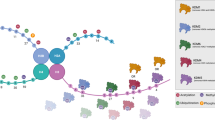
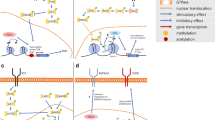
Abstract
Targeted therapies, including small molecule inhibitors directed against aberrant kinase signaling and chromatin regulators, are emerging treatment options for high-grade gliomas (HGG). However, when translating these inhibitors into the clinic, their efficacy is generally limited to partial and transient responses. Recent studies in models of high-grade gliomas reveal a convergence of epigenetic regulators and kinase signaling networks that often cooperate to promote malignant properties and drug resistance. This review examines the interplay between five well-characterized groups of chromatin regulators, including the histone deacetylase (HDAC) family, bromodomain and extraterminal (BET)-containing proteins, protein arginine methyltransferase (PRMT) family, Enhancer of zeste homolog 2 (EZH2), and lysine-specific demethylase 1 (LSD1), and various signaling pathways essential for cancer cell growth and progression. These specific epigenetic regulators were chosen for review due to their targetability via pharmacological intervention and clinical relevance. Several studies have demonstrated improved efficacy from the dual inhibition of the epigenetic regulators and signaling kinases. Overall, the interactions between epigenetic regulators and kinase signaling pathways are likely influenced by several factors, including individual glioma subtypes, preexisting mutations, and overlap**/interdependent functions of the chromatin regulators. The insights gained by understanding how the genome and epigenome cooperate in high-grade gliomas will guide the design of future therapeutic strategies that utilize dual inhibition with improved efficacy and overall survival.
Similar content being viewed by others
Introduction
High-grade gliomas (HGGs) are central nervous system (CNS) tumors that occur in both children and adults, although bear distinct molecular features and neuroanatomy in younger compared with older patients [1,2,3,4]. While these types of cancers are relatively rare, patient prognosis is quite poor, with an average 2-year overall survival of only 20% [5], despite multimodal treatment regimens consisting of surgery, radiation therapy, and chemotherapy [6, 7]. Tumor location or disease progression can further complicate therapeutic interventions; therefore, novel treatment modalities such as targeted therapies, including epigenetically directed therapies, are critical to improve patient outcomes.
The identification of cancer-specific targets supporting tumor growth is a major research objective to develop therapeutic options in HGG. Advances in sequencing technology and single-cell analyses of HGGs and subsets of medulloblastoma have revealed frequent alterations in kinase signaling proteins and proteins which regulate their activity (i.e., tumor suppressors) [8,9,10,11]. For example, several receptor tyrosine kinases (RTKs) are frequently overexpressed or mutated in HGG [8, 11], causing hyperactivity of downstream signaling cascades leading to increased cell proliferation, growth, and survival of cancer cells. To date, there has been limited clinical efficacy from single-agent inhibition of dysregulated signaling pathways in HGG [12].
In addition to aberrant proliferative signaling pathways, disruption of the epigenome has been identified as a contributor to tumorigenesis, cancer progression, and chemotherapy resistance [13, 14]. Epigenetic regulators, termed “writers” and “readers”, catalyze the reversible chemical modifications of histones and DNA [15]. The most predominant epigenetic alterations are post-translational modifications to histones, involving the addition and removal of methyl and acetyl marks, and DNA methylation [16]. The epigenetic “readers” recognize specific modifications and translate their effects on gene expression and other cellular processes. Pharmacological inhibitors have been developed to target various chromatin regulators, such as those directed against the histone deacetylase (HDAC) family, bromodomain and extraterminal (BET)-containing proteins, protein arginine methyltransferase (PRMT) family, Enhancer of zeste homolog 2 (EZH2), and lysine-specific demethylase 1 (LSD1).
Understanding the complex interactions between the cancer genome and epigenome is paramount when designing novel therapeutic strategies. Targeted therapies directed solely at epigenetic regulators or dysregulated kinases have shown limited success in sustaining clinical responses in HGG [17,18,19,20,21,22]. Evidence increasingly shows that epigenetic modulators cooperate with several relevant kinase signaling pathways in gliomas to promote cancer progression and contribute to therapeutic resistance. In this review, we systematically explore the epigenetic regulators and their interactions with kinase signaling networks in HGG and how combination strategies have developed and could be envisioned via existing small molecule inhibitors.
Molecular dysfunction of the genome and epigenome
Kinase signaling pathway alterations
Over the past two decades, substantial effort has been made to sequence and molecularly characterize primary cancer samples across all cancer types, including adult and pediatric HGG. This initiative has identified frequent alterations in several kinase signaling pathways and their regulators (Table 1.) One of the more common alterations detected involve the RTKs, including epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor (PDGFRA), and KIT, also known as mast/stem cell growth factor receptor. These alterations are composed of gene mutations and/or copy number amplification, which hyperactivate downstream signaling networks involved in cell proliferation, differentiation, cell growth, metabolism, and survival. One convergent activating pathway downstream of these RTKs is phosphatidylinositol-3 kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) signaling. Alterations are found in the catalytic subunit of PI3K, PIK3CA, and the regulatory subunit, PIK3R1, which can activate downstream signaling at AKT and mTOR. Additionally, copy number deletions are found within Phosphatase and Tensin Homolog (PTEN), a tumor suppressor that negatively regulates PI3K/AKT signaling. A common deletion in PTEN includes homozygous deletion, which contributes to hyperactivation of the PI3K/AKT/mTOR pathway.
In addition to the PI3K/AKT pathway, the mitogen-activated protein kinase (MAPK) cascade is downstream of RTKs and involved in HGGs. In this pathway, loss of function mutation in neurofibromin 1 (NF1), a small GTPase activating protein that regulates signal transduction through RAS (Rat sarcoma virus), affects the downstream MAPK signaling leading to activation. Lastly, the cell cycle control gene, cyclin-dependent kinase inhibitor 2 A (CDKN2A), is frequently found to have homozygous deletion. Beyond the aforementioned kinase alterations, several other gene alterations form the HGG genomic landscape. When comparing adult and pediatric gene alterations, there are many overlap** changes within the kinase signaling pathways; however, the frequency at which they occur differs with age.
Epigenetic abnormalities from histone modifiers
Unlike genetic changes, epigenetic dysregulation is not typically the result of mutations and instead occurs through alterations in chromatin accessibility and gene expression [25]. Transcriptional dysregulation can result from overexpression of chromatin modulators and their subsequent hyperactivity. The absence or presence of specific histone modifications, including acetylation and methylation of critical amino acids, governs chromatin structure leading to changes in gene transcription.
Numerous enzymes and protein complexes have been identified to be responsible for regulating the expression of various genes. These epigenetic proteins are over- or under-expressed in tumors, including HGG. One of the most well-studied epigenetic modulators is the HDAC family of enzymes. In gliomas, HDAC activity generally suppresses the expression of regulatory proteins and DNA repair genes as a component of repressive transcriptional complexes. Several HDAC family members have been identified as having altered gene expression in HGG. For example, HDAC1, 2, 3, and 7 have been found to be overexpressed in grade IV gliomas compared to normal brain tissue and low-grade gliomas [26]. Meanwhile, other enzymes that act as readers of these acetylated amino acids are often dysregulated in gliomas. These sets of proteins include the BET proteins. Two BET proteins, BRD2 and BRD4, are significantly overexpressed in gliomas, and the knockout of BRD4 diminishes glioma proliferation [27]. Similarly, PRMT enzymes, such as PRMT1, 2, and 5, function to methylate arginine residues and can promote dysregulation in brain tumors arising from the aberrant expression or activity [28,29,30,31]. Another epigenetic regulator relevant to HGG is the methyltransferase, EZH2, which is overexpressed in gliomas and correlated with high-grade gliomas [32, 33]. EZH2’s activity contributes to glioma progression by silencing tumor-suppressor genes [34]. An additional epigenetic modulator that is commonly dysregulated in gliomas is LSD1, a histone demethylase that is a component of several repressive complexes and is associated with reduced gene transcription. LSD1 is found to be overexpressed in several cancer types, including glioblastoma [35,36,37], and has been associated with poor patient prognosis in certain types of cancer [53]. For example, methylation profiling can be used as a surrogate to identify the mutation status of isocitrate dehydrogenase (IDH) [53]. IDH mutations, associated with the production of an oncometabolite (2-hydroxyglutarate), leads to global hypermethylation of the CpG islands, thereby causing gene silencing [54, 55]. The presence of this methylation pattern in gliomas is called the glioma – CpG island methylator phenotype (G-CIMP) and occurs frequently in low-grade gliomas [54, 55]. Large cohort studies have shown that G-CIMP is highly associated with the presence of an IDH mutation and correlated with a favorable prognosis [56]. Similarly, histone mutations common to pediatric HGGs lead to global changes in DNA methylation that can be detected through DNA methylation profiling [55, 57]. Methylation profiling can be used to derive copy number profiles inclusive of gene amplifications/deletions and chromosome alterations (7+/10- and 1p/19q codeletion) associated with different glioma subtypes [53]. Finally, the DNA methylation status of O6-methylguanine-DNA methyltransferase (MGMT) is widely used as a predictive biomarker for therapeutic response to the alkylating agent, temozolomide, and as a prognostic marker in glioblastoma patients [55, 58]. Thus, the value of methylation profiling in gliomas is expanding beyond its impact on gene expression, to help determine tumor phenotype and prognosis.
The World Health Organization (WHO) is beginning to adopt DNA methylation profiling in their classification of CNS tumors to provide a robust classification method and identify new tumor types and subtypes [53]. Notably, the emergence of DNA methylation profiling reveals an intriguing overlap with distinct kinase mutations traditionally associated with gliomas. For example, through methylation profiling, HGG is grouped into eight classes, including three classes characterized by RTK alterations, such as PDGFRA and EGFR amplification [59]. Furthermore, in pediatric HGGs with histone alterations, methylation profiling revealed several alterations in kinase signaling pathways, including PDGFRA, EGFR, KIT, MET, KRAS, PTEN, PIK3CA, and CDK4/6 [57]. Additional studies of pediatric HGGs used DNA methylation alongside whole genome sequencing and RNA sequencing in a clinical trial to molecularly characterize tumors and determine a treatment approach based on the identified alterations [60]. Overall, these studies highlight the potential of methylation profiling and its utility in understanding glioma subtypes, their associated kinase alterations, and appropriate therapeutic selection.
Single-agent targeted therapies in clinical development
Kinase inhibitors
Clinical trials have been implemented and are currently underway to assess the safety and efficacy of small molecule inhibitors directed against protein or lipid kinases in HGG patients with kinase dysregulation and aberrant signaling activation (Table 3). Several clinical studies have tested the effects of inhibition of RTKs, including small molecule inhibition of EGFR, PDGFR, FGFR (fibroblast growth factor receptor), c-MET (mesenchymal-epithelial transition factor), KIT (also referred to as CD117), AXL, and VEGFR (vascular endothelial growth factor receptor). Numerous inhibitors have assessed inhibition of EGFR in HGG as single agents and predominantly show a tolerable safety profile without improvements in survival. Likewise, the results from available clinical trials targeting other RTKs, PDGFR and MET, show tolerable safety profiles but limited efficacy. Currently, other RTK inhibitors targeting FGFR and KIT are being investigated in early-phase trials to determine the safety and efficacy of these small molecule inhibitors for adult (FGFR and AXL) and pediatric HGGs (KIT). Studies are also investigating the inhibition of intracellular signaling kinases in HGG downstream of RTKs. For example, clinical studies are ongoing in pediatric HGG patients receiving small molecule inhibitors against MEK and PI3K. Overall, many completed clinical trials conclude that single-agent inhibitors have tolerable toxicities in phase I but limited efficacy when assessed in phase II trials. This lack of clinical efficacy has been extensively reviewed elsewhere [61,126, 153]. Thus, this idea of identifying predictive biomarkers, or precision medicine, by analyzing a patient’s genomic landscape will improve patient selection and spare non-responders from toxicity. Inhibitors need to be more effective at lower doses and given to patients with a high likelihood of a response to improve the toxicity profile of targeted therapy.
Beyond safety, resistance is another obstacle to targeting chromatin modifiers and kinase signaling proteins. Due to the redundancy in kinase signaling pathways, inhibiting a single kinase is often unsuccessful, as compensatory pathways mitigate the effects of single-agent kinase inhibition. An additional mechanism of resistance to kinase inhibition is through epigenetic changes. Furthermore, epigenetic regulation is highly interdependent between the writers, readers, and erasers and can produce unexpected effects that may limit therapeutic efficacy. For example, inhibition of HDAC via vorinostat can increase H3K4 methylation, making it vulnerable to LSD1 demethylation [35]. One solution to overcome resistance and improve drug toxicity profile is to design combination treatment strategies inclusive of kinase inhibition and epigenetically directed inhibition.
Several in vitro and in vivo models have identified synergistic relationships between kinase and epigenetic inhibition. However, there is still a need to explore the interplay between epigenetic regulators and kinase signaling pathways and understand their specific mechanisms. Identifying safe drug combinations with synergistic or additive effects may afford improvements in efficacy and create an opportunity in the clinical trial setting for HGGs. In the clinic, treatment combinations could allow for dose reductions that exert a clinical effect and decrease unwanted side effects. Certain drug combinations may also circumvent resistance mechanisms associated with single-agent inhibition to extend a drug response. Additionally, other combination treatment strategies to overcome the lack of single-agent success may include introducing immunotherapies to either kinase inhibition or epigenetically directed agents. Previous studies have shown that epigenetic alterations change the tumor microenvironment to contribute to the immune suppressive niche for tumor cells. More recently, studies in several cancer types have shown inhibition of epigenetic regulators (LSD1, EZH2, BET proteins) can enhance the anti-tumor immune response of anti-PD1 therapy [13, 154,155,156]. Interestingly, studies in other cancer models highlight that kinase inhibition can enhance anti-tumor effects with immunotherapies [157,158,159]. Investigating triple therapy targeting chromatin modulators and kinase pathways and using immunotherapy in the ongoing efforts to attenuate tumor cell proliferation to improve patient outcomes and increase overall survivability would be worthwhile. In conclusion, enhancing our understanding of the cooperation across the HGG epigenome and genome will guide the development of new therapeutic strategies.
Data Availability
Not applicable.
Abbreviations
- 4-EBP1:
-
Eukaryotic translation initiation factor 4E-binding protein 1
- AMP:
-
Amplification
- AURKA:
-
Aurora kinase A
- BBB:
-
Blood-brain-barrier
- BET:
-
Bromodomain and extraterminal
- BRD) c-Jun N-terminal kinase (JNK:
-
Bromodomain
- c-MET:
-
Mesenchymal-epithelial transition factor
- CDKN2A:
-
Cyclin-dependent kinase inhibitor 2 A
- CDKs:
-
Cyclin-dependent kinases
- CNA:
-
Copy number alterations
- CNS:
-
Central nervous system
- CoREST:
-
REST corepressor 1
- DCR:
-
Disease control rate
- DIPG:
-
Diffuse intrinsic pontine glioma
- DLT:
-
Dose-limiting toxicity
- DMG:
-
Diffuse midline gliomas
- DNMT:
-
DNA methyltransferase
- DZNep:
-
3-Deazaneplanocin A
- EGFR:
-
Epidermal growth factor receptor
- ERK:
-
Extracellular signal-regulated kinase
- EZH2:
-
Enhancer of zeste homolog 2
- FGFR:
-
Fibroblast growth factor receptor
- FOXM1:
-
Forkhead box M1
- G-CIMP:
-
Glioma – CpG island methylator phenotype
- GBM:
-
Glioblastoma
- H3K4/9/27:
-
Histone 3 lysine 4/9/27
- H4K5/8/12:
-
Histone 4 lysine 5/8/12
- HATs:
-
Histone acetyltransferase
- HDAC:
-
Histone deacetylase
- HGGs:
-
High-grade gliomas
- HMBA:
-
Hexamethylene bisacetamide
- HOMDEL:
-
Homozygous deletion
- HOTAIR:
-
Hox transcript antisense intergenic RNA HOTAIR
- IDH:
-
Isocitrate dehydrogenase
- JAK:
-
Janus kinase
- KIT:
-
Mast/stem cell growth factor receptor
- lnRNA:
-
Long noncoding RNA
- LSD1/KDM1A:
-
Lysine-specific demethylase 1
- MAPK:
-
Mitogen-activated protein kinase
- MEK:
-
Mitogen-activated protein kinase kinase
- MELK:
-
Maternal embryonic leucine-zipper kinases
- MGMT:
-
O6-methylguanine-DNA methyltransferase
- MKP1:
-
MAPK phosphatase 1
- MTD:
-
Maximum tolerated dose
- mTOR:
-
Mammalian target of rapamycin
- NF1:
-
Neurofibromin 1
- NuRD:
-
Nucleosome remodeling and deacetylase
- ORR:
-
Overall response rate
- OS:
-
Overall survival
- P-TEFb:
-
Positive transcription elongation factor b
- PcG:
-
Polycomb-group
- PD:
-
Pharmacodynamic
- PDGFRA:
-
Platelet-derived growth factor receptor
- PFS:
-
Progression-free survival
- pHGG:
-
Pediatric high-grade glioma
- PI3K:
-
Phosphatidylinositol-3 kinase
- PK:
-
Pharmacokinetic
- PRC2:
-
Polycomb repressive complex
- PRMT:
-
Protein arginine methyltransferase
- PTEN:
-
Phosphatase and tensin homolog
- RAS:
-
Rat sarcoma virus
- RB:
-
Retinoblastoma
- RCC1:
-
Regulator of chromosome condensation 1
- RT:
-
Radiation therapy
- RTKs:
-
Receptor tyrosine kinases
- S6K1:
-
S6 kinase beta-1
- SAH:
-
S-adenosyl homocysteine
- SAM:
-
S-adenosyl-L-methionine
- STAT:
-
Signal transducer and activator of transcription
- VEGFR:
-
Vascular endothelial growth factor receptor
- WHO:
-
World Health Organization
References
Ceccarelli M, Barthel FP, Malta TM, Sabedot TS, Salama SR, Murray BA, et al. Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell. 2016;164(3):550–63.
Aggarwal P, Luo W, Pehlivan KC, Hoang H, Rajappa P, Cripe TP, et al. Pediatric versus adult high grade glioma: immunotherapeutic and genomic considerations. Front Immunol. 2022;13:1038096.
Sturm D, Bender S, Jones DT, Lichter P, Grill J, Becher O, et al. Paediatric and adult glioblastoma: multiform (epi)genomic culprits emerge. Nat Rev Cancer. 2014;14(2):92–107.
Sturm D, Witt H, Hovestadt V, Khuong-Quang DA, Jones DT, Konermann C, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22(4):425–37.
Marra JS, Mendes GP, Yoshinari GH, da Silva Guimarães F, Mazin SC, de Oliveira HF. Survival after radiation therapy for high-grade glioma. Rep Pract Oncol Radiother. 2019;24(1):35–40.
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96.
Tan AC, Ashley DM, López GY, Malinzak M, Friedman HS, Khasraw M. Management of glioblastoma: state of the art and future directions. CA Cancer J Clin. 2020;70(4):299–312.
Sakthikumar S, Roy A, Haseeb L, Pettersson ME, Sundström E, Marinescu VD, et al. Whole-genome sequencing of glioblastoma reveals enrichment of non-coding constraint mutations in known and novel genes. Genome Biol. 2020;21(1):127.
Northcott PA, Buchhalter I, Morrissy AS, Hovestadt V, Weischenfeldt J, Ehrenberger T, et al. The whole-genome landscape of medulloblastoma subtypes. Nature. 2017;547(7663):311–7.
Buczkowicz P, Hoeman C, Rakopoulos P, Pajovic S, Letourneau L, Dzamba M, et al. Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat Genet. 2014;46(5):451–6.
Paugh BS, Broniscer A, Qu C, Miller CP, Zhang J, Tatevossian RG, et al. Genome-wide analyses identify recurrent amplifications of receptor tyrosine kinases and cell-cycle regulatory genes in diffuse intrinsic pontine glioma. J Clin Oncol. 2011;29(30):3999–4006.
Wang H, Xu T, Jiang Y, Xu H, Yan Y, Fu D, et al. The challenges and the promise of molecular targeted therapy in malignant gliomas. Neoplasia. 2015;17(3):239–55.
Lu Y, Chan YT, Tan HY, Li S, Wang N, Feng Y. Epigenetic regulation in human cancer: the potential role of epi-drug in cancer therapy. Mol Cancer. 2020;19(1):79.
Muntean AG, Hess JL. Epigenetic dysregulation in cancer. Am J Pathol. 2009;175(4):1353–61.
Cheng Y, He C, Wang M, Ma X, Mo F, Yang S, et al. Targeting epigenetic regulators for cancer therapy: mechanisms and advances in clinical trials. Signal Transduct Target Ther. 2019;4:62.
Gibney ER, Nolan CM. Epigenetics and gene expression. Heredity (Edinb). 2010;105(1):4–13.
Pan PC, Magge RS. Mechanisms of EGFR resistance in glioblastoma. Int J Mol Sci. 2020;21(22).
Hottinger A, Sanson M, Moyal E, Delord J-P, Micheli RD, Rezai K, et al. Dose optimization of MK-8628 (OTX015), a small molecule inhibitor of bromodomain and extra-terminal (BET) proteins, in patients (pts) with recurrent glioblastoma (GB). J Clin Oncol. 2016;34(15):e14123.
Monje M, Cooney T, Glod J, Huang J, Peer CJ, Faury D et al. A phase I trial of panobinostat in children with diffuse intrinsic pontine glioma: a report from the Pediatric Brain Tumor Consortium (PBTC-047). Neuro Oncol. 2023.
Lassman AB, Sepúlveda-Sánchez JM, Cloughesy TF, Gil-Gil MJ, Puduvalli VK, Raizer JJ, et al. Infigratinib in patients with recurrent gliomas and FGFR alterations: a multicenter phase II study. Clin Cancer Res. 2022;28(11):2270–7.
Tinkle CL, Broniscer A, Chiang J, Campagne O, Huang J, Orr BA, et al. Phase I study using crenolanib to target PDGFR kinase in children and young adults with newly diagnosed DIPG or recurrent high-grade glioma, including DIPG. Neurooncol Adv. 2021;3(1):vdab179.
Hu H, Mu Q, Bao Z, Chen Y, Liu Y, Chen J, et al. Mutational landscape of secondary glioblastoma guides MET-targeted trial in brain tumor. Cell. 2018;175(6):1665–78e18.
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–4.
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1.
Zoghbi HY, Beaudet AL. Epigenetics and human disease. Cold Spring Harb Perspect Biol. 2016;8(2):a019497.
Was H, Krol SK, Rotili D, Mai A, Wojtas B, Kaminska B, et al. Histone deacetylase inhibitors exert anti-tumor effects on human adherent and stem-like glioma cells. Clin Epigenetics. 2019;11(1):11.
Pastori C, Daniel M, Penas C, Volmar CH, Johnstone AL, Brothers SP, et al. BET bromodomain proteins are required for glioblastoma cell proliferation. Epigenetics. 2014;9(4):611–20.
Yan F, Alinari L, Lustberg ME, Martin LK, Cordero-Nieves HM, Banasavadi-Siddegowda Y, et al. Genetic validation of the protein arginine methyltransferase PRMT5 as a candidate therapeutic target in glioblastoma. Cancer Res. 2014;74(6):1752–65.
Gu X, He M, Lebedev T, Lin CH, Hua ZY, Zheng YG, et al. PRMT1 is an important factor for medulloblastoma cell proliferation and survival. Biochem Biophys Rep. 2022;32:101364.
Wang S, Tan X, Yang B, Yin B, Yuan J, Qiang B, et al. The role of protein arginine-methyltransferase 1 in gliomagenesis. BMB Rep. 2012;45(8):470–5.
Dong F, Li Q, Yang C, Huo D, Wang X, Ai C, et al. PRMT2 links histone H3R8 asymmetric dimethylation to oncogenic activation and tumorigenesis of glioblastoma. Nat Commun. 2018;9(1):4552.
Chen YN, Hou SQ, Jiang R, Sun JL, Cheng CD, Qian ZR. EZH2 is a potential prognostic predictor of glioma. J Cell Mol Med. 2021;25(2):925–36.
Zhang J, Chen L, Han L, Shi Z, Pu P, Kang C. EZH2 is a negative prognostic factor and exhibits pro-oncogenic activity in glioblastoma. Cancer Lett. 2015;356(2 Pt B):929–36.
Chang CJ, Hung MC. The role of EZH2 in tumour progression. Br J Cancer. 2012;106(2):243–7.
Singh MM, Manton CA, Bhat KP, Tsai WW, Aldape K, Barton MC, et al. Inhibition of LSD1 sensitizes glioblastoma cells to histone deacetylase inhibitors. Neuro Oncol. 2011;13(8):894–903.
Sareddy GR, Viswanadhapalli S, Surapaneni P, Suzuki T, Brenner A, Vadlamudi RK. Novel KDM1A inhibitors induce differentiation and apoptosis of glioma stem cells via unfolded protein response pathway. Oncogene. 2017;36(17):2423–34.
Stitzlein LM, Gangadharan A, Walsh LM, Nam D, Espejo AB, Singh MM, et al. Comparison of pharmacological inhibitors of lysine-specific demethylase 1 in glioblastoma stem cells reveals inhibitor-specific efficacy profiles. Front Neurol. 2023;14:1112207.
Ding J, Zhang ZM, **a Y, Liao GQ, Pan Y, Liu S, et al. LSD1-mediated epigenetic modification contributes to proliferation and metastasis of colon cancer. Br J Cancer. 2013;109(4):994–1003.
Nagasawa S, Sedukhina AS, Nakagawa Y, Maeda I, Kubota M, Ohnuma S, et al. LSD1 overexpression is associated with poor prognosis in basal-like breast cancer, and sensitivity to PARP inhibition. PLoS ONE. 2015;10(2):e0118002.
Zhao ZK, Yu HF, Wang DR, Dong P, Chen L, Wu WG, et al. Overexpression of lysine specific demethylase 1 predicts worse prognosis in primary hepatocellular carcinoma patients. World J Gastroenterol. 2012;18(45):6651–6.
Jie D, Zhongmin Z, Guoqing L, Sheng L, Yi Z, **g W, et al. Positive expression of LSD1 and negative expression of E-cadherin correlate with metastasis and poor prognosis of colon cancer. Dig Dis Sci. 2013;58(6):1581–9.
Yu Y, Wang B, Zhang K, Lei Z, Guo Y, **ao H, et al. High expression of lysine-specific demethylase 1 correlates with poor prognosis of patients with esophageal squamous cell carcinoma. Biochem Biophys Res Commun. 2013;437(2):192–8.
Lee MG, Wynder C, Bochar DA, Hakimi MA, Cooch N, Shiekhattar R. Functional interplay between histone demethylase and deacetylase enzymes. Mol Cell Biol. 2006;26(17):6395–402.
Shi YJ, Matson C, Lan F, Iwase S, Baba T, Shi Y. Regulation of LSD1 histone demethylase activity by its associated factors. Mol Cell. 2005;19(6):857–64.
You A, Tong JK, Grozinger CM, Schreiber SL. CoREST is an integral component of the CoREST- human histone deacetylase complex. Proc Natl Acad Sci U S A. 2001;98(4):1454–8.
Hakimi MA, Bochar DA, Chenoweth J, Lane WS, Mandel G, Shiekhattar R. A core-BRAF35 complex containing histone deacetylase mediates repression of neuronal-specific genes. Proc Natl Acad Sci U S A. 2002;99(11):7420–5.
Wang Y, Zhang H, Chen Y, Sun Y, Yang F, Yu W, et al. LSD1 is a subunit of the NuRD complex and targets the metastasis programs in breast cancer. Cell. 2009;138(4):660–72.
Basta J, Rauchman M. The nucleosome remodeling and deacetylase complex in development and disease. Transl Res. 2015;165(1):36–47.
Yang Y, Li G. Post-translational modifications of PRC2: signals directing its activity. Epigenetics Chromatin. 2020;13(1):47.
Friesen WJ, Paushkin S, Wyce A, Massenet S, Pesiridis GS, Van Duyne G, et al. The methylosome, a 20S complex containing JBP1 and pICln, produces dimethylarginine-modified sm proteins. Mol Cell Biol. 2001;21(24):8289–300.
Liao Y, Luo Z, Lin Y, Chen H, Chen T, Xu L, et al. PRMT3 drives glioblastoma progression by enhancing HIF1A and glycolytic metabolism. Cell Death Dis. 2022;13(11):943.
Huang T, Yang Y, Song X, Wan X, Wu B, Sastry N, et al. PRMT6 methylation of RCC1 regulates mitosis, tumorigenicity, and radiation response of glioblastoma stem cells. Mol Cell. 2021;81(6):1276–91e9.
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–51.
Noushmehr H, Weisenberger DJ, Diefes K, Phillips HS, Pujara K, Berman BP, et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17(5):510–22.
Wenger A, Carén H. Methylation profiling in diffuse gliomas: diagnostic value and considerations. Cancers (Basel). 2022;14(22).
Malta TM, de Souza CF, Sabedot TS, Silva TC, Mosella MS, Kalkanis SN, et al. Glioma CpG island methylator phenotype (G-CIMP): biological and clinical implications. Neuro Oncol. 2018;20(5):608–20.
Chai RC, Yan H, An SY, Pang B, Chen HY, Mu QH, et al. Genomic profiling and prognostic factors of H3 K27M-mutant spinal cord diffuse glioma. Brain Pathol. 2023;33(4):e13153.
Della Monica R, Cuomo M, Buonaiuto M, Costabile D, Franca RA, Del Caro B. M, MGMT and whole-genome DNA methylation impacts on diagnosis, prognosis and therapy of Glioblastoma multiforme. Int J Mol Sci. 2022;23(13).
Capper D, Jones DTW, Sill M, Hovestadt V, Schrimpf D, Sturm D, et al. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555(7697):469–74.
Kline C, Jain P, Kilburn L, Bonner ER, Gupta N, Crawford JR, et al. Upfront biology-guided therapy in diffuse intrinsic pontine glioma: therapeutic, molecular, and biomarker outcomes from PNOC003. Clin Cancer Res. 2022;28(18):3965–78.
Aldaz P, Arozarena I. Tyrosine kinase inhibitors in adult glioblastoma: an (un)closed chapter? Cancers (Basel). 2021;13(22).
Qin A, Musket A, Musich PR, Schweitzer JB, **e Q. Receptor tyrosine kinases as druggable targets in glioblastoma: do signaling pathways matter? Neurooncol Adv. 2021;3(1):vdab133.
Yang K, Wu Z, Zhang H, Zhang N, Wu W, Wang Z, et al. Glioma targeted therapy: insight into future of molecular approaches. Mol Cancer. 2022;21(1):39.
Fabro F, Lamfers MLM, Leenstra S. Advancements, challenges, and future directions in tackling glioblastoma resistance to small kinase inhibitors. Cancers (Basel). 2022;14(3).
Geoerger B, Hargrave D, Thomas F, Ndiaye A, Frappaz D, Andreiuolo F, et al. Innovative therapies for children with cancer pediatric phase I study of erlotinib in brainstem glioma and relapsing/refractory brain tumors. Neuro Oncol. 2011;13(1):109–18.
Kesavabhotla K, Schlaff CD, Shin B, Mubita L, Kaplan R, Tsiouris AJ, et al. Phase I/II study of oral erlotinib for treatment of relapsed/refractory glioblastoma multiforme and anaplastic astrocytoma. J Exp Ther Oncol. 2012;10(1):71–81.
Qaddoumi I, Kocak M, Pai Panandiker AS, Armstrong GT, Wetmore C, Crawford JR, et al. Phase II trial of erlotinib during and after radiotherapy in children with newly diagnosed high-grade gliomas. Front Oncol. 2014;4:67.
van den Bent MJ, Brandes AA, Rampling R, Kouwenhoven MC, Kros JM, Carpentier AF, et al. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J Clin Oncol. 2009;27(8):1268–74.
Chakravarti A, Wang M, Robins HI, Lautenschlaeger T, Curran WJ, Brachman DG, et al. RTOG 0211: a phase 1/2 study of radiation therapy with concurrent gefitinib for newly diagnosed glioblastoma patients. Int J Radiat Oncol Biol Phys. 2013;85(5):1206–11.
Geyer JR, Stewart CF, Kocak M, Broniscer A, Phillips P, Douglas JG, et al. A phase I and biology study of gefitinib and radiation in children with newly diagnosed brain stem gliomas or supratentorial malignant gliomas. Eur J Cancer. 2010;46(18):3287–93.
Uhm JH, Ballman KV, Wu W, Giannini C, Krauss JC, Buckner JC, et al. Phase II evaluation of gefitinib in patients with newly diagnosed grade 4 astrocytoma: Mayo/North central cancer treatment group study N0074. Int J Radiat Oncol Biol Phys. 2011;80(2):347–53.
Hegi ME, Diserens AC, Bady P, Kamoshima Y, Kouwenhoven MC, Delorenzi M, et al. Pathway analysis of glioblastoma tissue after preoperative treatment with the EGFR tyrosine kinase inhibitor gefitinib–a phase II trial. Mol Cancer Ther. 2011;10(6):1102–12.
Sepúlveda-Sánchez JM, Vaz M, Balañá C, Gil-Gil M, Reynés G, Gallego Ó, et al. Phase II trial of dacomitinib, a pan-human EGFR tyrosine kinase inhibitor, in recurrent glioblastoma patients with EGFR amplification. Neuro Oncol. 2017;19(11):1522–31.
Wang Y, Liang D, Chen J, Chen H, Fan R, Gao Y, et al. Targeted therapy with anlotinib for a patient with an oncogenic FGFR3-TACC3 fusion and recurrent glioblastoma. Oncologist. 2021;26(3):173–7.
Galanis E, Jaeckle KA, Maurer MJ, Reid JM, Ames MM, Hardwick JS, et al. Phase II trial of vorinostat in recurrent glioblastoma multiforme: a north central cancer treatment group study. J Clin Oncol. 2009;27(12):2052–8.
Mueller S, Kline C, Stoller S, Lundy S, Christopher L, Reddy AT et al. PNOC015: repeated convection enhanced delivery (CED) of MTX110 (aqueous panobinostat) in children with newly diagnosed diffuse intrinsic pontine glioma (DIPG). Neuro Oncol. 2023.
Berenguer-Daizé C, Astorgues-Xerri L, Odore E, Cayol M, Cvitkovic E, Noel K, et al. OTX015 (MK-8628), a novel BET inhibitor, displays in vitro and in vivo antitumor effects alone and in combination with conventional therapies in glioblastoma models. Int J Cancer. 2016;139(9):2047–55.
Bukowinski A, Chang B, Reid JM, Liu X, Minard CG, Trepel JB, et al. A phase 1 study of entinostat in children and adolescents with recurrent or refractory solid tumors, including CNS tumors: trial ADVL1513, Pediatric Early Phase-Clinical Trial Network (PEP-CTN). Pediatr Blood Cancer. 2021;68(4):e28892.
Park SY, Kim JS. A short guide to histone deacetylases including recent progress on class II enzymes. Exp Mol Med. 2020;52(2):204–12.
Eckschlager T, Plch J, Stiborova M, Hrabeta J. Histone deacetylase inhibitors as anticancer drugs. Int J Mol Sci. 2017;18(7).
Piao J, Chen L, Quan T, Li L, Quan C, Piao Y, et al. Superior efficacy of co-treatment with the dual PI3K/mTOR inhibitor BEZ235 and histone deacetylase inhibitor trichostatin A against NSCLC. Oncotarget. 2016;7(37):60169–80.
Rahmani M, Aust MM, Benson EC, Wallace L, Friedberg J, Grant S. PI3K/mTOR inhibition markedly potentiates HDAC inhibitor activity in NHL cells through BIM- and MCL-1-dependent mechanisms in vitro and in vivo. Clin Cancer Res. 2014;20(18):4849–60.
Meng W, Wang B, Mao W, Wang J, Zhao Y, Li Q, et al. Enhanced efficacy of histone deacetylase inhibitor panobinostat combined with dual PI3K/mTOR inhibitor BEZ235 against glioblastoma. Nagoya J Med Sci. 2019;81(1):93–102.
Pal S, Kozono D, Yang X, Fendler W, Fitts W, Ni J, et al. Dual HDAC and PI3K inhibition abrogates NFκB- and FOXM1-mediated DNA damage response to radiosensitize pediatric high-grade gliomas. Cancer Res. 2018;78(14):4007–21.
Qian C, Lai CJ, Bao R, Wang DG, Wang J, Xu GX, et al. Cancer network disruption by a single molecule inhibitor targeting both histone deacetylase activity and phosphatidylinositol 3-kinase signaling. Clin Cancer Res. 2012;18(15):4104–13.
Pei Y, Liu KW, Wang J, Garancher A, Tao R, Esparza LA, et al. HDAC and PI3K antagonists cooperate to inhibit growth of MYC-driven medulloblastoma. Cancer Cell. 2016;29(3):311–23.
da Cunha Jaeger M, Ghisleni EC, Cardoso PS, Siniglaglia M, Falcon T, Brunetto AT, et al. HDAC and MAPK/ERK inhibitors cooperate to reduce viability and stemness in medulloblastoma. J Mol Neurosci. 2020;70(6):981–92.
Li JY, Wang H, May S, Song X, Fueyo J, Fuller GN. Constitutive activation of c-Jun N-terminal kinase correlates with histologic grade and EGFR expression in diffuse gliomas. J Neurooncol. 2008;88(1):11–7.
Yoon CH, Kim MJ, Kim RK, Lim EJ, Choi KS, An S, et al. c-Jun N-terminal kinase has a pivotal role in the maintenance of self-renewal and tumorigenicity in glioma stem-like cells. Oncogene. 2012;31(44):4655–66.
Huang Z, **a Y, Hu K, Zeng S, Wu L, Liu S, et al. Histone deacetylase 6 promotes growth of glioblastoma through the MKK7/JNK/c-Jun signaling pathway. J Neurochem. 2020;152(2):221–34.
Arrizabalaga O, Moreno-Cugnon L, Auzmendi-Iriarte J, Aldaz P, Ibanez de Caceres I, Garros-Regulez L, et al. High expression of MKP1/DUSP1 counteracts glioma stem cell activity and mediates HDAC inhibitor response. Oncogenesis. 2017;6(12):401.
Meel MH, de Gooijer MC, Metselaar DS, Sewing ACP, Zwaan K, Waranecki P, et al. Combined therapy of AXL and HDAC inhibition reverses mesenchymal transition in diffuse intrinsic pontine glioma. Clin Cancer Res. 2020;26(13):3319–32.
Buendia Duque M, Pinheiro KV, Thomaz A, da Silva CA, Freire NH, Brunetto AT, et al. Combined inhibition of HDAC and EGFR reduces viability and proliferation and enhances STAT3 mRNA expression in glioblastoma cells. J Mol Neurosci. 2019;68(1):49–57.
Luwor RB, Stylli SS, Kaye AH. The role of Stat3 in glioblastoma multiforme. J Clin Neurosci. 2013;20(7):907–11.
Liffers K, Kolbe K, Westphal M, Lamszus K, Schulte A. Histone deacetylase inhibitors resensitize EGFR/EGFRvIII-overexpressing, erlotinib-resistant glioblastoma cells to tyrosine kinase inhibition. Target Oncol. 2016;11(1):29–40.
Wu SY, Chiang CM. The double bromodomain-containing chromatin adaptor Brd4 and transcriptional regulation. J Biol Chem. 2007;282(18):13141–5.
Dhalluin C, Carlson JE, Zeng L, He C, Aggarwal AK, Zhou MM. Structure and ligand of a histone acetyltransferase bromodomain. Nature. 1999;399(6735):491–6.
Dey A, Chitsaz F, Abbasi A, Misteli T, Ozato K. The double bromodomain protein Brd4 binds to acetylated chromatin during interphase and mitosis. Proc Natl Acad Sci U S A. 2003;100(15):8758–63.
Yang Z, Yik JH, Chen R, He N, Jang MK, Ozato K, et al. Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol Cell. 2005;19(4):535–45.
Jang MK, Mochizuki K, Zhou M, Jeong HS, Brady JN, Ozato K. The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol Cell. 2005;19(4):523–34.
Denis GV, Vaziri C, Guo N, Faller DV. RING3 kinase transactivates promoters of cell cycle regulatory genes through E2F. Cell Growth Differ. 2000;11(8):417–24.
Kanno T, Kanno Y, Siegel RM, Jang MK, Lenardo MJ, Ozato K. Selective recognition of acetylated histones by bromodomain proteins visualized in living cells. Mol Cell. 2004;13(1):33–43.
Shorstova T, Foulkes WD, Witcher M. Achieving clinical success with BET inhibitors as anti-cancer agents. Br J Cancer. 2021;124(9):1478–90.
Iniguez AB, Alexe G, Wang EJ, Roti G, Patel S, Chen L, et al. Resistance to epigenetic-targeted therapy engenders tumor cell vulnerabilities associated with enhancer remodeling. Cancer Cell. 2018;34(6):922–38e7.
Zawistowski JS, Bevill SM, Goulet DR, Stuhlmiller TJ, Beltran AS, Olivares-Quintero JF, et al. Enhancer remodeling during adaptive bypass to MEK inhibition is attenuated by pharmacologic targeting of the P-TEFb complex. Cancer Discov. 2017;7(3):302–21.
Funck-Brentano E, Vizlin-Hodzic D, Nilsson JA, Nilsson LM. BET bromodomain inhibitor HMBA synergizes with MEK inhibition in treatment of malignant glioma. Epigenetics. 2021;16(1):54–63.
Wen N, Guo B, Zheng H, Xu L, Liang H, Wang Q, et al. Bromodomain inhibitor jq1 induces cell cycle arrest and apoptosis of glioma stem cells through the VEGF/PI3K/AKT signaling pathway. Int J Oncol. 2019;55(4):879–95.
Čančer M, Drews LF, Bengtsson J, Bolin S, Rosén G, Westermark B, et al. BET and aurora kinase A inhibitors synergize against MYCN-positive human glioblastoma cells. Cell Death Dis. 2019;10(12):881.
Nikonova AS, Astsaturov I, Serebriiskii IG, Dunbrack RL, Golemis EA. Aurora A kinase (AURKA) in normal and pathological cell division. Cell Mol Life Sci. 2013;70(4):661–87.
Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146(6):904–17.
Bolin S, Borgenvik A, Persson CU, Sundström A, Qi J, Bradner JE, et al. Combined BET bromodomain and CDK2 inhibition in MYC-driven medulloblastoma. Oncogene. 2018;37(21):2850–62.
Hydbring P, Bahram F, Su Y, Tronnersjö S, Högstrand K, von der Lehr N, et al. Phosphorylation by Cdk2 is required for Myc to repress ras-induced senescence in cotransformation. Proc Natl Acad Sci U S A. 2010;107(1):58–63.
Sjostrom SK, Finn G, Hahn WC, Rowitch DH, Kenney AM. The Cdk1 complex plays a prime role in regulating N-myc phosphorylation and turnover in neural precursors. Dev Cell. 2005;9(3):327–38.
Tewary SK, Zheng YG, Ho MC. Protein arginine methyltransferases: insights into the enzyme structure and mechanism at the atomic level. Cell Mol Life Sci. 2019;76(15):2917–32.
Guccione E, Richard S. The regulation, functions and clinical relevance of arginine methylation. Nat Rev Mol Cell Biol. 2019;20(10):642–57.
Chen Z, Gan J, Wei Z, Zhang M, Du Y, Xu C, et al. The emerging role of PRMT6 in cancer. Front Oncol. 2022;12:841381.
Stopa N, Krebs JE, Shechter D. The PRMT5 arginine methyltransferase: many roles in development, cancer and beyond. Cell Mol Life Sci. 2015;72(11):2041–59.
Favia A, Salvatori L, Nanni S, Iwamoto-Stohl LK, Valente S, Mai A, et al. The protein arginine methyltransferases 1 and 5 affect Myc properties in glioblastoma stem cells. Sci Rep. 2019;9(1):15925.
Dong J, Duan J, Hui Z, Garrido C, Deng Z, **e T, et al. An updated patent review of protein arginine N-methyltransferase inhibitors (2019–2022). Expert Opin Ther Pat. 2022;32(12):1185–205.
Banasavadi-Siddegowda YK, Russell L, Frair E, Karkhanis VA, Relation T, Yoo JY, et al. PRMT5-PTEN molecular pathway regulates senescence and self-renewal of primary glioblastoma neurosphere cells. Oncogene. 2017;36(2):263–74.
Honda M, Nakashima K, Katada S. PRMT1 regulates astrocytic differentiation of embryonic neural stem/precursor cells. J Neurochem. 2017;142(6):901–7.
Duan R, Du W, Guo W. EZH2: a novel target for cancer treatment. J Hematol Oncol. 2020;13(1):104.
Satijn DP, Gunster MJ, van der Vlag J, Hamer KM, Schul W, Alkema MJ, et al. RING1 is associated with the polycomb group protein complex and acts as a transcriptional repressor. Mol Cell Biol. 1997;17(7):4105–13.
Kuzmichev A, Nishioka K, Erdjument-Bromage H, Tempst P, Reinberg D. Histone methyltransferase activity associated with a human multiprotein complex containing the enhancer of Zeste protein. Genes Dev. 2002;16(22):2893–905.
Margueron R, Reinberg D. The polycomb complex PRC2 and its mark in life. Nature. 2011;469(7330):343–9.
Dhar S, Gadd S, Patel P, Vaynshteyn J, Raju GP, Hashizume R, et al. A tumor suppressor role for EZH2 in diffuse midline glioma pathogenesis. Acta Neuropathol Commun. 2022;10(1):47.
Mohammad F, Weissmann S, Leblanc B, Pandey DP, Højfeldt JW, Comet I, et al. EZH2 is a potential therapeutic target for H3K27M-mutant pediatric gliomas. Nat Med. 2017;23(4):483–92.
Simon JA, Lange CA. Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutat Res. 2008;647(1–2):21–9.
Yang R, Wang M, Zhang G, Bao Y, Wu Y, Li X, et al. E2F7-EZH2 axis regulates PTEN/AKT/mTOR signalling and glioblastoma progression. Br J Cancer. 2020;123(9):1445–55.
Ganguly R, Mohyeldin A, Thiel J, Kornblum HI, Beullens M, Nakano I. MELK-a conserved kinase: functions, signaling, cancer, and controversy. Clin Transl Med. 2015;4:11.
Liu H, Sun Y, Qi X, Gordon RE, O’Brien JA, Yuan H, et al. EZH2 phosphorylation promotes self-renewal of glioma stem-like cells through NF-κB methylation. Front Oncol. 2019;9:641.
Speers C, Zhao SG, Kothari V, Santola A, Liu M, Wilder-Romans K, et al. Maternal embryonic leucine zipper kinase (MELK) as a novel mediator and biomarker of radioresistance in human breast cancer. Clin Cancer Res. 2016;22(23):5864–75.
Liu H, Sun Q, Sun Y, Zhang J, Yuan H, Pang S, et al. MELK and EZH2 cooperate to regulate medulloblastoma cancer stem-like cell proliferation and differentiation. Mol Cancer Res. 2017;15(9):1275–86.
Kim E, Kim M, Woo DH, Shin Y, Shin J, Chang N, et al. Phosphorylation of EZH2 activates STAT3 signaling via STAT3 methylation and promotes tumorigenicity of glioblastoma stem-like cells. Cancer Cell. 2013;23(6):839–52.
** W. Role of JAK/STAT3 signaling in the regulation of metastasis, the transition of cancer stem cells, and chemoresistance of cancer by epithelial-mesenchymal transition. Cells. 2020;9(1).
Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119(7):941–53.
Karakaidos P, Verigos J, Magklara A. LSD1/KDM1A, a gate-keeper of cancer stemness and a promising therapeutic target. Cancers (Basel). 2019;11(12).
Kim D, Kim KI, Baek SH. Roles of lysine-specific demethylase 1 (LSD1) in homeostasis and diseases. J Biomed Sci. 2021;28(1):41.
Bailey CP, Figueroa M, Gangadharan A, Yang Y, Romero MM, Kennis BA, et al. Pharmacologic inhibition of lysine-specific demethylase 1 as a therapeutic and immune-sensitization strategy in pediatric high-grade glioma. Neuro Oncol. 2020;22(9):1302–14.
Alejo S, Palacios BE, Venkata PP, He Y, Li W, Johnson JD, et al. Lysine-specific histone demethylase 1A (KDM1A/LSD1) inhibition attenuates DNA double-strand break repair and augments the efficacy of temozolomide in glioblastoma. Neuro Oncol. 2023;25(7):1249–61.
Yang M, Culhane JC, Szewczuk LM, Jalili P, Ball HL, Machius M, et al. Structural basis for the inhibition of the LSD1 histone demethylase by the antidepressant trans-2-phenylcyclopropylamine. Biochemistry. 2007;46(27):8058–65.
Fang Y, Liao G, Yu B. LSD1/KDM1A inhibitors in clinical trials: advances and prospects. J Hematol Oncol. 2019;12(1):129.
Mohammad HP, Smitheman KN, Kamat CD, Soong D, Federowicz KE, Van Aller GS, et al. A DNA hypomethylation signature predicts antitumor activity of LSD1 inhibitors in SCLC. Cancer Cell. 2015;28(1):57–69.
Binda C, Valente S, Romanenghi M, Pilotto S, Cirilli R, Karytinos A, et al. Biochemical, structural, and biological evaluation of tranylcypromine derivatives as inhibitors of histone demethylases LSD1 and LSD2. J Am Chem Soc. 2010;132(19):6827–33.
Bailey CP, Figueroa M, Gangadharan A, Lee DA, Chandra J. Scaffolding LSD1 inhibitors impair NK cell metabolism and cytotoxic function through depletion of glutathione. Front Immunol. 2020;11:2196.
Maes T, Mascaró C, Tirapu I, Estiarte A, Ciceri F, Lunardi S, et al. ORY-1001, a potent and selective covalent KDM1A inhibitor, for the treatment of acute leukemia. Cancer Cell. 2018;33(3):495–511e12.
Johnston G, Ramsey HE, Liu Q, Wang J, Stengel KR, Sampathi S, et al. Nascent transcript and single-cell RNA-seq analysis defines the mechanism of action of the LSD1 inhibitor INCB059872 in myeloid leukemia. Gene. 2020;752:144758.
Sacilotto N, Dessanti P, Lufino MMP, Ortega A, Rodríguez-Gimeno A, Salas J, et al. Comprehensive in vitro characterization of the LSD1 small molecule inhibitor class in oncology. ACS Pharmacol Transl Sci. 2021;4(6):1818–34.
Sehrawat A, Gao L, Wang Y, Bankhead A, McWeeney SK, King CJ, et al. LSD1 activates a lethal prostate cancer gene network independently of its demethylase function. Proc Natl Acad Sci U S A. 2018;115(18):E4179–E88.
Kanouni T, Severin C, Cho RW, Yuen NY, Xu J, Shi L, et al. Discovery of CC-90011: a potent and selective reversible inhibitor of Lysine Specific Demethylase 1 (LSD1). J Med Chem. 2020;63(23):14522–9.
Saccà CD, Gorini F, Ambrosio S, Amente S, Faicchia D, Matarese G, et al. Inhibition of lysine-specific demethylase LSD1 induces senescence in glioblastoma cells through a HIF-1α-dependent pathway. Biochim Biophys Acta Gene Regul Mech. 2019;1862(5):535–46.
Zhao J, ** W, Yi K, Wang Q, Zhou J, Tan Y, et al. Combination LSD1 and HOTAIR-EZH2 inhibition disrupts cell cycle processes and induces apoptosis in glioblastoma cells. Pharmacol Res. 2021;171:105764.
Eich ML, Athar M, Ferguson JE, Varambally S. EZH2-targeted therapies in cancer: hype or a reality. Cancer Res. 2020;80(24):5449–58.
Sheng W, LaFleur MW, Nguyen TH, Chen S, Chakravarthy A, Conway JR, et al. LSD1 ablation stimulates anti-tumor immunity and enables checkpoint blockade. Cell. 2018;174(3):549–63e19.
Wang H, Liu G, ** X, Song S, Chen S, Zhou P, et al. BET inhibitor JQ1 enhances anti-tumor immunity and synergizes with PD-1 blockade in CRC. J Cancer. 2022;13(7):2126–37.
Zhou L, Mudianto T, Ma X, Riley R, Uppaluri R. Targeting EZH2 enhances antigen presentation, antitumor immunity, and circumvents Anti-PD-1 resistance in head and neck cancer. Clin Cancer Res. 2020;26(1):290–300.
Liu L, Mayes PA, Eastman S, Shi H, Yadavilli S, Zhang T, et al. The BRAF and MEK inhibitors dabrafenib and trametinib: effects on immune function and in combination with immunomodulatory antibodies targeting PD-1, PD-L1, and CTLA-4. Clin Cancer Res. 2015;21(7):1639–51.
Galbán S, Apfelbaum AA, Espinoza C, Heist K, Haley H, Bedi K, et al. A bifunctional MAPK/PI3K antagonist for inhibition of tumor growth and metastasis. Mol Cancer Ther. 2017;16(11):2340–50.
Yan C, Saleh N, Yang J, Nebhan CA, Vilgelm AE, Reddy EP, et al. Novel induction of CD40 expression by tumor cells with RAS/RAF/PI3K pathway inhibition augments response to checkpoint blockade. Mol Cancer. 2021;20(1):85.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Institutes of Health (R21 NS093387, P50 CA127001, TL1 TR003169, R61/R33NS111058 and P30 CA016672), by the Cancer Prevention and Research Institute of Texas (DP160014) and by the Department of Defense (W81XWH2110728).
Author information
Authors and Affiliations
Contributions
Conception and design of the review article: L.S., R.D., and J.C. Interpretation of relevant literature and manuscript writing: L.S., J.A., E.S., R.D., and J.C.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare none.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Stitzlein, L.M., Adams, J.T., Stitzlein, E.N. et al. Current and future therapeutic strategies for high-grade gliomas leveraging the interplay between epigenetic regulators and kinase signaling networks. J Exp Clin Cancer Res 43, 12 (2024). https://doi.org/10.1186/s13046-023-02923-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13046-023-02923-7