Abstract
Background
In this study, we aimed to combine transcriptomic and network pharmacology to explore the crucial mRNAs and specific regulatory molecules of Buyang Huanwu Decoction (BYHWD) in intracerebral hemorrhage (ICH) treatment.
Methods
C57BL/6 mice were randomly divided into three groups: sham, ICH, and BYHWD. BYHWD (43.29 g/kg) was administered once a day for 7 days. An equal volume of double-distilled water was used as a control. Behavioural and histopathological experiments were conducted to confirm the neuroprotective effects of BYHWD. Brain tissues were collected for transcriptomic detection. Bioinformatics analysis were performed to illustrate the target gene functions. Network pharmacology was used to predict potential targets for BYHWD. Next, transcriptomic assays were combined with network pharmacology to identify the potential differentially expressed mRNAs. Immunofluorescence staining, real-time polymerase chain reaction, western blotting, and transmission electron microscopy were performed to elucidate the underlying mechanisms.
Results
BYHWD intervention in ICH reduced neurological deficits. Network pharmacology analysis identified 203 potential therapeutic targets for ICH, whereas transcriptomic assay revealed 109 differentially expressed mRNAs post-ICH. Among these, cathepsin B, ATP binding cassette subfamily B member 1, toll-like receptor 4, chemokine (C–C motif) ligand 12, and baculoviral IAP repeat-containing 5 were identified as potential target mRNAs through the integration of transcriptomics and network pharmacology approaches. Bioinformatics analysis suggested that the beneficial effects of BYHWD in ICH may be associated with apoptosis, animal autophagy signal pathways, and PI3K-Akt and mTOR biological processes. Furthermore, BYHWD intervention decreased Ctsb expression levels and increased autophagy levels in ICH.
Conclusions
Animal experiments in combination with bioinformatics analysis confirmed that BYHWD plays a neuroprotective role in ICH by regulating Ctsb to enhance autophagy.
Similar content being viewed by others
Background
Spontaneous nontraumatic intracerebral hemorrhage(ICH) is a serious subtype that accounts for approximately 10–15% of all types of strokes [1]. The overall incidence of ICH is 24.6 per 100 000 person-years, with a mortality rate of 40% [2, 3]. Moreover, ICH presents acutely and progresses rapidly, resulting in hemiplegia and altered consciousness, thus imposing a substantial global clinical and economic burden [4, 5]. Current treatments for ICH involve surgical and conservative medical approaches [6]. However, the results of multiple clinical trials have indicated that surgical interventions did not significantly improve the adverse outcomes in ICH [7]. Furthermore, no specific therapeutic medication has been approved for ICH treatment, and supportive therapy remains the primary approach for patients. Despite increased attention to ICH management, the available treatment options and effective targets are currently limited [8].
Brain injuries resulting from ICH encompass intricate pathophysiological processes, categorised as either primary or secondary brain injury based on pathological patterns [9]. Primary injury refers to the local tissue damage caused by an intracerebral haematoma, primarily occurring within minutes to hours after the onset of the disease [9]. Secondary brain injury is primarily induced by inflammation, oxidative stress, and excitotoxicity, all of which can initiate substantial neuronal cell death [10]. In such instances, the extensive cellular and tissue debris cannot be timely cleared, leading to the accumulation of detrimental substances that can result in persistent neurological dysfunction [11,12,13,14,15,16,17]. The neurological deficit caused by brain injury may induce autophagy. Autophagy plays a critical role in the elimination of cellular debris and waste following injury, thereby facilitating neuronal repair in nervous system diseases [18,19,20,21,22,23].
Buyang Huanwu Decoction (BYHWD) is widely recognised as a clinically effective formula for treating various types of strokes. Previous systematic reviews and meta-analyses have consistently demonstrated the efficacy and safety of BYHWD for treating ICH. [24, 25]. As a neuroprotective agent, BYHWD modulates the non-classical NF-κB pathway, attenuating the inflammatory response following ICH [23]. Furthermore, previous experimental studies have indicated that BYHWD promotes lactate accumulation and activates the HIF-1α/VEGF signal pathway to induce angiogenesis post-ICH [24]. Additionally, BYHWD downregulates the expression of leukemia inhibitory factors, thereby reducing glial scar formation induced by ICH [33]. In this study, we combined transcriptomics and network pharmacology analyses to explore crucial target mRNAs and specific regulatory molecules associated with BYHWD in ICH treatment. This research is expected to provide deeper insights into the transcriptional-level effects of BYHWD in ICH treatment.
Methods
BYHWD preparation
The original prescription of BYHWD is available in the ‘TCM Prescriptions Dictionary’, which lists the following seven Chinese herbs: Astragalus mongholicus Bunge, Angelicae sinensis (Oliv.) Diels, Ligusticumi chuanxiong Hort, Paeonia lactiflora Pall, Prunus persica (L.) Batsch, Carthamus tinctorius L, and Pheretima aspergillum (E. Perrier) (Table 1). The raw herbs were sourced from the ** targets: cathepsin B (Ctsb), ATP binding cassette subfamily B member 1 (Abcb1b), toll-like receptor 4 (Tlr4), chemokine (C–C motif) ligand 12 (Ccl12), and baculoviral IAP repeat-containing 5 (Birc5). Disease-drug-target network analysis of BYHWD and ICH was performed to visualise the link between disease and drugs (Fig. 4b). The clustering heatmap (Fig. 4c) was used to observe trends among the three groups. To verify the results derived from bioinformatics analysis, haemojuvelin (Hfe2), scavenger receptor class A member 5 (Scara5), Ras-related protein Rab-7b, transferrin, PYD and CARD Domain Containing (Pycard), Ccl12, Abcb1b, Tlr4, Ctsb, and Birc5 were randomly selected for RT-qPCR (Fig. 4d–l). In particular, Abcb1b, Birc5, Ccl12, Trf, Tlr4, Rab7b, Pycard, and Ctsb expressions were upregulated after ICH; however, a significant decrease was observed after BYHWD treatment. The expression of Hfe2 and Scara5 demonstrated opposite trends in that they were downregulated after ICH but upregulated after BYHWD treatment. The results of the experiment were consistent with the microarray results. Among the five core targets, Ctsb showed the most significant change in mRNA expression (P < 0.001).
Differential expressed genes of Combined network pharmacology and Illumina sequencing display in Venn Diagram a. Disease-Drug-Targets network analysis of BYHWD and ICH b. Heat map illustrating target mRNAs c. RT-qPCR is performed to validate the results of Illumina sequencing d–m. n = 3, *P < 0.05 **P < 0.01 ***P < 0.001
BYHWD enhances autophagy by regulating Ctsb in ICH
As shown in Fig. 4d, the significant change in mRNA expression of Ctsb was also visualised using immunofluorescence and western blot (Fig. 5a–c). The increase in Ctsb expression following ICH was reduced after BYHWD treatment. Due to its localisation on lysosomes, Ctsb may potentially influence the autophagic process [34]. Western blotting was used to assess changes in autophagy-related proteins to further investigate the potential mechanism of BYHWD regulation of Ctsb (Fig. 5b). After ICH on day 7, the expression of the autophagy substrate P62 (Fig. 5d) decreased in the ICH group than in the sham group, whereas the expression of autophagy-related proteins such as Beclin1, Atg5, and Lc3b increased (Fig. 5e–g). The trends were reinforced in the BYHWD group. Autophagosomes were observed by TEM, the gold standard for studying autophagy. The experimental results suggested that autophagic activity was active and autophagy levels were enhanced after BYHWD treatment (Fig. 6a). Ctsb cleaves the adhesive protein 1 (mucolipin 1, Mcoln1) in lysosomal calcium channels, inhibiting the synthesis of transcription factor EB (Tfeb) and reducing the expression of lysosomal and autophagy-related proteins, thus impacting cellular autophagy [35]. The mRNA expression of Tfeb (Fig. 6b) and Mcoln1 decreased in the ICH group but increased in the BYHWD group (Fig. 6c), which was negatively correlated with the mRNA expression of Ctsb. These experiments suggest that BYHWD exerts a neuroprotective effect by inhibiting Ctsb and enhancing the expression of Tfeb and Mcoln1 to increase autophagy.
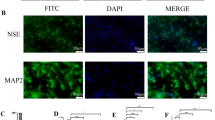
Immunofluorescence was utilized to detect Ctsb-positive cells in different groups, the white solid line in the figure represents the hematoma area, and the magnified image in the top left white box shows a local enlargement a. The sample and pathological observation position of mouse brain in immunofluorescence b. Western blotting (WB) was conducted to assess the levels of Ctsb and autophagy-related molecules c. The quantitative analysis in WB of CTSB d, P62 e, BECLIN f, ATG5 g and LC3B h, n = 3–6, *P < 0.05 **P < 0.01
Discussion
In this study, we identified five critical differentially expressed mRNAs following BYHWD intervention after ICH using a transcriptomic-combined network pharmacology strategy. Among these mRNAs, the most prominently altered mRNA was Ctsb, which acts therapeutically by enhancing autophagy. We have attempted to elucidate the possible mechanisms of the pharmacotherapeutic efficacy of BYHWD for ICH treatment and to achieve precise therapy at the transcriptional level.
Previous studies have shown that the administration of BYHWD can attenuate neuronal death and improve neurological deficits after ICH [27, 32]. In this study, BYHWD significantly enhanced the recovery of neurological function defects, as determined by several classical behavioural tests. Furthermore, BYHWD effectively reversed the neurofunctional and pathological impairments induced by ICH. These findings underscore the crucial role of BYHWD in exerting a neuroprotective response against ICH.
The mRNA alterations caused by BYHWD treatment are a complex process. Five differentially expressed mRNAs, namely Abcb1b, Ccl12, Birc5, Tlr4, and Ctsb, overlapped (Table 3). Our research confirmed an association between Abcb1b and BYHWD on ICH. Abcb1b is an ATP-dependent drug efflux pump that maintains intracellular homeostasis and belongs to subfamily B of the ATP-binding cassette transporter superfamily. In the nervous system, Abcb1b is present in the blood–brain barrier (BBB), where it restricts drug penetration into the brain [36, 37]. Recent clinical cross-sectional findings have suggested an association between two SNPs in Abcb1b and post-thrombolytic hemorrhagein ischaemic stroke [38]. However, the specific mechanism of action remains unclear. Our results indicated that the crucial role Abcb1b plays may be attributed to the modulation of drug penetration across the BBB by affecting proton pump activity. Inhibitor of apoptosis (IAP) repeat containing 5 (Birc5), also known as survivin, is a member of the IAP family and is highly expressed in most tumor cells [36]. Birc5 negatively regulates apoptosis by inhibiting the expression of caspase 3 [37]. The therapeutic effect of BYHWD on ICH may be attributed to its ability to induce apoptosis through Birc5 downregulation. Ccl12 belongs to the C–C (or beta) chemokine family, which modulates immune regulation and inflammation [38]. During ICH, Ccl12 induces inflammation and exacerbates brain damage by recruiting macrophages and T cells [39]. In this study, BYHWD intervention reversed the upregulation of Ccl12 after modelling.
The neuroprotective effect of BYHWD may be achieved by reducing the expression of Ccl12, resulting in the inhibition of inflammation. Tlr4 is an innate immune receptor belonging to the toll-like receptor family [40, 41]. Tlr4 is a marker of inflammation and apoptosis in ICH and is increased following secondary brain injury. In turn, high Tlr4 expression exacerbates neurological defects, consistent with our results [42]. We speculate that BYHWD downregulates Tlr4 expression to alleviate neuroinflammation and improve the prognosis of patients with ICH. In conclusion, although we have confirmed the association between key genes such as Abcb1b, Birc5, Ccl12, Tlr4, and Ctsb and BYHWD treatment for ICH in our study. Further experimental validation is still required to elucidate their functional significance and mechanisms of action in ICH pathophysiology. The specific roles and interrelationships of these genes are crucial for understanding the pathogenesis of ICH and the therapeutic effects of BYHWD.
The most significant alteration in gene expression following BYHWD intervention in ICH was observed in Ctsb during the study. Cathepsin B, a member of the cysteine cathepsin family, is primarily located in lysosomes and endosomes. It plays a role in autophagy, antigen presentation, cellular stress signalling, and lysosome-dependent cell death [43]. Previous studies have reported that the deletion of the Ctsb gene has a significant positive impact on behavioural deficits in conditions such as ischaemic neuropathic damage, inflammatory pain, opioid tolerance, epilepsy, and Alzheimer's disease [44]. Additionally, research has indicated the involvement of Ctsb in neuronal cell death following ICH. Selective Ctsb inhibition improves neurological function in rats, potentially by promoting cell survival at the ICH border and restoring neuroplasticity and angiogenesis [45]. Ctsb directly activates several matrix metalloproteinases (MMPs), and its absence inhibits MMP-9 expression [35]. MMP-9 is associated with ICH enlargement and the development of perihaematomal oedema [46]. Interestingly, a previous study revealed that Ctsb plays a role in correct chromosome segregation, which is closely related to lysosomal function [47]. This finding aligns with the biological enrichment results of transcriptomics analysis, specifically highlighting the involvement of Ctsb in chromosomal maintenance. Collectively, these findings support the notion that Ctsb is involved in neural restoration following ICH through multiple pathways. This further underscores the potential of Ctsb as a critical target for BYHWD therapy. The localisation of Ctsb within lysosomes suggests its potential impact on the autophagy process [34]. Autophagy plays a vital role in degrading intracellular materials and maintaining cellular homeostasis by utilising lysosomal enzymes [48]. The production and transportation of autophagosomes primarily occur in axons, and proper axonal transport is crucial for clearing damaged mitochondria [19]. Ctsb is closely associated with autophagy and negatively regulates neurite outgrowth by modulating lysosomal trafficking in neurons [49]. In our study, autophagy levels were elevated following ICH injury. However, concomitant increases in both Ctsb mRNA and protein expressions suggest that Ctsb may not be the sole factor influencing autophagy levels, as the autophagy process is regulated by multiple factors [50]. Conversely, after intervention with BYHWD, a significant enhancement in autophagy levels was observed, accompanied by a decrease in Ctsb levels. This indicates that BYHWD can partially inhibit Ctsb expression and enhance autophagy.
Under normal homeostatic conditions, Ctsb cleaves Mcoln1 within the lysosome, inhibiting calcium efflux from the lysosomal lumen to the cytoplasm. This process restricts the expression of Tfeb and subsequently reduces the expression of lysosomal and autophagy-related proteins [34]. Tfeb is a key regulator of autophagy and lysosomal biogenesis [51], and its activation alleviates neurodegeneration by promoting lysosomal function and inducing autophagy [52, 53]. Mcoln1 is a ubiquitously expressed lysosomal calcium channel, which is activated during autophagy and is involved in lysosomal calcium transport [54]. Furthermore, the Mcoln1/calcineurin-dependent mechanism is implicated in autophagy initiation [52].
In our study, the mRNA expression levels of Mcoln1 and Tfeb decreased after BYHWD intervention. Thus, we hypothesised that BYHWD moderates the expression of Ctsb, Mcoln1, and Tfeb to regulate autophagy after ICH. Autophagy plays a bidirectional role in ICH. Some studies have demonstrated that autophagy is involved in neuronal death following brain injury [55]; others have revealed that enhancing autophagy 7 days after ICH improves disease prognosis [21]. However, the integrity of autophagic flux and the autophagy level are critical factors that influence the effectiveness of autophagy [56].
Basic experimental research has suggested that in the early stages after injury, many autophagic vesicles around the haematoma show impaired autophagic flux and production of cellular waste. At this point, high levels of autophagy and endoplasmic reticulum stress are in a positive feedback loop, exacerbating nerve damage. However, 7 days after ICH, the autophagic flux returns to normal, allowing autophagy to efficiently clear the metabolic waste and play a neuroprotective role [57]. These studies collectively demonstrate that cellular autophagy activation has a beneficial effect 7 days post-ICH. In summary, the therapeutic mechanism of BYHWD at 7 days after ICH likely involves enhancing cellular autophagy through the downregulation of Ctsb expression.
Conclusions
In summary, based on a combination of bioinformatics analyses and validation experiments, we have elucidated the key target proteins and specific mechanisms of BYHWD treatment for ICH from multiple perspectives, which are associated with apoptosis, signal pathways, and PI3K-Akt/mTOR biological processes. In addition, we confirmed that BYHWD improves the prognosis of ICH by inhibiting Ctsb and enhancing cell autophagy.
Availability of data and materials
The data used to support the findings of this study are available from the corresponding author upon request.
Abbreviations
- ICH:
-
Intracerebral hemorrhage
- BYHWD:
-
Buyang Huanwu Decoction
- mNSS:
-
Modified neurological severity score
- H&E:
-
Haematoxylin and eosin
- TCMSP:
-
Traditional Chinese Medicine Systems Pharmacology
- RT-qPCR:
-
Real-time quantitative polymerase chain reaction
- TEM:
-
Transmission electron microscopy
- TDD:
-
Therapeutic target database
- Ctsb:
-
Cathepsin B
- Abcb1b:
-
ATP binding cassette subfamily B member 1
- Tlr4:
-
Toll-like receptor 4
- Ccl12:
-
Chemokine (C–C motif) ligand 12
- Birc5:
-
Baculoviral IAP repeat-containing 5
- Tfeb:
-
Transcription factor EB
- BBB:
-
Blood brain barrier
- IAP:
-
Inhibitor of apoptosis
- Birc5:
-
Inhibitor of apoptosis (IAP) repeat containing 5
- MMPs:
-
Matrix metalloproteinases
- Mcoln1:
-
Mucolipin 1
- Hfe2:
-
Haemojuvelin,
- Scara5:
-
Scavenger receptor class A member 5
References
Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2022 update: a report from the american heart association. Circulation. 2022;145(8):e153–639.
Gregório T, Pipa S, Cavaleiro P, et al. Prognostic models for intracerebral hemorrhage: systematic review and meta-analysis. BMC Med Res Methodol. 2018;18(1):145.
An SJ, Kim TJ, Yoon BW. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J Stroke. 2017;19(1):3–10.
Garg R, Biller J. Recent advances in spontaneous intracerebral hemorrhage. F1000Res. 2019. https://doi.org/10.12688/f1000research.16357.1.
Lo BWY, Teitelbaum JS. Hyperthermia, cerebral edema, and outcome in intracerebral hemorrhage: too darn hot. Neurology. 2020;94(16):687–8.
Greenberg SM, Ziai WC, Cordonnier C, et al. 2022 Guideline for the management of patients with spontaneous intracerebral hemorrhage: a guideline from the american heart association/american stroke association. Stroke. 2022;53(7):e282–361.
Mendelow AD, Gregson BA, Rowan EN, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. 2013;382(9890):397–408.
Sheth KN. Spontaneous intracerebral hemorrhage. N Engl J Med. 2022;387(17):1589–96.
Wang T, Nowrangi D, Yu L, et al. Activation of dopamine D1 receptor decreased NLRP3-mediated inflammation in intracerebral hemorrhage mice. J Neuroinflammation. 2018;15(1):2.
Wu X, Cui W, Guo W, et al. Acrolein aggravates secondary brain injury after intracerebral hemorrhage through Drp1-mediated mitochondrial oxidative damage in mice. Neurosci Bull. 2020;36(10):1158–70.
Dasari R, Zhi W, Bonsack F, et al. A combined proteomics and bioinformatics approach reveals novel signaling pathways and molecular targets after intracerebral hemorrhage. J Mol Neurosci. 2020;70(8):1186–97.
Fu X, Zhou G, Zhuang J, et al. White matter injury after intracerebral hemorrhage. Front Neurol. 2021;12: 562090.
Shao L, Chen S, Ma L. Secondary brain injury by oxidative stress after cerebral hemorrhage: recent advances. Front Cell Neurosci. 2022;16: 853589.
Ting WK, Fadul FA, Fecteau S, et al. Neurostimulation for stroke rehabilitation. Front Neurosci. 2021;15: 649459.
Yuan XL, Zhao YP, Huang J, et al. A Kv13 channel-specific blocker alleviates neurological impairment through inhibiting T-cell activation in experimental autoimmune encephalomyelitis. CNS Neurosci Ther. 2018;24(10):967–77.
Antonioli M, di Rienzo M, Piacentini M, et al. Emerging mechanisms in initiating and terminating autophagy. Trends Biochem Sci. 2017;42(1):28–41.
Li R, Li D, Wu C, et al. Nerve growth factor activates autophagy in Schwann cells to enhance myelin debris clearance and to expedite nerve regeneration. Theranostics. 2020;10(4):1649–77.
Shen X, Ma L, Dong W, et al. Autophagy regulates intracerebral hemorrhage induced neural damage via apoptosis and NF-Œ∫B pathway. Neurochem Int. 2016;96:100–12.
Fu K, Xu W, Lenahan C, et al. Autophagy regulates inflammation in intracerebral hemorrhage: enemy or friend? Front Cell Neurosci. 2022;16:1036313.
Zhang Y, Liu C. Autophagy and hemorrhagic stroke. Adv Exp Med Biol. 2020;1207:135–47.
Duan XC, Wang W, Feng DX, et al. Roles of autophagy and endoplasmic reticulum stress in intracerebral hemorrhage-induced secondary brain injury in rats. CNS Neurosci Ther. 2017;23(7):554–66.
Zhao M, Gao J, Cui C, et al. Inhibition of PTEN ameliorates secondary hippocampal injury and cognitive deficits after intracerebral hemorrhage: involvement of AKT/FoxO3a/ATG-mediated autophagy. Oxid Med Cell Longev. 2021;2021:5472605.
Xu W, Ocak U, Gao L, et al. Selective autophagy as a therapeutic target for neurological diseases. Cell Mol Life Sci. 2021;78(4):1369–92.
Shao L, She Y, Yong S, et al. An evidence-based evaluation of Buyang Huanwu decoction for the treatment of the sequelae of stroke: a PRISMA-compliant systematic review and meta-analysis of randomized controlled trials. Phytomedicine. 2022;104: 154312.
Wang R, Ren J, Li S, et al. Efficacy evaluation of Buyang Huanwu Decoction in the treatment of ischemic stroke in the recovery period: a systematic review of randomized controlled trials. Front Pharmacol. 2022;13: 975816.
**ao W, He Z, Luo W, et al. BYHWD alleviates inflammatory response by NIK-mediated repression of the noncanonical NF-κB pathway during ICH recovery. Front Pharmacol. 2021;12: 632407.
Zhou J, Guo H, Yang A, et al. Buyang Huanwu decoction: a traditional chinese medicine, promotes lactate-induced angiogenesis in experimental intracerebral hemorrhage. Evid Based Complement Alternat Med. 2022;2022:4063315.
Kang X, Zhou HJ, Yang J, et al. Buyang huanwu decoction () attenuates glial scar by downregulating the expression of leukemia inhibitory factor in intracerebral hemorrhagic rats. Chin J Integr Med. 2019;25(4):264–9.
Nie Y, Fan Y, Zhang X, et al. Buyang Huanwu decoction improves neural recovery after spinal cord injury in rats through the mTOR signaling pathway and autophagy. J Spinal Cord Med. 2023;46(1):99–106.
Perrino C, Barabási L, Condorelli G, et al. Epigenomic and transcriptomic approaches in the post-genomic era: path to novel targets for diagnosis and therapy of the ischaemic heart? position paper of the european society of cardiology working group on cellular biology of the heart. Cardiovasc Res. 2017;113(7):725–36.
Cui H, Liu T, Li P, et al. An intersectional study of LncRNAs and mRNAs reveals the potential therapeutic targets of buyang huanwu decoction in experimental intracerebral hemorrhage. Cell Physiol Biochem. 2018;46(5):2173–86.
Li P, Tang T, Liu T, et al. Systematic analysis of tRNA-derived small RNAs reveals novel potential therapeutic targets of traditional chinese medicine (Buyang-Huanwu-Decoction) on intracerebral hemorrhage. Int J Biol Sci. 2019;15(4):895–908.
Chen D, Wu Y, Chen Y, et al. Exploration of the molecular targets and mechanisms of suxiao xintong drop** pills for myocardial infarction by network pharmacology method. 2021. Biosci Rep. https://doi.org/10.1042/BSR20204211.
Man SM, Kanneganti TD. Regulation of lysosomal dynamics and autophagy by CTSB/cathepsin B. Autophagy. 2016;12(12):2504–5.
Wu JS, Li ZF, Wang HF, et al. Cathepsin B defines leader cells during the collective invasion of salivary adenoid cystic carcinoma. Int J Oncol. 2019;54(4):1233–44.
Xu L, Yu W, **ao H, et al. BIRC5 is a prognostic biomarker associated with tumor immune cell infiltration. Sci Rep. 2021;11(1):390.
Vizeacoumar FS, Guo H, Dwernychuk L, et al. Mining the plasma-proteome associated genes in patients with gastro-esophageal cancers for biomarker discovery. Sci Rep. 2021;11(1):7590.
Adams DH, Lloyd AR. Chemokines: leucocyte recruitment and activation cytokines. Lancet. 1997;349(9050):490–5.
Huang J, Yang G, **ong X, et al. Age-related CCL12 aggravates intracerebral hemorrhage-induced brain injury via recruitment of macrophages and T lymphocytes. Aging Dis. 2020;11(5):1103–15.
Zhang P, Yang M, Chen C, et al. Toll-like receptor 4 (TLR4)/Opioid receptor pathway crosstalk and impact on opioid analgesia, immune function, and gastrointestinal motility. Front Immunol. 2020;11:1455.
Liu G, Zhang H, Zhao C, et al. Evolutionary history of the toll-like receptor gene family across vertebrates. Genome Biol Evol. 2020;12(1):3615–34.
Yu SJ, Wu KJ, Wang YS, et al. Protective effect of CXCR4 antagonist CX807 in a rat model of hemorrhagic stroke. Int J Mol Sci. 2020;21(19):7085.
Poreba M, Rut W, Vizovisek M, et al. Selective imaging of cathepsin L in breast cancer by fluorescent activity-based probes. Chem Sci. 2018;9(8):2113–29.
Hook G, Reinheckel T, Ni J, et al. Cathepsin B gene knockout improves behavioral deficits and reduces pathology in models of neurologic disorders. Pharmacol Rev. 2022;74(3):600–29.
Yang D, Han Y, Zhang J, et al. Improvement in recovery after experimental intracerebral hemorrhage using a selective cathepsin B and L inhibitor. J Neurosurg. 2011;114(4):1110–6.
Hsueh PJ, Wang MH, Hsiao CJ, et al. Ergosta-7,9(11),22-trien-3β-ol alleviates intracerebral hemorrhage-induced brain injury and BV-2 microglial activation. Molecules. 2021;26(10):2970.
HäMäLISTö S, Stahl JL, Favaro E, et al. Spatially and temporally defined lysosomal leakage facilitates mitotic chromosome segregation. Nat Commun. 2020;11(1):229.
Iwama H, Mehanna S, Imasaka M, et al. Cathepsin B and D deficiency in the mouse pancreas induces impaired autophagy and chronic pancreatitis. Sci Rep. 2021;11(1):6596.
Jiang M, Meng J, Zeng F, et al. Cathepsin B inhibition blocks neurite outgrowth in cultured neurons by regulating lysosomal trafficking and remodeling. J Neurochem. 2020;155(3):300–12.
Mizushima N. The ATG conjugation systems in autophagy. Curr Opin Cell Biol. 2020;63:1–10.
Tao J, Yang P, **e L, et al. Gastrodin induces lysosomal biogenesis and autophagy to prevent the formation of foam cells via AMPK-FoxO1-TFEB signalling axis. J Cell Mol Med. 2021;25(12):5769–81.
Zhuang XX, Wang SF, Tan Y, et al. Pharmacological enhancement of TFEB-mediated autophagy alleviated neuronal death in oxidative stress-induced Parkinson’s disease models. Cell Death Dis. 2020;11(2):128.
Fu Z, Zhang Z, Wu X, et al. Hydrogen-rich saline inhibits lipopolysaccharide-induced acute lung injury and endothelial dysfunction by regulating autophagy through Mtor/Tfeb signaling pathway. Biomed Res Int. 2020;2020:9121894.
Wilden AR, Molina JA, Feuerborn M, et al. Glutamine-dependent lysosome homeostatic changes induced by starvation and lysosome inhibition. Biochim Biophys Acta Mol Cell Res. 2018;1865(9):1356–67.
Han X, Ren H, Nandi A, et al. Analysis of glucose metabolism by (18)F-FDG-PET imaging and glucose transporter expression in a mouse model of intracerebral hemorrhage. Sci Rep. 2021;11(1):10885.
Li H, Wu J, Shen H, et al. Autophagy in hemorrhagic stroke: Mechanisms and clinical implications. Prog Neurobiol. 2018;163–164:79–97.
He Y, Wan S, Hua Y, et al. Autophagy after experimental intracerebral hemorrhage. J Cereb Blood Flow Metab. 2008;28(5):897–905.
Acknowledgements
This work was financially supported by Hunan Provincial Administration of Traditional Chinese Medicine (C2022038) and National Natural Science Foundation of China (81874425).
Funding
This research was supported by Hunan Provincial Administration of Traditional Chinese Medicine (C2022038) and National Natural Science Foundation of China (81874425).
Author information
Authors and Affiliations
Contributions
YC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing—original draft, Writing—review and editing. ZY: Data curation, Investigation, Funding acquisition. Writing—review and editing. XY: Conceptualization, Methodology, Software, Validation, Formal analysis. WL: Methodology, Software, Writing—review and editing. EH: Methodology, Software, Funding acquisition. TL: Methodology, Funding acquisition. WZ: Methodology, Writing—review and editing. YW: Resources, Project administration, Supervision. TT: Conceptualization, Funding acquisition. JL: Conceptualization, Supervision, Project administration.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal experiments were approved by the Institutional Animal Ethics Committee of.
Central South University (NO.2020sydw0929).
Consent for publication
All authors consent to the publication of this work in Chinese Medicine.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cai, Y., Yu, Z., Yang, X. et al. Integrative transcriptomic and network pharmacology analysis reveals the neuroprotective role of BYHWD through enhancing autophagy by inhibiting Ctsb in intracerebral hemorrhage mice. Chin Med 18, 150 (2023). https://doi.org/10.1186/s13020-023-00852-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13020-023-00852-3