry. In addition to this comfort and well-being, unfortunately, the excessive use of toxic and dangerous substances has caused serious and irreparable damage to the environment and humans. Since the relationship between chemical knowledge and human life is a two-way relationship, the appropriate solution to reduce the risks along with enjoying the benefits of chemistry is the use of green chemistry [1]. Environmental protection has become a very important and popular topic for organic chemists in recent decades. That's why scientists are designing reactions by following green chemistry. Except for green chemistry methods, many of the methods reported for the synthesis of the heterocyclic compound often need hard experimental conditions or have poor yields and by-products [2].
The ball milling method is new and environmentally friendly and is a mechanicochemical method that has recently become very popular and is considered by organic chemists for the synthesis of various organic materials [3]. The advantages of the mechanicochemical methods are that the reactions can be performed under solvent-free conditions, in a short time, and with pure products [4]. In addition, ball milling methods have different applications in medicinal and pharmaceutical chemistry [5, 6], it is also widely used in the polymerization reaction by mediating ball milling [7, 8], multi-component organic synthesis [9,10,11], including gas reagents [12], carbon materials [13,14,15], and the preparation of crystals [16, 17].
Pyranopyrazole (PPz) is a fused heterocyclic framework comprising pyran and pyrazole moieties [18,19,20,21]. PPzs have shown important roles in the field of medicinal and pharmaceutical chemistry [18] including antimicrobial [22], anti-cancer [23], and anti-fungal [24], and many drugs containing sulfaphenazole (antibacterial) [25], celecoxib (anti-inflammatory) [26, 27], rimonabant (antiobesity) [28], and mepiprazole (antidepressant) [29] are derived from pyrazole core [30, 31]. Several catalysts have been applied for the synthesis of PPz such as Nano-AlPO4/Ti(IV) [32], nano-eggshell/Ti(IV) [33], Fe3O4@SiO2@(CH2)3NH@CC@Imidazole@SO3H [34], AC-SO3H/[Choline-Cl][Urea]2 [35], PAN@melamine/Fe3O4 [36] isonicotinic acid [37], nano‐Fe‐ [phenylsalicylaldiminemethylpyranopyrazole]Cl2 [38], CaO@SiO2-SO3H [39], IRMOF-3/GO/CuFe2O4 [40], [(EMIM)Ac)] [41], RuIII@CMC/Fe3O4 [42], PS-DABCO [43], Fe3O4@rGO-NH [44], and NiFe2O4@SiO2-H14[NaP5W30O110] [45].
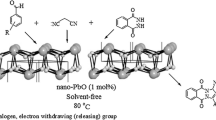
In this work, we wish to report an efficient method for the synthesis of PPz using nano-silica/aminoethylpiperazine (Nano-SiO2/AEP) under solvent-free ball milling conditions (Fig. 1). The heterogeneous nano-catalyst was identified by the FT-IR, FESEM, TGA, EDX, EDS map**, XRD, and pH techniques.
Results and discussion
To prepare the Nano-SiO2/AEP nano-catalyst, first, a mixture of nano-silica gel and thionyl chloride was refluxed for 48 h to prepare the nano-silica chloride. In the next step, the dried silica-chloride was reacted with 2-aminoethylpiperazine (AEP) in DMF at 80 °C (Fig. 2).
FT-IR of nano-SiO2/AEP
According to the FT-IR 2-AEP (Fig. 3a), the peaks at 2804 cm−1 and 2936 cm−1 are due to the symmetric and asymmetric stretching vibration of the CH2 group, respectively, and the peak at 1594 cm−1 is related to N–H bending vibration. The broad peak at the range of 3200–3400 cm−1 is related to N–H stretching vibration. In the spectrum of nano-silica chloride (Fig. 3b), two bands at 806 cm−1 and 1055 cm−1 are assigned to the stretching and bending vibration of the Si–O–Si, respectively. After immobilization of AEP on nano-silica chloride (Fig. 3c), the peak at 1452 cm−1 is assigned to the stretching vibration of the C-N. All of these observations indicate that the Nano-SiO2/AEP heterogeneous catalyst has been successfully prepared.
XRD of nano-SiO2/AEP
XRD analysis of the Nano-SiO2/AEP spectrum is shown in Fig. 4. A broad peak was observed at 2θ = 23–30° which proved that the catalyst is predominantly in the amorphous form. This is in agreement with the literature [46].
Thermal gravimetric analysis (TGA) of nano-SiO2/AEP
The thermal stability of the Nano-SiO2/AEP nano-catalyst was determined by TGA and DTA (Fig. 5) in the temperature range (50–800 °C). The DTA curve shows the endothermic processes in the temperature range of 50–100 °C and 150–350 °C. According to the TGA curve, two stages of weight loss occurred. The first weight loss (less than 100 °C) can be related to the loss of surface water and other solvents on the catalyst surface. The second weight loss of 150–390 °C can be attributed to the destruction of the organic part of the catalyst.
FESEM of heterogeneous nano-SiO2/AEP
The morphological properties and size of Nano-SiO2/AEP nano-catalysts were investigated by FESEM and the results are compiled in Fig. 6. The results show that the Nano-SiO2/AEP is composed of a nanoparticle scale that shows a quasi-spherical morphology with diameters in the range of 16–27 nm.
EDS-map of nano-SiO2/AEP and energy dispersive X-ray (EDX)
The EDX spectrum of Nano-SiO2/AEP (Fig. 7a), shows the presence of the elements O, Si, C, and N with the corresponding weight percentages (47.70, 21.68, 16.56, 10.99%). The elemental map** of the Nano-SiO2/AEP nano-catalyst is shown in Fig. 7b. According to obtained textures, the elements are homogeneously distributed within the nano-catalyst.
To confirm the basic property of nano-catalyst Nano-SiO2/AEP, 0.04 g of it was added to 20 mL of deionized water (pH = 7) and stirred for 1 h at room temperature. Then, the pH of the obtained mixture is 8.62.
After the characterization of the basic nano-catalyst Nano-SiO2/AEP, it was used for the synthesis of pyranopyrazoles. To optimize the reaction conditions, 4-nitrobenzaldehyde, hydrazine hydrate, ethylacetoacetate, and malononitrile were performed under various conditions such as the catalyst amount, temperature, and solvent (Table 1). Meanwhile, the model reaction was done in a stainless steel vial and milled with two stainless steel balls of 0.8 mm diameter at 10, 15, and 20 Hz at room temperature (Fig. 8). At the end of the reaction, hot ethanol was added and the entire reaction mixture was scraped, then the catalyst was separated. When the reaction was performed at lower frequencies such as 10 Hz, a few substrates were still present, probably due to the reduction in the amount of energy per impact.
With the optimal reaction conditions (Table 1, entry 5), the best result was achieved by using a mixer-milling (frequency of 20 Hz) and 0.04 g of catalyst without any solvent.
As Table 2 shows, a wide range of aromatic aldehydes containing electron donors and electron-withdrawing groups were applied for the synthesis of PPz derivatives under optimized conditions.
Reusability of nano-SiO2/AEP nano-catalyst
The reusability of nano-catalyst Nano-SiO2/AEP was investigated as an important factor for green synthesis (Fig. 9). After completion of the reaction, hot ethanol was added to the reaction mixture and then filtered to separate the nano-catalyst from it. The separated nano-catalyst was washed three times with hot ethanol, dried at ambient temperature, and reused three times with a low loss of its activity.
The catalytic activity of the Nano-SiO2/AEP heterogeneous nano-catalyst was compared with other catalysts. As shown in Table 3, Nano-SiO2/AEP nano-catalyst acts with relatively high catalytic activity in a short reaction time.
According to relevant literature reports [18], the proposed mechanism for the synthesis of PPz is shown in Fig. 10. Pyranopyrazole formation is a three-step process. Initially, under ball milling conditions, ethylacetoacetate reacts with hydrazine to form an active pyrazolone intermediate (IM1). Then in the second step, aldehyde with malononitrile is condensed and forms an active intermediate IM2. In the third step, both intermediates, IM1, and IM2 are condensed to produce the desired product.
Conclusions
In summary, we have developed Nano-SiO2/AEP as a new heterogeneous nano-catalyst and identified it by FT-IR, FESEM, TGA, XRD, EDX, EDS-map, and pH techniques. A highly efficient, environmentally friendly practical method for the synthesis of PPz derivatives was developed in the presence of Nano-SiO2/AEP nano-catalysts. The reaction proceeds well by ball milling under solvent and metal-free conditions. Other key features of this green approach include high performance, easy preparation, and low temperature.
Experimental section
Materials and methods
Chemicals were purchased from Merck, Fluka, and Aldrich Chemical Companies. FT-IR spectra were run on a Bruker, Equinox 55 spectrometer. A Bruker (DRX-400 Avanes) NMR was applied to record the 1H-NMR and 13C-NMR spectra. Melting points were determined on a Büchi B-540 apparatus. X-ray diffraction (XRD) pattern was obtained by the BRUKER, (Avance). Field Emission Scanning Electron Microscopy (FESEM) images were obtained by a TESCAN, Mira III. The EDS-MAP micrographs were obtained on MIRA II detector SAMX from TESCAN company (France). Thermal gravimetric analysis (TGA) was conducted using the “STA 503” instrument from BAHR company. The pH of the nano-catalyst was matured by the Metrohm model 691 pH/mv meter. The Reactions were conducted using the Mixer Mill model Retsch MM 400 which consisted of two stainless steel vials, each containing two stainless steel balls.
Preparation of nano-silica chloride (SC)
In a 250 mL round-bottomed flask equipped with a condenser, 10 g of Silica gel and 40 mL of thionyl chloride were added and refluxed for 48 h. Then, the reaction mixture was filtered and the resulting mixture was washed three times with dichloromethane (40 mL × 3) to remove unreacted thionyl chloride from the reaction mixture, the resulting white-grayish powder was dried at ambient temperature and stored in a tight sample tube.
Preparation of nano-silica chloride/aminoethylpiperazine (nano-SiO2/AEP)
In a 100 mL round-bottomed flask, 15 mmol nano-Silica chloride (1.433 g) and 15 mmol of 2-AEP (1.938 g, pH = 12), 10 mL of DMF were heated for 24 h at 80 °C. Then, the resulting mixture was washed three times with dichloromethane (10 mL × 3) and dried at ambient temperature.
General procedure for the synthesis of PPz
In a stainless steel ball mill vessel, a mixture of ethylacetoacetate (1 mmol), hydrazine hydrate (1.5 mmol), aldehydes (1 mmol), malononitrile (1 mmol), and Nano-SiO2/AEP (0.04 g) was milled at 20 Hz. The reaction progress was monitored by TLC (n-hexane: ethyl acetate [8:2]). After checking out the reaction, hot ethanol was added and the reaction mixture was scraped and filtrated to separate the catalyst. Then, the obtained solution was poured into cold water. The crude products appeared as solids that were filtered and washed with water. The crude products were recrystallized in ethanol.
Availability of data and materials
All the methods were carried out in accordance with relevant local/national/international institutional guidelines and regulations. All data generated or analyzed during this study are not publicly available due to DATA NOT PUBLIC but are available from the corresponding author on reasonable request.
Abbreviations
- PPz:
-
Pyranopyrazole
- FESEM:
-
Field emission scanning electron microscopy
- TGA:
-
Thermo gravimetric analysis
- XRD:
-
X-ray diffraction
- AEP:
-
2-Aminoethylpiperazine
- DMF:
-
N, N-Dimethylformamide
- IM:
-
Intermediate
References
Ahluwalia VK. Green chemistry: environmentally benign reactions. India: Springer Nature ND; 2021.
Kurniawan YS, Priyangga KTA, Krisbiantoro PA, Imawan AC. Green chemistry influences in organic synthesis: a review. J Multidiscip Appl Nat Sci. 2021. https://doi.org/10.47352/jmans.v1i1.2.
Ould M’hamed, M. Ball milling for heterocyclic compounds synthesis in green chemistry: a review. Synth. Commun. 2015;45:2511–28.
Stolle A, Szuppa T, Leonhardt SE, Ondruschka B. Ball milling in organic synthesis: solutions and challenges. Chem Soc Rev. 2011;40:2317–29.
Eguaogie O, Vyle JS, Conlon PF, Gîlea MA, Liang Y. Mechanochemistry of nucleosides, nucleotides and related materials. Beilstein J Org Chem. 2018;14:955–70.
Tan D, Loots L, Friščić T. Towards medicinal mechanochemistry: evolution of milling from pharmaceutical solid form screening to the synthesis of active pharmaceutical ingredients (APIs). Chem Comm. 2016;52:7760–81.
Li J, Nagamani C, Moore JS. Polymer mechanochemistry: from destructive to productive. Acc Chem Res. 2015;48:2181–90.
Willis-Fox N, Rognin E, Aljohani TA, Daly R. Polymer mechanochemistry: manufacturing is now a force to be reckoned with. Chem. 2018;4:2499–537.
Leonardi M, Villacampa M, Menéndez JC. Multicomponent mechanochemical synthesis. Chem Sci. 2018;9:2042–64.
Achar TK, Bose A, Mal P. Mechanochemical synthesis of small organic molecules. Beilstein J Org Chem. 2017;13:1907–31.
Sarkar A, Santra S, Kundu SK, Hajra A, Zyryanov GV, Chupakhin ON, Charushin VN, Majee A. A decade update on solvent and catalyst-free neat organic reactions: a step forward towards sustainability. Green Chem. 2016;18:4475–525.
Bolm C, Hernández JG. Mechanochemistry of gaseous reactants. Angew Chem Int Ed. 2019;58:3285–99.
Zhu SE, Li F, Wang GW. Mechanochemistry of fullerenes and related materials. Chem Soc Rev. 2013;42:7535–70.
Burk L, Gliem M, Mülhaupt R. Mechanochemical routes to functionalized graphene nanofillers tuned for lightweight carbon/hydrocarbon composites. Macromol Mater Eng. 2019;304:1800496.
Baklanova ON, Knyazheva OA, Lavrenov AV, Drozdov VA, Trenikhin MV, Arbuzov AB, Kuznetsova Y, Rempel AA. Mechanical treatment as highly effective method of physico-chemical properties control of carbon black. Micropor Mesopor Mat. 2019;279:193–200.
Hasa D, Schneider Rauber G, Voinovich D, Jones W. Cocrystal formation through mechanochemistry: from neat and liquid-assisted grinding to polymer-assisted grinding. Angew Chem. 2015;127:7479–83.
Friščić T. Supramolecular concepts and new techniques in mechanochemistry: cocrystals, cages, rotaxanes, open metal–organic frameworks. Chem Soc Rev. 2012;41:3493–510.
Ganta RK, Kerru N, Maddila S, Jonnalagadda SB. Advances in pyranopyrazole scaffolds’ syntheses using sustainable catalysts. Molecules. 2021;26:3270.
Mamaghani M, Hossein Nia R. A review on the recent multicomponent synthesis of pyranopyrazoles. Polycycl Aromat Compd. 2021;41:223–91.
Sikandar S, Zahoor AF. Synthesis of pyrano [2, 3-c] pyrazoles: a review. J Heterocycl Chem. 2021;58:685–705.
Singh R, Kaur R, Ahlawat P, Kaushik P, Singh K. Green methods for the synthesis of pyrazoles. Org Prep Proced Int. 2021;53:317–51.
Fischer DS, Allan GM, Bubert C, Vicker N, Smith A, Tutill HJ, Purohit A, Wood L, Packham G, Mahon MF, Reed MJ. E-Ring modified steroids as novel potent inhibitors of 17b-hydroxysteroid dehydrogenase type 1. J Med Chem. 2005;48:5749–70.
Bansode TN, Ansari RM, Gawale YK. Synthesis and biological activity of some new pyrazole derivatives. J Pharm Res. 2011;4:1141–2.
Wang JL, Liu D, Zhang ZJ, Shan S, Han X, Srinivasula SM, Croce CM, Alnemri ES, Huang Z. Structure-based discovery of an organic compound that binds bcl-2 protein and induces apoptosis of tumor cells. PNAS. 2000;97:7124–9.
Abraham DJ, editor. Burger’s medicinal chemistry and drug discovery. 6th ed. John Wiley & Sons; 2003.
Schönthal AH, Chen TC, Hofman FM, Louie SG, Petasis NA. Celecoxib analogs that lack COX-2 inhibitory function: preclinical development of novel anticancer drugs. Expert Opin Investing drugs. 2008;17:197–208.
Penning TD, Talley JJ, Bertenshaw SR, Carter JS, Collins PW, Docter S, Graneto MJ, Lee LF, Malecha JW, Miyashiro JM, Rogers RS. Synthesis and biological evaluation of the 1, 5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1 H-pyrazol-1-yl] benzenesulfonamide (SC-58635, celecoxib). J Med Chem. 1997;40:1347–65.
Kang JG, Park CY. Anti-obesity drugs: a review about their effects and safety. Diabetes Metab J. 2012;36:13–25.
Fuxe K, Agnati LF, Ungerstedt U. The effect of mepiprazole on central monoamine neurons. Evidence for increased 5-hydroxytryptamine and dopamine receptor activity. Eur J Pharmacol. 1976;35:93–107.
Beerappa M, Shivashankar K. Four component synthesis of highly functionalized pyrano [2, 3-c] pyrazoles from benzyl halides. Synth Commun. 2018;48:146–54.
Kamel MM. Convenient synthesis, characterization, cytotoxicity and toxicity of pyrazole derivatives. Acta Chim Slov. 2015;62:136–51.
Mehravar M, Mirjalili BF, Babaei E, Bamoniri A. Preparation and application of nano-alpo4/ti (iv) as a new and recyclable catalyst for the four-component synthesis of dihydropyrano [2, 3-c] pyrazoles. Polycycl Aromat Compd. 2020. https://doi.org/10.1080/10406638.2020.1856149.
Tafti AD, Mirjalili BF, Bamoniri A, Salehi N. Rapid four-component synthesis of dihydropyrano [2, 3-c] pyrazoles using nano-eggshell/Ti(IV) as a highly compatible natural based catalyst. BMC Chem. 2021. https://doi.org/10.1186/s13065-021-00734-5.
Akbarpour T, Yousefi Seyf J, Khazaei A, Sarmasti N. Synthesis of pyrano [2, 3-c] pyrazole derivatives using a novel ionic-liquid based nano-magnetic catalyst (Fe3O4@SiO2@(CH2)3NH@CC@Imidazole@SO3H+Cl−). Polycycl Aromat Compd. 2021. https://doi.org/10.1080/10406638.2021.1873152.
Van Nguyen HT, Le T, Tran PH. AC-SO3H/[CholineCl][Urea]2 as a green catalytic system for the synthesis of pyrano [2, 3-c] pyrazole scaffolds. J Environ Chem Eng. 2021;9: 105228.
Hassanzadeh-Afruzi F, Dogari H, Esmailzadeh F, Maleki A. Magnetized melamine-modified polyacrylonitrile (PAN@ melamine/Fe3O4) organometallic nanomaterial: preparation, characterization, and application as a multifunctional catalyst in the synthesis of bioactive dihydropyrano [2, 3-c] pyrazole and 2-amino-3-cyano 4H-pyran derivatives. Appl Organomet Chem. 2021;35: e6363.
Zolfigol MA, Tavasoli M, Moosavi-Zare AR, Moosavi P, Kruger HG, Shiri M, Khakyzadeh V. Synthesis of pyranopyrazoles using isonicotinic acid as a dual and biological organocatalyst. RSC Adv. 2013;3:25681–5.
Moosavi-Zare AR, Goudarziafshar H, Saki K. Synthesis of pyranopyrazoles using nano-Fe-[phenylsalicylaldiminemethylpyranopyrazole] Cl2 as a new Schiff base complex and catalyst. Appl Organomet Chem. 2018;32: e3968.
Sameri F, Mobinikhaledi A, Bodaghifard MA. Preparation of core/shell CaO@ SiO2-SO3H as a novel and recyclable nanocatalyst for one-pot synthesize of dihydropyrano [2, 3-c] pyrazoles and tetrahydrobenzo [b] pyrans. SILICON. 2022;14:1395–406.
Ghasemzadeh MA, Mirhosseini-Eshkevari B, Dadashi J. IRMOF-3 functionalized GO/CuFe2O4: a new and recyclable catalyst for the synthesis of dihydropyrano [2, 3-c] pyrazoles under ultrasound irradiations. J Mol Struct. 2022;1261: 132843.
Katariya AP, Katariya MV, Sangshetti J, Deshmukh SU. Ionic liquid [(EMIM) Ac] catalyzed green and efficient synthesis of Pyrano [2, 3-c] pyrazole derivatives. Polycycl Aromat Compd 2022;1–15.
Chen Y, Zhang Z, Jiang W, Zhang M, Li Y. Ru III@CMC/Fe3O4 hybrid: an efficient, magnetic, retrievable, self-organized nanocatalyst for green synthesis of pyranopyrazole and polyhydroquinoline derivatives. Mol Divers. 2019;23:421–42.
Khairnar BJ, Mane DV, Chaudhari BR. Heterogeneous PS-DABCO catalyzed one pot four-component synthesis of pyranopyrazole. J Appl Chem. 2019;8:425–34.
Rezaei-Seresht E, Bakhshi-Noroozi M, Maleki B. Piperazine-grafted magnetic reduced graphene oxide (Fe3O4@ rGO-NH) as a reusable heterogeneous catalyst for gewald three-component reaction. Polycycl Aromat Compd. 2021;41:1944–52.
Maleki B, Baghayeri M, Abadi SAJ, Tayebee R, Khojastehnezhad A. Ultrasound promoted facile one pot synthesis of highly substituted pyran derivatives catalyzed by silica-coated magnetic NiFe2O4 nanoparticle-supported H14 [NaP5W30O110] under mild conditions. RSC Adv. 2016;6:96644–61.
Morsi RE, Mohamed RS. Nanostructured mesoporous silica: influence of the preparation conditions on the physical-surface properties for efficient organic dye uptake. R Soc Open Sci. 2018;5: 172021.
Vasuki G, Kumaravel K. Rapid four-component reactions in water: synthesis of pyranopyrazoles. Tetrahedron Lett. 2008;49:5636–8.
Litvinov YM, Shestopalov AA, Rodinovskaya LA, Shestopalov AM. New convenient four-component synthesis of 6-amino-2,4-dihydropyrano [2,3-c] pyrazol-5-carbonitriles and one-pot synthesis of 6′-aminospiro [(3H)-indol-3,4′-pyrano [2,3-c] pyrazol]-(1H)-2-on-5′-carbonitriles. J Comb Chem. 2009;11:914–9.
Acknowledgements
The Research Council of Yazd University is gratefully acknowledged for the financial support of this work.
Funding
This study was financially supported by Yazd University. The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
DM and BFM designed and performed the research, analyzed the data, interpreted the results, and prepared the manuscript. DM performed the assay and conducted the optimization, and purification of compounds. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication.
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary file containing FTIR and NMR of products.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mallah, D., Mirjalili, B.B.F. A green protocol ball milling synthesis of dihydropyrano[2,3-c]pyrazole using nano-silica/aminoethylpiperazine as a metal-free catalyst. BMC Chemistry 17, 10 (2023). https://doi.org/10.1186/s13065-023-00934-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-023-00934-1