Abstract
Background
We investigated the association of several air pollution measures with postmenopausal breast cancer (BCa) risk.
Methods
This study included 155,235 postmenopausal women (of which 6146 with BCa) from UK Biobank. Cancer diagnoses were ascertained through the linkage to the UK National Health Service Central Registers. Annual exposure averages were available from 2005, 2006, 2007, and 2010 for NO2, from 2007 and 2010 for PM10, and from 2010 for PM2.5, NOX, PM2.5–10 and PM2.5 absorbance. Information on BCa risk factors was collected at baseline. Cox proportional hazards regression was used to evaluate the associations of year-specific and cumulative average exposures with BCa risk, overall and with 2-year exposure lag, while adjusting for BCa risk factors.
Results
PM10 in 2007 and cumulative average PM10 were positively associated with BCa risk (2007 PM10: Hazard ratio [HR] per 10 µg/m3 = 1.18, 95% CI 1.08, 1.29; cumulative average PM10: HR per 10 µg/m3 = 1.99, 95% CI 1.75, 2.27). Compared to women with low exposure, women with higher 2007 PM10 and cumulative average PM10 had greater BCa risk (4th vs. 1st quartile HR = 1.15, 95% CI 1.07, 1.24, p-trend = 0.001 and HR = 1.35, 95% CI 1.25, 1.44, p-trend < 0.0001, respectively). No significant associations were found for any other exposure measures. In the analysis with 2-year exposure lag, both 2007 PM 10 and cumulative average PM10 were positively associated with BCa risk (4th vs. 1st quartile HR = 1.19, 95% CI 1.10, 1.28 and HR = 1.29, 95% CI 1.19, 1.39, respectively).
Conclusion
Our findings suggest a positive association of 2007 PM10 and cumulative average PM10 with postmenopausal BCa risk.
Similar content being viewed by others
Introduction
Established breast cancer risk factors explain only 30–50% of breast cancer cases [1,2,3,4,5], and previous studies in immigrants have demonstrated the importance of environmental factors in the etiology of breast cancer [6]. Air pollution is classified as a human carcinogen by the International Agency for Research on Cancer (IARC) with strongest evidence for lung and bladder cancers, and some studies linking it also to the risk of liver, gastric, cervical, and brain cancers [7,8,9,10,11,12,13]. However, the relationship between air pollution and breast cancer remains unclear. Notably, more than half of the worlds’ population continue to be exposed to increased levels of air pollution [14].
Of the common air pollutants used for air quality monitoring, particulate matter (PM), and nitrogen oxides (NOx), including nitrogen dioxide (NO2) are of interest with respect to breast carcinogenesis. PM, including fine inhalable particles of ≤ 2.5 µm in diameter (PM2.5) and inhalable particles ≤ 10 µm in diameter (PM10), have biological properties relevant to breast carcinogenesis and other chronic diseases [15,16,17,18,19,20,21,22], while NOx and NO2 represent biomarkers of exposure to PM, polycyclic aromatic hydrocarbons (PAHs) or benzene from traffic related air pollution [23]. Despite known carcinogenic properties of PM constituents, experimental evidence and strong biological plausibility, the epidemiologic evidence on the association of PM with breast cancer remains very limited and inconsistent. Some previous studies reported positive associations of PM10 and PM2.5, while others found none [24,25,26,27,28,29,30,31,32,33,34]. However, most of the previous observational studies were relatively small (< 3450 breast cancer cases) and/or limited to specific sub-populations of women (nurses or breast cancer-free women with a family history of breast cancer) [9, 24,25,26,27,28, 35,36,37,38]. Similarly, for NO2, some studies found significant associations, while others found no associations with breast cancer, often with trends suggesting a positive association [26, 27, 31,32,33, 35,36,37,38].
Epidemiologic evidence for biological role of the individual chemicals found in PMs suggests an increase in breast cancer risk in different populations, some even at the lowest detectable levels [39,40,41,42,43,44,45,46,47,48]. PAHs have endocrine disrupting properties and interfere with normal DNA damage repair [15,16,17,18, 49, 50]. Among other compounds found in PMs, polychlorinated dibenzodioxins (dioxin), dibenzofurans (PCDF), polychlorinated biphenyls (PCB) and heavy metals (cadmium, arsenic, and mercury) have also been shown to have endocrine disrupting properties and associations with breast cancer in some studies [16, 51,52,53,54,55,56,57,58,59].
Although their intrinsic carcinogenicity is not clearly established, NOx and NO2 represents biomarkers of exposure to diesel exhaust, which contains many carcinogenic components such as PM, PAHs, and benzene [60, 61]. An important source of NOx and NO2 is fossil fuel combustion, mainly from engine vehicles and energy production; therefore, NOx and NO2 are considered as the best road traffic tracers and markers of exposure to components with plausible biological mechanisms, without being directly involved in cancer pathophysiology. Further, it has been previously shown that air concentrations of nickel or vanadium in PM2.5 were more highly correlated with NO2 or NOx concentration levels than with total PM2.5 concentration levels [62]. Thus, NO2 might be a better marker of exposure to specific PM species or heavy metals with carcinogenic properties found in vehicles exhaust than ambient total PM concentration levels.
The goal of this study was to explore the association between air pollution (PM2.5, PM10, PMcoarse 2.5–10, PM2.5 absorbance, NO2, and NOx) and breast cancer risk in a large population-based sample of postmenopausal women from an established prospective cohort. These associations were examined overall as well as with 2-year and 5-year exposure lag.
Methods
Study population
Women in this study were selected from UK Biobank, an established population-based prospective cohort. UK Biobank contains more than 500,000 (44% women) volunteers who were aged 40–69 years when recruited during 2006–2010 from England, Scotland and Wales via National Health Service (NHS) patient registers [63]. A detailed description of the enrollment process has been previously described [64]. Briefly, at enrollment all participants provided health, lifestyle, and socio-demographic data through questionnaires and interviews, underwent physical examination, provided blood, urine and saliva samples and agreed to be followed for health outcomes. Between 2012 and 2013, 20,346 (20%) participants completed their first repeat assessment. Participants’ outcomes were ascertained via record linkage to the NHS Central Registers. The latest cancer registry record linkage to UK Biobank data was completed on February 29, 2020 for participants from England and Wales, and on August 31, 2021 for Scotland.
As premenopausal and postmenopausal breast cancers are different and as the follow-up data did not capture updates on woman’s menopausal status or reproductive history, only postmenopausal women were included in this study. Women were considered to be postmenopausal at baseline if they reported (1) having menopause (periods stopped), (2) bilateral oophorectomy, or (3) hysterectomy with one or both ovaries retained and being 54 years or older for ever smokers or 56 years or older for never smokers [65]. We included postmenopausal women without a history of breast cancer or any other type of cancer (except non-melanoma skin) at recruitment. A breast cancer diagnosis (invasive and in-situ) was identified based on diagnostic codes according to the 9th revision of the International Classification of Diseases (ICD-9: 174, 2330) or the 10th revision (ICD-10: C50, D05).
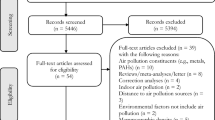
Out of 158,979 eligible women, we further excluded participants with missing information on all air pollution assessments and selected breast cancer risk factors. The final sample included 155,235 women (98% of all eligible women in UK Biobank) of which 6,146 developed breast cancer during the follow-up through the last linkage to the cancer registries (Fig. 1). UK Biobank protocol was approved by the NorthWest Multi-centre Research Ethics Committee (MREC), which covers the UK. All participants of UK Biobank provided written consent at recruitment.
Air pollution data
In the UK Biobank, the annual averages of PM10 and NO2 were available for the baseline assessment period (2005, 2006, 2007, and 2010 for NO2; 2007 and 2010 for PM10), while PM2.5, NOX, PM coarse (PM with an aerodynamic diameter > 2.5 µm but ≤ 10 µm, PM2.5–10) and PM2.5 absorbance (measurement of the blackness of PM2.5 filters, a proxy for elemental carbon [the dominant light absorbing substance]) were available only for 2010 (Fig. 2).
Air pollution estimates for 2005–2007 were derived from EU-wide air pollution maps (resolution 100 m × 100 m) [66]. The X and Y coordinates of UK Biobank participants were overlaid with these maps (projected to British National Grid) and the corresponding air pollution concentration of the 100 m × 100 m grid cell was assigned to the coordinate. EU-wide air pollution maps were modelled based on a land use regression (LUR) models for Western Europe [66]. The dependent variables in LUR models were ambient concentrations of NO2 and PM10, obtained from EuroAirnet, the regulatory air pollution monitoring network in Europe [67]. Air pollution estimates for 2010 were modeled for each address using a LUR models developed as part of the European Study of Cohorts for Air Pollution Effects (ESCAPE) [68, 69]. Within the ESCAPE project, PM2.5, PM10, PM2.5 absorbance, and nitrogen oxides (NO2 and NOx) were measured between October 2008 and April 2011 during standardized specific PM monitoring campaigns [70, 71]. The estimates from the LUR models were used to calculate the annual averages of air pollutants. The LUR estimates for PM were not valid beyond 400 km from Greater London, the initial ESCAPE study area; therefore, participants living beyond 400 km (mainly from northern England or Scotland), were not assigned PM10, PM2.5, PM2.5 absorbance, and PMcoarse concentrations for 2010 to prevent exposure misclassification.
Covariates information
Information on breast cancer risk factors was extracted for all the participants from baseline questionnaires, interviews, and physical examinations (2006–2010). The following covariates were included: race/ethnicity, Body Mass Index (BMI), age at menarche, age at menopause, parity/age at first child’s birth, postmenopausal hormone therapy, alcohol use, smoking status, and family history of breast cancer. Since there was a small proportion (< 0.5%) of women with missing values on race, BMI, parity, alcohol, and smoking, those women were excluded from the sample, while for variables with relatively larger proportion of missing values (> 3%), we created an “Unknown” category to retain these observations in the models.
For variables that could potentially change with time in postmenopausal women (BMI, alcohol use, smoking, and family history of breast cancer), we found a high correlation in the values for these covariates at baseline with those collected at the repeat assessments on the subset of participants thus justifying the use of baseline data in the analysis. The correlations between baseline values and values collected at first, second, or third subsequent assessments were 0.92, 0.89, and 0.86 for BMI, 0.91, 0.88, and 0.89 for smoking status, 0.76, 0.70, and 0.69 for alcohol use status, respectively; for family history (yes/no), the agreement between first and second assessment was 0.94.
Statistical analyses
Cox proportional hazards regression models were used to analyze the association between air pollution and breast cancer risk while adjusting for known breast cancer risk factors: age at recruitment (years, continuous), age at menarche (years, continuous), BMI (kg/m2, continuous), race (Caucasian [reference], other), parity/age at first child’s birth (nulliparous [reference], parous with age at first birth ≤ 25 years, parous with age at first birth > 25 years, parous with unknown age at first birth), family history of breast cancer in first degree relatives (none [reference], any, unknown), age at menopause (< 46 [reference], 46 to < 50, 50 to < 55, ≥ 55 years, unknown), postmenopausal hormone use (never [reference], past, current, unknown), smoking (never [reference], past, current), and alcohol consumption (never [reference], past, current). The risk estimates were presented as hazard ratios (HRs) and their corresponding 95% confidence intervals (CIs). The regression models were developed for all available measures of air pollution preceding the breast cancer diagnosis in two ways. First, air pollution from specific years was examined in relation to breast cancer risk. Second, the cumulative average exposure for pollutants with multiple assessment years (PM10 and NO2) was used in the analyses, calculated as the average across all available measures. Each analysis included only incident breast cancer cases diagnosed in the same year or after the measurements of the air pollutant modeled were taken. The follow-up start time and the subset of women included in each analysis listed in Additional file 1: Table 1 are based upon the measure of air pollutant modeled. For example, when analyzing the associations of NO2 2006 with breast cancer risk, the follow-up started in 2006 and included all women who were cancer-free as of 2006, while analysis of NO2 2010 had a start of follow-up in 2010 and included only women who were cancer-free as of 2010. For analyses with the cumulative average exposure to PM10 or NO2, the follow-up time started at the last available exposure assessment year. In all the analyses, the follow-up time ended at the time of breast cancer diagnosis for women with breast cancer, at the time of death for women who died during the follow-up, at the time of cancer diagnosis for women who developed other cancer type during the follow-up, or at time of the last linkage to the cancer registries for everyone else.
Air pollution was modeled as a continuous variable and the estimates were reported per 5 µg/m3 and 10 µg/m3 increase of air pollutant, consistent with previous studies [24,25,26,27,28,29, 32, 33, 37]. Air pollution was also modeled as quartiles based on the distribution in the study sample, specific to assessment year or cumulative average exposure for PM10 and NO2. Tests for trends were performed using the median level of the exposure within each of the quartiles. Additional analyses were performed to allow 2-year and 5-year lag in air pollution exposures by including only breast cancer cases diagnosed two years and five years after the exposure measures, respectively, in order to exclude the most recent exposure measures. As no previous studies on air pollution and breast cancer used lagging, our decision was based on minimum lagging period used in other studies of environmental exposures with breast cancer [72] as well as minimum lagging in studies of air pollution and lung cancer [73]. Thus, in the analysis for NO2 exposure from 2006, only breast cancer cases diagnosed in 2008 or later were included. Additionally, as our sample included 17,122 women with bilateral oophorectomy (645 of which with breast cancer diagnosis during the follow-up), we conducted a sensitivity analysis that excluded these women. Finally, in the secondary analysis, we excluded 947 women who were diagnosed with ductal carcinoma in situ (DCIS, ICD-9 codes 2330 or ICD-10 code D05).
Prior to regression analysis, we tested the proportional hazards assumption; only age at recruitment violated this assumption. To account for non-proportionality, the interaction term between age at recruitment and time was included in the models [74]. All the tests were two-sided and significance of the effects was assessed at 5% level of significance. All analyses were performed using SAS (SAS Institute Inc. version 9.4).
Results
In this prospective study of 155,235 postmenopausal women, 6146 developed breast cancer and 149,089 women remained breast cancer-free during the follow-up. The mean age of the study population at enrollment was 60.1 years (range 40–71 years). The average follow-up time calculated for analyses of earliest exposures measured before or at baseline (2005–2006), was 5.4 years (standard deviation [SD] = 3.1 years) for breast cancer cases and 10.7 years (SD = 2.0 years) for cancer-free women. The average follow-up time calculated for analyses of exposures measured in 2010, was 4.9 years (standard deviation [SD] = 2.9 years) for breast cancer cases and 9.8 years (SD = 1.6 years) for cancer-free women. The distribution of air pollution measures (in µg/m3) are presented in Table 1 and their correlations are shown in Additional file 1: Table 2. Characteristics of the study population at baseline by breast cancer status are presented in Table 2. As compared to women without breast cancer, women with a breast cancer diagnosis were, on average, older (60.5 vs. 60.1 years, p for difference < 0.0001), had higher BMI (27.6 vs. 27.2 kg/m3, p for difference < 0.0001), were more likely to be current postmenopausal hormone therapy users at the time of enrollment (12.2% vs. 9.3%, p for difference < 0.0001), and more likely to have a family history of breast cancer (11.1% vs. 7.2%, p for difference < 0.0001, Table 2). We also present participants’ baseline characteristics by quartiles of the earliest exposure measure (2005 NO2) (Additional file 1: Table 3).
Overall associations of air pollution measures with breast cancer risk
In the main analyses, the risk of breast cancer increased by 18% per 10 µg/m3 increase in PM10 exposure in 2007 (HR = 1.18, 95%CI 1.08, 1.29, Table 3). Compared to women exposed to the lowest 2007 PM10 concentrations, women with higher exposure levels had a greater risk of breast cancer (HR for 4th vs. 1st quartile = 1.15, 95% CI 1.07, 1.24, p-trend = 0.001, Table 3). No association was found for 2010 PM10 exposure. The cumulative average exposure to PM10 was significantly positively associated with breast cancer risk, when modeled both as continuous (HR per 10 µg/m3 = 1.99, 95% CI 1.75, 2.27), and as quartiles (HR for 4th vs. 1st quartile = 1.35, 95% CI 1.25, 1.44, p-trend < 0.001, Table 3). We found no associations of PM2.5, PMcoarse 2.5–10, PM2.5 absorbance, NO2, or NOx with breast cancer risk (Tables 3 and 4). The results of associations were similar in sensitivity analysis excluding women with bilateral oophorectomy (data not shown).
Lagged exposure analyses
In the analysis with 2-year exposure lag (Tables 3 and 4), we observed a positive association between exposure to 2007 PM10 and breast cancer risk (HR per 10 µg/m3 = 1.23, 95% CI 1.12, 1.35, Table 3). Compared to women with lowest 2007 PM10 exposure, women with higher exposure levels had a greater risk of breast cancer (HR for 4th vs. 1st quartile = 1.19, 95% CI 1.10, 1.28, p-trend < 0.001, Table 3). The cumulative average exposure to PM10 was significantly positively associated with breast cancer risk when modeled both as continuous (HR per 10 µg/m3 = 1.86, 95% CI 1.61, 2.15), and as quartiles (HR for 4th vs. 1st quartile = 1.29, 95% CI 1.19, 1.39, p-trend < 0.001, Table 3). No significant associations were found for PM10 exposure in 2010 or any other air pollutant measures. The associations were similar with 5-year exposure lag (Additional file 1: Table 4).
Secondary analyses of associations with invasive breast cancer only
In the secondary analyses including 5,175 invasive breast cancer and 148,289 breast cancer-free women, the risk of invasive breast cancer increased by 19% per 10 µg/m3 increase in 2007 PM10 exposure (HR per 10 µg/m3 = 1.19, 95% CI 1.08, 1.31) (Additional file 1: Table 5). Compared to women exposed to lowest concentrations of 2007 PM10, women with higher exposure levels had a greater risk of invasive breast cancer (HR for 4th vs. 1st quartile = 1.16, 95% CI 1.07, 1.25, p-trend = 0.002) (Additional file 1: Table 5). The cumulative average exposure to PM10 was significantly associated with invasive breast cancer risk when modeled both as continuous (HR per 10 µg/m3 = 2.06, 95% CI 1.79, 2.37), and as quartiles (HR for 4th vs. 1st quartile = 1.35, 95% CI 1.25, 1.46, p-trend < 0.001) (Additional file 1: Table 5). We also found a suggestive inverse association between NOx and breast cancer risk (HR for 4th vs. 1st quartile = 0.92, 95% CI 0.84, 0.99, p-trend = 0.035). None of the other air pollution measures were associated with the risk of invasive breast cancer (Additional file 1: Table 5).
In the analysis with 2-year exposure lag, we observed a positive association between 2007 PM10 and invasive breast cancer risk (HR per 10 µg/m3 = 1.24, 95% CI 1.13, 1.37) (Additional file 1: Table 5). Compared to women with the lowest 2007 PM10, women with higher exposure levels had a greater risk of breast cancer (HR for 4th vs. 1st quartile = 1.19, 95% CI 1.10, 1.29, p-trend < 0.001) (Additional file 1: Table 5). The cumulative average exposure to PM10 was significantly associated with breast cancer risk, when modeled both as continuous (HR per 10 µg/m3 = 1.93, 95% CI 1.65, 2.26), and as quartiles (HR for 4th vs. 1st quartile = 1.30, 95% CI 1.19, 1.42, p-trend < 0.001) (Additional file 1: Table 5). No significant associations were found for PM10 exposure in 2010 or any other air pollutant and breast cancer risk (Additional file 1: Table 5).
Discussion
In this large prospective cohort of postmenopausal women enrolled in the UK Biobank, we investigated the association of air pollution with postmenopausal breast cancer risk. We found positive associations of 2007 PM10 and PM10 cumulative average with postmenopausal breast cancer risk. In the analysis with a 2-year exposure lag, the associations were stronger for 2007 PM10 as compared to overall analysis. No associations were found for other examined air pollution measures.
Our findings for the association of PM10 with breast cancer risk are consistent with some, but not all studies [24,25,26,27,28, 30,31,32, 35]. A recent cohort study from Germany found 19% breast cancer risk increase per 10 μg/m3 increase in PM10 (Relative Risk [RR] = 1.19, 95% CI 1.09, 1.31) [32]. However, the study population was not limited to postmenopausal women. In an ecological study that utilized region-based national census data that encompassed the entire female population of South Korea for 10 years, Hwang et al. found a 13% breast cancer risk increase per 10 μg/m3 increase in PM10 level (Odds Ratio [OR] = 1.13, 95% CI 1.09, 1.17) [31]. However, the findings from this ecological study cannot be applied to individuals (ecological fallacy); further, the risk estimates could not be adjusted for breast cancer risk factors. Studies that evaluated the relationship between PM10 and breast cancer risk among postmenopausal women have not found any associations. Andersen et al. pooled data from seven European prospective cohorts (overall 3,612 breast cancer cases) and found elevated non-significant associations of PM10 with postmenopausal breast cancer (HR per 10 μg/m3 = 1.07, 95% CI 0.89, 1.30) [25]. There was considerable heterogeneity between individual cohort estimates, with hazard ratios per 10 μg/m3 exposure increase ranging between 0.81 and 1.73. A null association between PM10 and postmenopausal breast cancer risk was also observed in the Nurses' Health Study II (HR per 10 μg/m3 = 0.97, 95% CI 0.86, 1.09) [28]. However, this cohort is limited to specific female nurses and thus the findings may not be generalizable to other populations. Finally, it is worth to note that there appeared to be a threshold effect rather than a dose–response relationship for associations of PM10 with breast cancer risk as the risk estimates for quartiles 2, 3, and 4 were similar.
While we found significant associations of 2007 PM10 and cumulative average with postmenopausal breast cancer risk, we did not find an association with 2010 PM10, most likely due to PM10 concentrations declining over time from a median of 21.72 μg/m3 in 2007 to a median of 16.01 μg/m3 in 2010 as well as fewer number of breast cancer cases in this analysis. Notably, only 0.07% (n = 95) of all 139,147 women (and 0.17% [n = 6] of breast cancer cases) had a 2010 PM10 concentration greater than the 2007 PM10 cutoff concentration for the 4th quartile (23.5 μg/m3).
We found no associations of PM2.5, PM2.5 absorbance, or PMcoarse 2.5–10 with breast cancer risk in any of the analyses performed, consistent with the previous studies in postmenopausal women, though some studies that were not limited to postmenopausal women reported some associations [24, 25, 28, 29]. In the largest cohort study to date including long-term residents of Ontario and registered with Ontario’s provincial health insurance plan on April 1, 1996, the hazard ratio was 1.01 per inter-quartile range (5.3 µg/m3) increase of PM2.5 (95% CI 1.00, 1.03) [33]. However, the average PM2.5 levels in this study were slightly higher than in our sample (10.8 [SD = 3.5] vs. 9.95 [SD = 1.04]) and the analyses were not stratified by menopausal status. Finally, PM2.5, PM2.5 absorbance and PM coarse 2.5–10 were only measured in 2010 and not 2007. Therefore, the same reasons for why PM10 in 2010 was not associated with breast cancer risk could potentially also explain the null associations for these other PM measures.
To our best knowledge, no other study looked at associations of PM10 lagged exposure with postmenopausal breast cancer. We found stronger associations when we included only breast cancer cases diagnosed two years as well as only those diagnosed 5 years after exposure to PM10 in 2007; thus, our findings provide further evidence that earlier exposures to higher concentrations of PM10 may be more relevant with respect to breast cancer etiology. Even though the confidence intervals for the estimates in overall analysis have some overlap with those in 2-year analysis, this overlap becomes less apparent with 5-year exposure lag further demonstrating that earlier exposures might be more relevant and that the observed differences in the strengths of association is unlikely to be the result of just random variation. However, we found a weaker effect of 2-year or 5-year lagged exposure for PM10 cumulative average compared to the effect of exposure for PM10 cumulative average without exposure lag, which could be potentially explained by declining PM10 concentrations over time combined with a reduction in the number of breast cancer cases included in these lagged analyses. Finally, we could not examine the associations with 7- and 10-year lag in the exposure as this would result in significant reduction of the number of breast cancer cases (by 50–70% of the original Ns, depending on the exposure) for 7-year lagged analysis and down to as few as 70 cases for 10-year lagged analysis), which would not be informative.
Our findings of significant association of PM10 with breast cancer risk are consistent with previous studies indicating relevant biological pathways as a possible explanation for potential effects of PM on breast cancer risk which could lead to tumor development. The biological effects of exposure to PMs could result from systemic inflammation, oxidative stress, and epigenetic changes that lead to formation of DNA adducts, disruption of DNA repair, induction of carcinogen-activating enzymes, and DNA methylation of tumor suppressor genes in breast tissue [15,16,17,18,19,20,21, 49, 50, 57,58,59, 75,76,77,78,79,80,81,82,83,84,85]. Importantly, some of these changes are stable and thus low dose long-term exposure would result in accumulation of these alterations over time. Further, some of these compounds have a very long half-life and accumulate in adipose tissue (including the breast) due to their lipophilic properties thus increasing target organ-specific dose [86,87,88].
Contrary to our hypothesis, we found a marginal association of NOx with breast cancer risk when only invasive cancers were included in analyses. Studies that assessed NO2 and NOx exposures reported mixed findings. Six of the 12 studies that investigated the association of NO2 with breast cancer risk reported significant positive associations [36, 37]. Of these six studies, two studies investigated the association of NO2 stratified by menopausal status, with positive associations found in postmenopausal women [24, 27, 32, 36, 37, 89]. Two of the four studies that investigated the association of NOx with breast cancer risk [9, 25, 26, 30] also found significant positive associations [25, 30]. However, in contrast to our study, all the studies except one [25] included premenopausal women and did not stratify by menopausal status. Some of the studies were ecological with no adjustment for known risk factors for breast cancer, or hospital-based case–control studies that included patients diagnosed with bladder cancer among controls, a type of cancer that has been found to be associated with air pollution [30, 32, 33, 36, 90]. As fossil fuel combustion is a major source of NO2 and NOx in the air, these two air pollutants may represent a marker of exposure to a mixture of components with carcinogenetic properties, such as PAHs, benzene, metals and other chemicals, possibly acting on breast tissues [23, 61]. Some previous studies had showed that breast cancer risk increases with proximity to roadways and traffic volume [26, 28].
While our findings of suggestive inverse associations of NOx with breast cancer risk are puzzling, they could potentially be the result of residual confounding, for example, by mammographic breast density, a well-established breast cancer risk factor, as some studies reported inverse associations of NOx with high breast density [60]. Information on breast density, however, was not available in UKBiobank. On the other hand, some previous reports have linked long-term exposure to NOx to lower levels of interleukin (IL)-2, IL-8, 40 IL-10 and tumor necrosis factor-α [91], all of which have been implicated in breast carcinogenesis [92]. Importantly, because we observed only association of NOx with invasive breast cancer risk without a clear pattern, future studies are needed to confirm our findings.
Our study is the largest study to date to investigate the association of air pollution with breast cancer among postmenopausal women. The study utilized a population-based prospective cohort with a rigorously defined protocol, rigorous ascertainment of the endpoints through continuous linkages to the national registries, and well-validated methods for assessing air pollution. This study has a few limitations. Information on covariates was used from baseline assessment only; however, the correlations with the values available from follow-up assessments were high (as described in Covariate information section) and thus, use of baseline risk factor data is unlikely to introduce misclassification. We were also unable to investigate associations by breast cancer subtypes, such as estrogen receptor (ER)-positive versus ER-negative, because the information was not recorded within the UK Biobank. Further, exposures to air pollutants were estimated based on a single residential address at baseline; therefore, we cannot rule out potential exposure misclassification caused by outside activities performed away from home or change of residence. However, recent studies suggest very small contribution of commuting to total weekly exposure and demonstrate that omitting commute does not significantly underestimate health effects as compared with the model combining home and work [93, 94]. Any exposure misclassification due to the change in residence is expected to be non-differential and could lead to dilution of the effect. Additionally, the variability in air pollution over time (as demonstrated by the differences in PM10 and NO2 levels, Table 1) implies that repeated measurements of air pollution recorded over time could provide a better estimate of cumulative exposure. Finally, a large number of potential associations were examined, and some of the significant findings could be false positives as a result of multiple testing.
Conclusion
We found a significant association of exposure to PM10 with postmenopausal breast cancer risk. Our findings contribute to the limited evidence on the association of air pollution with the risk of breast cancer in postmenopausal women. More studies in diverse populations, including Black women and other racial/ethnic minorities, are needed to confirm our results and to elucidate the potential biological mechanisms underlying the observed associations.
Availability of data and materials
Data used in this study are available from UK Biobank according to standard controlled access procedure. More details can be found at https://www.ukbiobank.ac.uk/enable-your-research/apply-for-access.
Abbreviations
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- DCIS:
-
Ductal carcinoma in situ
- ER:
-
Estrogen receptor
- ESCAPE:
-
European Study of Cohorts for Air Pollution Effects
- EU:
-
European Union
- HR:
-
Hazard ratio
- IARC:
-
International Agency for Research on Cancer
- ICD:
-
International Classification of Diseases
- LUR:
-
Land use regression
- MREC:
-
NorthWest Multi-centre Research Ethics Committee
- NHS:
-
National Health Service
- NOx:
-
Nitrogen oxides
- NO2 :
-
Nitrogen dioxide
- OR:
-
Odds ratio
- PAHs:
-
Polycyclic aromatic hydrocarbons
- PCB:
-
Polychlorinated biphenyls
- PCDF:
-
Dibenzofurans
- PM:
-
Particulate matter
- RR:
-
Relative risk
- SD:
-
Standard deviation
- UK:
-
United Kingdom
References
Coyle YM. The effect of environment on breast cancer risk. Breast Cancer Res Treat. 2004;84(3):273–88.
Laden F, Hunter DJ. Environmental risk factors and female breast cancer. Annu Rev Public Health. 1998;19:101–23.
Brody JG, Rudel RA, Michels KB, Moysich KB, Bernstein L, Attfield KR, Gray S. Environmental pollutants, diet, physical activity, body size, and breast cancer: Where do we stand in research to identify opportunities for prevention? Cancer. 2007;109(12 Suppl):2627–34.
Engmann NJ, Golmakani MK, Miglioretti DL, Sprague BL, Kerlikowske K. Population-attributable risk proportion of clinical risk factors for breast cancer. JAMA Oncol. 2017;3(9):1228–36.
Sprague BL, Trentham-Dietz A, Egan KM, Titus-Ernstoff L, Hampton JM, Newcomb PA. Proportion of invasive breast cancer attributable to risk factors modifiable after menopause. Am J Epidemiol. 2008;168(4):404–11.
Andreeva VA, Unger JB, Pentz MA. Breast cancer among immigrants: a systematic review and new research directions. J Immigr Minor Health. 2007;9(4):307–22.
Christodoulakos GE, Lambrinoudaki IV, Vourtsi AD, Vlachou S, Creatsa M, Panoulis KP, Botsis D. The effect of low dose hormone therapy on mammographic breast density. Maturitas. 2006;54(1):78–85.
Loomis D, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Baan R, Mattock H, Straif K, et al. The carcinogenicity of outdoor air pollution. Lancet Oncol. 2013;14(13):1262–3.
Raaschou-Nielsen O, Andersen ZJ, Hvidberg M, Jensen SS, Ketzel M, Sørensen M, Hansen J, Loft S, Overvad K, Tjønneland A. Air pollution from traffic and cancer incidence: a Danish cohort study. Environ Health. 2011;10(1):67.
Pedersen M, Andersen ZJ, Stafoggia M, Weinmayr G, Galassi C, Sørensen M, Eriksen KT, Tjønneland A, Loft S, Jaensch A, et al. Ambient air pollution and primary liver cancer incidence in four European cohorts within the ESCAPE project. Environ Res. 2017;154:226–33.
Cicalese L, Curcuru G, Montalbano M, Shirafkan A, Georgiadis J, Rastellini C. Hazardous air pollutants and primary liver cancer in Texas. PLoS ONE. 2017;12(10):e0185610.
Pan W-C, Wu C-D, Chen M-J, Huang Y-T, Chen C-J, Su H-J, Yang H-I. Fine particle pollution, alanine transaminase, and liver cancer: a Taiwanese prospective cohort study (REVEAL-HBV). JNCI J Natl Cancer Inst. 2016;108(3):djv341.
Nagel G, Stafoggia M, Pedersen M, Andersen ZJ, Galassi C, Munkenast J, Jaensch A, Sommar J, Forsberg B, Olsson D, et al. Air pollution and incidence of cancers of the stomach and the upper aerodigestive tract in the European Study of Cohorts for Air Pollution Effects (ESCAPE). Int J Cancer. 2018;143(7):1632–43.
Shaddick G, Thomas ML, Mudu P, Ruggery G, Gumy S. Half the world’s population are exposed to increasing air pollution. npj Clim Atmos Sci. 2020;3:23.
Kozielska B, Rogula-Kozłowska W, Klejnowski K. Selected organic compounds in fine particulate matter at the regional background, urban background and urban traffic points in Silesia (Poland). Int J Environ Res. 2015;9(2):575–84.
Nair PRA, Sujatha CH. Organic Pollutants as Endocrine Disruptors: Organometallics, PAHs, Organochlorine, Organophosphate and Carbamate Insecticides, Phthalates, Dioxins, Phytoestrogens, Alkyl Phenols and Bisphenol A. In: Lichtfouse E, Schwarzbauer J, Robert D, editors. Environmental chemistry for a sustainable world: Volume 1: Nanotechnology and health risk edn. Dordrecht: Springer Netherlands; 2012. Pp. 259–309.
Zhang Y, Dong S, Wang H, Tao S, Kiyama R. Biological impact of environmental polycyclic aromatic hydrocarbons (ePAHs) as endocrine disruptors. Environ Pollut. 2016;213:809–24.
Qu SX, Stacey NH. Formation and persistence of DNA adducts in different target tissues of rats after multiple administration of benzo[a]pyrene. Carcinogenesis. 1996;17(1):53–9.
Chen ST, Lin CC, Liu YS, Lin C, Hung PT, Jao CW, Lin PH. Airborne particulate collected from central Taiwan induces DNA strand breaks, Poly(ADP-ribose) polymerase-1 activation, and estrogen-disrupting activity in human breast carcinoma cell lines. J Environ Sci Health Part A Toxic/Hazard Subst Environ Eng. 2013;48(2):173–81.
Li W, Dorans KS, Wilker EH, Rice MB, Ljungman PL, Schwartz JD, Coull BA, Koutrakis P, Gold DR, Keaney JF, et al. Short-term exposure to ambient air pollution and biomarkers of systemic inflammation. Arterioscler Thromb Vasc Biol. 2017;37(9):1793–800.
Chen H, Goldberg MS. The effects of outdoor air pollution on chronic illnesses. McGill J Med MJM. 2009;12(1):58–64.
Weber SA, Insaf TZ, Hall ES, Talbot TO, Huff AK. Assessing the impact of fine particulate matter (PM2.5) on respiratory-cardiovascular chronic diseases in the New York City Metropolitan area using Hierarchical Bayesian Model estimates. Environ Res. 2016;151:399–409.
Beckerman B, Jerrett M, Brook JR, Verma DK, Arain MA, Finkelstein MM. Correlation of nitrogen dioxide with other traffic pollutants near a major expressway. Atmos Environ. 2008;42(2):275–90.
Lemarchand C, Gabet S, Cénée S, Tvardik N, Slama R, Guénel P. Breast cancer risk in relation to ambient concentrations of nitrogen dioxide and particulate matter: results of a population-based case-control study corrected for potential selection bias (the CECILE study). Environ Int. 2021;155:106604.
Andersen ZJ, Stafoggia M, Weinmayr G, Pedersen M, Galassi C, Jorgensen JT, Oudin A, Forsberg B, Olsson D, Oftedal B, et al. Long-term exposure to ambient air pollution and incidence of postmenopausal breast cancer in 15 European Cohorts within the ESCAPE project. Environ Health Perspect. 2017;125(10):107005.
Cheng I, Tseng C, Wu J, Yang J, Conroy SM, Shariff-Marco S, Li L, Hertz A, Gomez SL, Le Marchand L, et al. Association between ambient air pollution and breast cancer risk: the multiethnic cohort study. Int J Cancer. 2020;146(3):699–711.
White A, Keller J, Zhao S, Kaufman J, Sandler D. Air pollution, clustering of particulate matter components and breast cancer. Cancer Epidemiol Biomark Prev. 2019;28(3):624–5.
Hart JE, Bertrand KA, DuPre N, James P, Vieira VM, Tamimi RM, Laden F. Long-term particulate matter exposures during adulthood and risk of breast cancer incidence in the Nurses’ Health Study II prospective cohort. Cancer Epidemiol Biomark Prev. 2016;25(8):1274–6.
Villeneuve PJ, Goldberg MS, Crouse DL, To T, Weichenthal SA, Wall C, Miller AB. Residential exposure to fine particulate matter air pollution and incident breast cancer in a cohort of Canadian women. Environ Epidemiol. 2018;2(3):e021.
Wei Y, Davis J, Bina WF. Ambient air pollution is associated with the increased incidence of breast cancer in US. Int J Environ Health Res. 2012;22(1):12–21.
Hwang J, Bae H, Choi S, Yi H, Ko B, Kim N. Impact of air pollution on breast cancer incidence and mortality: a nationwide analysis in South Korea. Sci Rep. 2020;10(1):5392.
Datzmann T, Markevych I, Trautmann F, Heinrich J, Schmitt J, Tesch F. Outdoor air pollution, green space, and cancer incidence in saxony: a semi-individual cohort study. BMC Public Health. 2018;18(1):715.
Bai L, Shin S, Burnett RT, Kwong JC, Hystad P, van Donkelaar A, Goldberg MS, Lavigne E, Weichenthal S, Martin RV, et al. Exposure to ambient air pollution and the incidence of lung cancer and breast cancer in the Ontario Population Health and Environment Cohort. Int J Cancer. 2020;146(9):2450–9.
Parikh PV, Wei Y. PAHs and PM emissions and female breast cancer incidence in metro Atlanta and rural Georgia. Int J Environ Health Res 2016;1–9.
Andersen ZJ, Ravnskjær L, Andersen KK, Loft S, Brandt J, Becker T, Ketzel M, Hertel O, Lynge E, Bräuner EV. Long-term exposure to fine particulate matter and breast cancer incidence in the Danish nurse cohort study. Cancer Epidemiol Biomark Prev. 2017;26(3):428–30.
Crouse DL, Goldberg MS, Ross NA, Chen H, Labreche F. Postmenopausal breast cancer is associated with exposure to traffic-related air pollution in Montreal, Canada: a case-control study. Environ Health Perspect. 2010;118(11):1578–83.
Hystad P, Villeneuve PJ, Goldberg MS, Crouse DL, Johnson K. Exposure to traffic-related air pollution and the risk of develo** breast cancer among women in eight Canadian provinces: a case–control study. Environ Int. 2015;74:240–8.
Goldberg MS, Labrèche F, Weichenthal S, Lavigne E, Valois MF, Hatzopoulou M, Van Ryswyk K, Shekarrizfard M, Villeneuve PJ, Crouse D, et al. The association between the incidence of postmenopausal breast cancer and concentrations at street-level of nitrogen dioxide and ultrafine particles. Environ Res. 2017;158:7–15.
White AJ, Bradshaw PT, Herring AH, Teitelbaum SL, Beyea J, Stellman SD, Steck SE, Mordukhovich I, Eng SM, Engel LS, et al. Exposure to multiple sources of polycyclic aromatic hydrocarbons and breast cancer incidence. Environ Int. 2016;89–90:185–92.
Shen J, Liao Y, Hopper JL, Goldberg M, Santella RM, Terry MB. Dependence of cancer risk from environmental exposures on underlying genetic susceptibility: an illustration with polycyclic aromatic hydrocarbons and breast cancer. Br J Cancer. 2017;116:1229.
Warner M, Eskenazi B, Mocarelli P, Gerthoux PM, Samuels S, Needham L, Patterson D, Brambilla P. Serum dioxin concentrations and breast cancer risk in the Seveso Women’s Health Study. Environ Health Perspect. 2002;110(7):625–8.
McElroy JA, Shafer MM, Trentham-Dietz A, Hampton JM, Newcomb PA. Cadmium exposure and breast cancer risk. JNCI J Natl Cancer Inst. 2006;98(12):869–73.
Nagata C, Nagao Y, Nakamura K, Wada K, Tamai Y, Tsuji M, Yamamoto S, Kashiki Y. Cadmium exposure and the risk of breast cancer in Japanese women. Breast Cancer Res Treat. 2013;138(1):235–9.
Byrne C, Divekar SD, Storchan GB, Parodi DA, Martin MB. Metals and breast cancer. J Mammary Gland Biol Neoplasia. 2013;18(1):63–73.
Khanjani N, Jafarnejad A-B, Tavakkoli L. Arsenic and breast cancer: a systematic review of epidemiologic studies. Rev Environ Health. 2017;32:267.
Luelsdorf A. Arsenic exposure promotes aggressive breast cancer phenotype. FASEB J. 2017;31(1_supplement):982.
Gaudet HM, Christensen E, Conn B, Morrow S, Cressey L, Benoit J. Methylmercury promotes breast cancer cell proliferation. Toxicol Rep. 2018;5:579–84.
Gallagher CM, Chen JJ, Kovach JS. Environmental cadmium and breast cancer risk. Aging. 2010;2(11):804–14.
Stowers SJ, Anderson MW. Formation and persistence of benzo(a)pyrene metabolite-DNA adducts. Environ Health Perspect. 1985;62:31–9.
Pfohl-Leszkowicz A. Chapter 7 formation, persistence and significance of DNA adduct formation in relation to some pollutants from a broad perspective. In: James CF, editor. Advances in molecular toxicology, vol. 2. Elsevier; 2008. p. 183–239.
White AJ, O’Brien KM, Niehoff NM, Carroll R, Sandler DP. Metallic air pollutants and breast cancer risk in a nationwide cohort study. Epidemiology. 2019;30(1):20–8.
Demers A, Ayotte P, Brisson J, Dodin S, Robert J, Dewailly É. Plasma concentrations of polychlorinated biphenyls and the risk of breast cancer: a congener-specific analysis. Am J Epidemiol. 2002;155(7):629–35.
Dorgan JF, Brock JW, Rothman N, Needham LL, Miller R, Stephenson HE, Schussler N, Taylor PR. Serum organochlorine pesticides and PCBs and breast cancer risk: results from a prospective analysis (USA). Cancer Causes Control. 1999;10(1):1–11.
Holford TR, Zheng T, Mayne ST, Zahm SH, Tessari JD, Boyle P. Joint effects of nine polychlorinated biphenyl (PCB) congeners on breast cancer risk. Int J Epidemiol. 2000;29(6):975–82.
Pesatori AC. TCDD exposure and cancer risk: current knowledge. Epidemiology 2006;17(6).
Warner M, Eskenazi B, Samuels S, Needham L, Brambilla P, Mocarelli P. Serum dioxin concentrations and breast cancer risk in the seveso women. Epidemiology. 2011;22(1):S102.
Swedenborg E, Rüegg J, Mäkelä S, Pongratz I. Endocrine disruptive chemicals: mechanisms of action and involvement in metabolic disorders. J Mol Endocrinol. 2009;43(1):1–10.
Dyer CA. Heavy metals as endocrine-disrupting chemicals. In: Gore AC, editor. Endocrine-disrupting chemicals: from basic research to clinical practice. Totowa: Humana Press; 2007. p. 111–33.
Iavicoli I, Fontana L, Bergamaschi A. The effects of metals as endocrine disruptors. J Toxicol Environ Health B Crit Rev. 2009;12(3):206–23.
Huynh S, von Euler-Chelpin M, Raaschou-Nielsen O, Hertel O, Tjønneland A, Lynge E, Vejborg I, Andersen ZJ. Long-term exposure to air pollution and mammographic density in the Danish Diet. Cancer Health Cohort Environ Health. 2015;14:31.
Muzyka V, Veimer S, Shmidt N. Particle-bound benzene from diesel engine exhaust. (0355–3140 (Print)).
Tsai MY, Hoek G, Eeftens M, de Hoogh K, Beelen R, Beregszászi T, Cesaroni G, Cirach M, Cyrys J, De Nazelle A, et al. Spatial variation of PM elemental composition between and within 20 European study areas–Results of the ESCAPE project. Environ Int. 2015;84:181–92.
Stanczyk FZ, Ploszaj S, Gentzschein E, Qian D, Mishell DR Jr. Effect of oral contraceptives containing 20 and 35 micrograms ethinyl estradiol on urinary prostacyclin and thromboxane metabolite levels in smokers and nonsmokers. Contraception. 1999;59(1):17–23.
Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779.
Willett W, Stampfer MJ, Bain C, Lipnick R, Speizer FE, Rosner B, Cramer D, Hennekens CH. Cigarette smoking, relative weight, and menopause. Am J Epidemiol. 1983;117(6):651–8.
Vienneau D, de Hoogh K, Bechle MJ, Beelen R, van Donkelaar A, Martin RV, Millet DB, Hoek G, Marshall JD. Western European land use regression incorporating satellite- and ground-based measurements of NO2 and PM10. Environ Sci Technol. 2013;47(23):13555–64.
Stanczyk FZ, Brenner PF, Mishell DR Jr, Ortiz A, Gentzschein EK, Goebelsmann U. A radioimmunoassay for norethindrone (NET): measurement of serum NET concentrations following ingestion of NET-containing oral contraceptive steroids. Contraception. 1978;18(6):615–33.
Eeftens M, Beelen R, de Hoogh K, Bellander T, Cesaroni G, Cirach M, Declercq C, Dėdelė A, Dons E, de Nazelle A, et al. Development of land use regression models for PM(2.5), PM(2.5) absorbance, PM(10) and PM(coarse) in 20 European study areas; results of the ESCAPE project. Environ Sci Technol. 2012;46(20):11195–205.
Tarnowska C, Stanczyk D, Wink R. Hypothyroidism after combined therapy of laryngeal cancer. Otolaryngol Pol. 2002;56(6):689–93.
Eeftens M, Tsai M-Y, Ampe C, Anwander B, Beelen R, Bellander T, Cesaroni G, Cirach M, Cyrys J, de Hoogh K, et al. Spatial variation of PM2.5, PM10, PM2.5 absorbance and PMcoarse concentrations between and within 20 European study areas and the relationship with NO2—Results of the ESCAPE project. Atmos Environ. 2012;62:303–17.
Cyrys J, Eeftens M, Heinrich J, Ampe C, Armengaud A, Beelen R, Bellander T, Beregszaszi T, Birk M, Cesaroni G, et al. Variation of NO2 and NOx concentrations between and within 36 European study areas: Results from the ESCAPE study. Atmos Environ. 2012;62:374–90.
Ahern TP, Broe A, Lash TL, Cronin-Fenton DP, Ulrichsen SP, Christiansen PM, Cole BF, Tamimi RM, Sørensen HT, Damkier P. Phthalate exposure and breast cancer incidence: a Danish nationwide cohort study. J Clin Oncol. 2019;37(21):1800–9.
Wang W, Meng L, Hu Z, Yuan X, Zeng W, Li K, Luo H, Tang M, Zhou X, Tian X et al. The association between outdoor air pollution and lung cancer risk in seven eastern metropolises of China: trends in 2006–2014 and sex differences. Front Oncol 2022;12.
Therneau T, Grambsch P. Modeling survival data: extending the cox model, vol. 48; 2000.
Xu X, Jiang SY, Wang TY, Bai Y, Zhong M, Wang A, Lippmann M, Chen LC, Rajagopalan S, Sun Q. Inflammatory response to fine particulate air pollution exposure: neutrophil versus monocyte. PLoS ONE. 2013;8(8):e71414.
Chuang KJ, Chan CC, Su TC, Lee CT, Tang CS. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med. 2007;176(4):370–6.
Zhang Z, Hoek G, Chang LY, Chan TC, Guo C, Chuang YC, Chan J, Lin C, Jiang WK, Guo Y et al. Particulate matter air pollution, physical activity and systemic inflammation in Taiwanese adults. Int J Hyg Environ Health 2017.
Pope CA 3rd, Bhatnagar A, McCracken JP, Abplanalp W, Conklin DJ, O’Toole T. Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circ Res. 2016;119(11):1204–14.
Viehmann A, Hertel S, Fuks K, Eisele L, Moebus S, Mohlenkamp S, Nonnemacher M, Jakobs H, Erbel R, Jockel KH, et al. Long-term residential exposure to urban air pollution, and repeated measures of systemic blood markers of inflammation and coagulation. Occup Environ Med. 2015;72(9):656–63.
Lanki T, Hampel R, Tiittanen P, Andrich S, Beelen R, Brunekreef B, Dratva J, De Faire U, Fuks KB, Hoffmann B, et al. Air pollution from road traffic and systemic inflammation in adults: a cross-sectional analysis in the European ESCAPE project. Environ Health Perspect. 2015;123(8):785–91.
Araujo JA. Particulate air pollution, systemic oxidative stress, inflammation, and atherosclerosis. Air Qual Atmos Health. 2010;4(1):79–93.
Callahan CL, Bonner MR, Nie J, Han D, Wang Y, Tao MH, Shields PG, Marian C, Eng KH, Trevisan M, et al. Lifetime exposure to ambient air pollution and methylation of tumor suppressor genes in breast tumors. Environ Res. 2017;161:418–24.
Roepstorff V, Ostenfeldt N, Autrup H. Extracts of airborne particulates collected at different locations in the Copenhagen area induce the expression of cytochrome P-450IA1. J Toxicol Environ Health. 1990;30(4):225–37.
Coventry BJ, Morton J. CD1a-positive infiltrating-dendritic cell density and 5-year survival from human breast cancer. Br J Cancer. 2003;89(3):533–8.
Sahay D, Terry MB, Miller R. Is breast cancer a result of epigenetic responses to traffic-related air pollution? A review of the latest evidence. Epigenomics. 2019;11(6):701–14.
La Merrill M, Emond C, Kim MJ, Antignac J-P, Le Bizec B, Clément K, Birnbaum LS, Barouki R. Toxicological function of adipose tissue: focus on persistent organic pollutants. Environ Health Perspect. 2013;121(2):162–9.
Morris JJ, Seifter E. The role of aromatic hydrocarbons in the genesis of breast cancer. Med Hypotheses. 1992;38(3):177–84.
Lopez-Espinosa MJ, Kiviranta H, Araque P, Ruokojärvi P, Molina-Molina JM, Fernandez MF, Vartiainen T, Olea N. Dioxins in adipose tissue of women in Southern Spain. Chemosphere. 2008;73(6):967–71.
Niehoff NM, Terry MB, Bookwalter DB, Kaufman JD, O’Brien KM, Sandler DP, White AJ. Air pollution and breast cancer: an examination of modification by underlying familial breast cancer risk. Cancer Epidemiol Biomark Prev. 2022;31(2):422–9.
Boffetta P, Nyberg F. Contribution of environmental factors to cancer risk. Br Med Bull. 2003;68(1):71–94.
Mostafavi N, Vlaanderen J, Chadeau-Hyam M, Beelen R, Modig L, Palli D, Bergdahl IA, Vineis P, Hoek G, Kyrtopoulos S, et al. Inflammatory markers in relation to long-term air pollution. Environ Int. 2015;81:1–7.
Esquivel-Velázquez M, Ostoa-Saloma P, Palacios-Arreola MI, Nava-Castro KE, Castro JI, Morales-Montor J. The role of cytokines in breast cancer development and progression. J Interferon Cytokine Res. 2015;35(1):1–16.
Ragettli MS, Phuleria HC, Tsai M-Y, Schindler C, de Nazelle A, Ducret-Stich RE, Ineichen A, Perez L, Braun-Fahrländer C, Probst-Hensch N, et al. The relevance of commuter and work/school exposure in an epidemiological study on traffic-related air pollution. J Expo Sci Environ Epidemiol. 2014;25:474.
Dibben C, Clemens T. Place of work and residential exposure to ambient air pollution and birth outcomes in Scotland, using geographically fine pollution climate map** estimates. Environ Res. 2015;140:535–41.
Acknowledgements
This research has been conducted using the UK Biobank Resource under Application Number 67356.
Funding
None.
Author information
Authors and Affiliations
Contributions
Conceptualization: CS, LY. Formal Analysis: CS, LY. Methodology: LY, CS, BS, SD. Supervision: LY. Writing—original draft: CS, LY. Writing—review & editing: LY, BS, SD, HQ, and DB.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
UK Biobank protocol was approved by the NorthWest Multi-centre Research Ethics Committee (MREC), which covers the UK. All participants of UK Biobank provided written consent at recruitment.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table 1.
Summary of the follow-up times and breast cancer case inclusions in different models for associations of air pollution measures with breast cancer risk. Table 2. Correlation between 2010 air pollution measures. Table 3. Characteristics of the study participants at baseline, by the quartiles of 2005 Nitrogen dioxidelevels. Table 4. Associations of 5-year lagged air pollution exposure with breast cancer risk. Table 5. Association of air pollution measures with invasive breast cancer risk
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Smotherman, C., Sprague, B., Datta, S. et al. Association of air pollution with postmenopausal breast cancer risk in UK Biobank. Breast Cancer Res 25, 83 (2023). https://doi.org/10.1186/s13058-023-01681-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13058-023-01681-w