Abstract
Background
Molecular biology has been applied to the diagnosis, prognosis and treatment of various diseases, and long noncoding RNA LINC00943 (lncRNA LINC00943; LINC00943) plays an important role in a variety of cancers. Therefore, this study explored the prognostic role of LINC00943 in lung squamous cell carcinoma (LUSC) and understood its impact on the development of LUSC.
Methods
There are 89 LUSC patients were involved in current assay. By detecting the expression of LINC00943 and miR-196b-5p in tissues and cells, LINC00943 and its correlation with the characteristics of clinical data were analyzed. The biological function of LINC00943 was studied by Transwell migration and invasion assays. In addition, Pearson correlation coefficient and luciferase activity experiments were chosen to characterize the relationship between LINC00943 and miR-196b-5p and explore the mechanism of LINC00943.
Results
Compared with normal controls, LINC00943 expression in LUSC tissues and cells was significantly reduced, miR-196b-5p was markedly increased, there was a negative correlation between LINC00943 and miR-196b-5p. According to the in vitro cell experiments, migration and invasion of LUSC cells were suppressed by overexpression of LINC00943. Besides, LINC00943 was demonstrated to have prognostic power and targeting miR-196b-5p was involved in the progression of LUSC.
Conclusions
Overexpression of LINC00943 was molecular sponge for miR-196b-5p that controlled the deterioration of LUSC, which had great potential as a prognostic biomarker for LUSC.
Similar content being viewed by others
Background
Lung squamous cell carcinoma (LUSC) is the second most common type of lung cancer after lung adenocarcinoma, with the highest morbidity and mortality in China, and the number of patients continues to increase [1, 2]. The patient eventually mutated into LUSC due to chronic stimulation and damage to the columnar epithelial cells of the bronchial mucosa, loss of cilia, and squamous metaplasia of basal cells [3]. The specific etiology of LUSC has not yet been determined, but smoking, air pollution, family inheritance and bad living habits are all high-risk factors for the disease [4]. Surveys have shown that surgical treatment, radiotherapy and chemotherapy can relieve symptoms and prolong life. However, the recurrence rate of patients is extremely high, the prognosis is poor, and the survival rate of approximately 60% [5]. Therefore, identifying high-risk groups with poor prognosis through prognostic factors as early as possible is of great practical significance for the treatment of LUSC and the improvement of patient survival rates.
For the treatment of LUSC patients, in addition to the commonly used surgical resection, radiotherapy and chemotherapy, and drug therapy, molecular therapy represented by lncRNAs has also received attention in recent decades [6]. For example, lncRNA IGKJ2-MALLP2 suppressed reproduction and angiogenesis of LUSC cells [7]. LncRNA AC026166.2-001 and RP11-169D4.1-001 have clinical significance in the treatment of LUSC patients, which can be used as independent prognostic factors for patient survival [8]. LncRNA HAGLROS has also been shown to accelerate the growth of LUSC cells through multiple signaling pathways [9]. Recent studies have found that lncRNAs coordinate with microRNAs (miRNAs) and protein-coding mRNAs to regulate the progress of lung cancer. Ni et al. demonstrated that the lncRNA-SOX2OT/miRNA-194-5p/RAC1 signaling axis synergistically promotes the metastasis of non-small cell lung cancer (NSCLC) [10]. **a et al. promoted the development of lung cancer by absorbing different miRNAs through LINC01140, providing a new ideal target for tumor therapy [11].
Moreover, LINC00943 was found to be involved in the regulation of Parkinson’s disease [12], gastric cancer [13], clear cell renal cell carcinoma [14] and breast cancer [15] in the existing literature. However, the expression and function of LINC00943 in LUSC, and whether it can play a role in affecting tumor progression have not been documented. This experiment focused on exploring the potential of LINC00943 as a prognostic biomarker in LUSC and to understand the mechanism of LINC00943 binding to miRNA in LUSC.
Methods
Collection of tissue specimens
LUSC tissue specimens confirmed by professional pathologists were collected in KaiLuan General Hospital from 2015 to 2016. The 89 LUSC patients who confirmed to participate in this study did not receive any anti-tumor treatment before sampling, while patients with multiple diseases were excluded. The LUSC tissues and adjacent normal tissues removed from the patients were immediately frozen in liquid nitrogen and stored in a refrigerator at -80°C for subsequent experiments.
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of KaiLuan General Hospital approved this study, and all participating patients signed an informed consent form as required. The clinical indicators of LUSC patients, such as age, gender, tumor size, and differentiation are summarized in Table 1. All LUSC patients enrolled in the study were followed for 5 consecutive years by face-to-face interview and telephone communication, and survival information was recorded.
Cell lines and transfection
Four human LUSC cell lines (H520, H1703, EBC-1, H2170) and human bronchial epithelial cells BEAS2B were derived from the Shanghai Cell Bank of the Chinese Academy of Sciences. All cells were grown in 6-well cell culture plates in RPMI 1640 medium (Gibco/Life Technologies, USA), which contains 10% fetal bovine serum (FBS; Gibco, USA) and 1% MEM non-essential amino acids (MEM NEAA; Gibco, USA) at 37°C in a 5% CO2 incubator.
The overexpression vector plasmid pcDNA3.1 required for transfection was derived from GenePharma Co. Ltd. (Shanghai, China). In the transfection experiment, the constructed plasmid overexpressing LINC00943 (pcDNA3.1-LINC00943) was transferred into H520 and H2170 cells using Lipofectamine 3000 reagent (Invitrogen, USA), and the untreated cells were regarded as the control group.
Real-time quantitative PCR assay
The TRIZOL (Sigma-Aldrich) method was chosen to obtain total RNA, and precipitate and wash RNA with 85% ethanol to obtain miRNAs. Precision nanoScript2 Reverse Transcription Kit (Primerdesign) and miRNA 1st Strand cDNA Synthesis Kit (Vazyme) were selected to reverse transcription to synthesize cDNA (A260/A280 ratio: between 1.8 and 2.0) for lncRNA and miRNA respectively. The reaction system was configured using ChamQ SYBR qPCR Green Master Mix and miRNA Universal SYBR qPCR Master Mix (Vazyme), and RT-qPCR detection was performed on a 7500 Real-Time PCR system. Cycling parameters: incubation at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, 60°C for 60 s, and 72°C for 15 s, and finally extension at 72°C for 10 min. The endogenous control of LINC00943 and miR-196b-5p utilized glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and U6, while the LINC00943 and miR-196b-5p expression were calculated by the 2−ΔΔCt method. The primer sequences are as follows: LINC00943, F-5’-GATGAACCACCCATGGCCT-3’; R-5’-CTTCCAGGAATGGAAGCCA-3’. miR-196b-5p, F-5’-ATCCTTCCTAGTCCAGCC-3’; R-5’-ACCTGGCGGCACTCCTTA-3’.
Transwell assay
The number of LUSC cells migration and invasion was detected by Transwell assay, and the steps were similar, both of which were performed in a 24-well Transwell chamber. The specific steps are as follows: first add RPMI-1640 medium in which the transfected cells (2 × 104 cells/well) have been suspended in the upper chamber; then add 10% FBS and RPMI-1640 medium to the lower chamber to induce cell migration; 24 h later, use 4% paraformaldehyde was fixed for 30 min to migrate cells in the lower chamber, then the cells were washed with PBS; finally, stained with 0.1% crystal violet for 20 min at room temperature. It should be noted that in cell invasion assay, the upper chamber needs to be coated with Matrigel (200 mg/ml, BD Biosciences, Franklin Lakes, NJ, USA) 30 min in advance at 37°C. Under the optical microscope, observe the number of cells in five random fields of view.
Luciferase reporter assay
The luciferase reporter vector pmirGLO (Promega, Shanghai, China) was selected to construct WT-LINC00943 (wild-type) and MUT-LINC00943 (mutant-type). The H520 cells were seeded in 24-well plates and respectively co-transfected with miR-196b-5p mimic, miR-196b-5p inhibitor, mimic NC, and inhibitor NC with the help of Lipofectamine 3000 reagent. After 48 h, the measurement was performed by a dual-luciferase reporter assay kit (Promega) following the manufacturer’s protocol.
Statistical analysis
The statistical analysis was analyzed via the SPSS 20.0 software (SPSS, USA) and GraphPad Prism 5.0 software (GraphPad Software, USA). All experimental data are expressed as mean ± standard deviation (SD) deviation, and the correlation between the expression of LINC00943 and the clinical data of patients was compared by the χ2 test. The prognostic significance of LINC00943 can be analyzed by Kaplan-Meier and Cox regression analysis. P value lower than 0.05 is considered statistically significant. Each experiment was repeated at least three times.
Results
Expression of LINC00943 in LUSC tissues
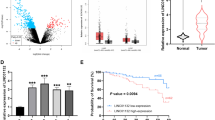
LINC00943 level in tumor tissues and normal tissues was detected via RT-qPCR. As exhibited in Fig. 1, LINC00943 were significantly reduced in tumor tissues, compared with adjacent normal tissues. According to the average expression of LINC00943, LUSC patients were divided into high expression group (n = 44) and low expression group (n = 45), and the correlation between LINC00943 expression and clinical characteristics of patients was analyzed (Table 1). The data showed that lymph node metastasis (P = 0.025) and TNM stage (P = 0.012) were associated with low expression of LINC00943.
Prognostic potential of LINC00943 in LUSC
The prognostic value of LINC00943 in LUSC was evaluated by Kaplan-Meier curve analysis and log-rank test. According to the analysis of LINC00943 expression and progression-free survival probability, the survival probability of high LINC00943 expression group (n = 44) was higher than that of low LINC00943 expression group (n = 45) in five years (Fig. 2). Additionally, Table 2 illustrated that LINC00943 (HR = 4.007, 95% CI = 1.568–10.237, P = 0.004), differentiation (HR = 2.805, 95% CI = 1.180–6.671, P = 0.020), lymph node metastasis (HR = 3.241, 95% CI = 1.384–7.591, P = 0.007), and TNM stage (HR = 3.874, 95% CI = 1.267–11.845, P = 0.018) were independent prognostic factors for five-year overall survival in LUSC after multivariate Cox analysis.
Assay in vitro of LUSC cells
To further confirm the expression of LINC00943 in cells, Fig. 3A showed downregulation of LINC00943 detected by RT-qPCR compared to human bronchial epithelial cells BEAS2B in LUSC cell lines (H520, H1703, EBC-1, H2170). Based on the relatively lower expression of LINC00943 in H520 and H2170 cells, all of them were chosen for subsequent experiments. The overexpression pcDNA3.1-LINC00943 was constructed with pcDNA3.1 as the vector, LINC00943 expression in H520 and H2170 cells was detected, and the function and influence of LINC00943 in LUSC were studied. In Fig. 3B, LINC00943 was obviously increased in H520 and H2170 cells after transfection. Moreover, as displayed in Fig. 3C and D, Transwell assay results demonstrated that pcDNA3.1-LINC00943 suppressed the migratory level and invasive abilities of H520 and H2170 cells. That is, LINC00943 overexpression may alleviate the exacerbation of LUSC.
The effect of overexpression of LINC00943 on LUSC cells was analyzed in vitro assays. (A) LINC00943 level in four LUSC cell lines (H520, H1703, EBC-1, H2170) and normal cell BEAS2B was detected. (B) LINC00943 expression ensured the transfection efficiency. (C) and (D) Migratory level and invasive ability of H520 and H2170 cells were measured via Transwell assay. ***P < 0.001
LINC00943 interacted with miR-196b-5p
Online prediction and bioinformatics analysis revealed that LINC00943 and miR-196b-5p form multiple base pairs. According to the luciferase assay showed that miR-196b-5p mimic significantly reduced the luciferase activity of cells transfected with WT-LINC00943, miR-485-5p inhibitor increased luciferase activity, whereas luciferase activity was not affected in MUT-LINC00943 transfected H520 cells (Fig. 4A). The content of miR-196b-5p was tested, and the increased expression of miR-196b-5p in LUSC tissues and cells was shown in Fig. 4B and C. Figure 4D characterizes the negative correlation between LINC00943 and miR-196b-5p through the Pearson correlation coefficient, indicating that high expression of LINC00943 inhibited the expression of miR-196b-5p (r = -0.7157, P < 0.0001). Simultaneously, the relative expression of miR-196b-5p in H520 cells after transfection with pcDNA3.1-LINC00943 elaborated that the high expression of LINC00943 inhibited the expression of miR-196b-5p (Fig. 4E).
Interaction of LINC00943 and miR-196b-5p. (A) Luciferase activity was examined in H520 cell co-transfected with miR-196b-5p mimic, miR-196b-5p inhibitor, mimic NC or inhibitor NC and WT-LINC00943 or MUT-LINC00943. (B) and (C) Compared with normal tissues adjacent to cancer, miR-196b-5p expression was up-regulated in tumor tissues and cells. (D) The reverse correlation between LINC00943 and miR-196b-5p expression (r = -0.7157, P < 0.0001). (E) Overexpression of LINC00943 inhibited the expression of miR-196b-5p in H520 cell. ***P < 0.001
Discussion
LUSC is a unique subtype of NSCLC that accounts for 25% to 30% of lung cancers [16, 17]. LUSC mostly occurs in elderly men who are addicted to smoking and is greatly affected by personal living habits [18, 19]. Most patients have no significant symptoms in the early stage, or are accompanied by symptoms such as cough, bloody sputum, chest pain, and fever [20]. With the gradual deterioration of the disease will appear corresponding respiratory symptoms or cancer metastasis expansion. Traditional surgery and drug therapy have certain limitations, so for better treatment and prognosis, researchers focus on the research and evaluation of molecular biomarkers. In the past few years, many studies have shown that lncRNAs play important role in tumor growth and metastasis.
Li et al. stated that under-expression of STARD13-AS in LUSC that affected tumor cells growth and motility [21]. In the research of Yin et al., it was found that the decrease of SFTA1P expression was related to the occurrence and metastasis of LUSC and was expected to become a new prognostic target for LUSC [22]. Here, we demonstrated that LINC00943 was lowly expressed in LUSC tissues and cells and proved to be a prognostic factor for LUSC, which was similar to the existing evidence. In addition, the significantly high expression of LINC00943 in this study negatively affected the migration and invasion of tumor cells, which was consistent with Wan et al. ‘s detection that overexpression of WT1-AS reduced the migration and invasion of NSCLC cells [23]. It is inferred that LINC00943 may regulate the occurrence and development of LUSC.
It has been reported that miRNAs, as conserved non-coding single-stranded RNA molecules, are involved in a variety of physiological and pathological processes [24]. As a sponge of miRNAs, lncRNA forms the corresponding lncRNA/miRNA axis to regulate transcription and tumor cell proliferation [25, 26]. This study predicted that LINC00943 and miR-196b-5p have binding sites. Liang et al. learned that miR-196b-5p mediates TSPAN12 and GATA6 factors to accelerate the development of NSCLC [27]. The downregulation of RSPO2 by miR-196b-5p also provided a new theoretical basis for the treatment of lung adenocarcinoma [28]. In addition, studies on miR-196b-5p elucidated that miR-196b-5p targets and negatively regulates NFKBIA to accelerate the growth of NSCLC tumor cells [29]. Existing evidence also described the differential expression and regulation of miR-196b-5p in diseases such as medulloblastoma, keloid tumor, infantile hemangioma [30,31,32]. We proved that miR-196b-5p was upregulated in LUSC, that LINC00943 directly targets miR-196b-5p to mediate disease in LUSC patients, and that LINC00943 and miR-196b-5p are negatively correlated. These results indicate that LINC00943/miR-196b-5p axis plays a regulatory role in LUSC, suggesting a new therapeutic target.
In particular, the process design of this study is relatively complete, but the lack of in vivo cell experiments limits the clinical application and promotion of LINC00943 as a prognostic marker. In addition, it is necessary to recruit more volunteers to participate in our subsequent research to improve the persuasiveness of the research results. There is still a long way to go before theoretical research can actually be applied to clinical experiments.
Conclusions
In summary, all data and results indicate that LINC00943 was downregulated and miR-196b-5p was significantly upregulated in LUSC tissues and cells. Overexpression of LINC00943 inhibited the progress of LUSC by negatively regulating miR-196b-5p. LINC00943 is promising to be a biomarker for the prognosis of LUSC.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Gao Y, Lyu Q, Luo P, Li M, Zhou R, Zhang J, et al. Applications of machine learning to Predict Cisplatin Resistance in Lung Cancer. Int J Gen Med. 2021;14:5911–25.
Wu J, Hong S, **e X, Liu W. A Network Pharmacology-based study on the Anti-lung Cancer Effect of Dipsaci Radix. Evidence-based Complement Altern Medicine: eCAM. 2020;2020:7424061.
Zhang W, Cui Q, Qu W, Ding X, Jiang D, Liu H. TRIM58/cg26157385 methylation is associated with eight prognostic genes in lung squamous cell carcinoma. Oncol Rep. 2018;40(1):206–16.
Liang S, Zhou G, Hu W. Research Progress of Heavy Ion Radiotherapy for Non-small-cell Lung Cancer. Int J Mol Sci. 2022;23(4).
Huang C, He J, Dong Y, Huang L, Chen Y, Peng A, et al. Identification of novel prognostic markers Associated with laryngeal squamous cell Carcinoma using Comprehensive Analysis. Front Oncol. 2021;11:779153.
Cui X, Yu H, Yu T, **ao D, Wang X. LncRNA MNX1-AS1 drives aggressive laryngeal squamous cell carcinoma progression and serves as a ceRNA to target FoxM1 by sponging microRNA-370. Aging. 2021;13(7):9900–10.
Cao J, Yang Z, An R, Zhang J, Zhao R, Li W, et al. lncRNA IGKJ2-MALLP2 suppresses LSCC proliferation, migration, invasion, and angiogenesis by sponging miR-1911-3p/p21. Cancer Sci. 2020;111(9):3245–57.
Shen Z, Li Q, Deng H, Lu D, Song H, Guo J. Long non-coding RNA profiling in laryngeal squamous cell carcinoma and its clinical significance: potential biomarkers for LSCC. PLoS ONE. 2014;9(9):e108237.
Ma Y, Zhang H, Li X, Liu Y. HAGLROS promotes cell proliferation and angiogenesis and inhibits apoptosis by activating multiple signaling pathways in LSCC cells. J oral Pathol Medicine: Official Publication Int Association Oral Pathologists Am Acad Oral Pathol. 2022;51(6):510–9.
Ni J, Zhang X, Li J, Zheng Z, Zhang J, Zhao W, et al. Tumour-derived exosomal lncRNA-SOX2OT promotes bone metastasis of non-small cell lung cancer by targeting the miRNA-194-5p/RAC1 signalling axis in osteoclasts. Cell Death Dis. 2021;12(7):662.
**a R, Geng G, Yu X, Xu Z, Guo J, Liu H et al. LINC01140 promotes the progression and tumor immune escape in lung cancer by sponging multiple microRNAs. J Immunother Cancer. 2021;9(8).
Meng C, Gao J, Ma Q, Sun Q, Qiao T. LINC00943 knockdown attenuates MPP(+)-induced neuronal damage via miR-15b-5p/RAB3IP axis in SK-N-SH cells. Neurol Res. 2021;43(3):181–90.
Xu Y, Ji T, An N, Wang X, Zhang H, Xu F. LINC00943 is correlated with gastric cancer and regulates cancer cell proliferation and chemosensitivity via hsa-miR-101-3p. Int J Clin Oncol. 2021;26(9):1650–60.
Zhang Y, Dai J, Huang W, Chen Q, Chen W, He Q, et al. Identification of a competing endogenous RNA network related to immune signature in clear cell renal cell carcinoma. Aging. 2021;13(24):25980–6002.
Chen Z, Feng R, Kahlert UD, Chen Z, Torres-Dela Roche LA, Soliman A, et al. Construction of ceRNA Networks Associated with CD8 T cells in breast Cancer. Front Oncol. 2022;12:883197.
Zhang J, Pan Y, Shi Q, Zhang G, Jiang L, Dong X, et al. Paclitaxel liposome for injection (Lipusu) plus cisplatin versus gemcitabine plus cisplatin in the first-line treatment of locally advanced or metastatic lung squamous cell carcinoma: a multicenter, randomized, open-label, parallel controlled clinical study. Cancer Commun (London England). 2022;42(1):3–16.
Yu G, Zhong N, Huang B, Mi Y. PEBP4 gene expression in lung squamous cell carcinoma: a meta-analysis-based study of the molecular pathways involved. Oncol Lett. 2020;19(4):2825–31.
Liu Y, Yuan X, Chen K, Zhou F, Yang H, Yang H, et al. Clinical significance and prognostic value of Porphyromonas gingivalis infection in lung cancer. Translational Oncol. 2021;14(1):100972.
**a Z, **ao J, Dai Z, Chen Q. Membrane progesterone receptor α (mPRα) enhances hypoxia-induced vascular endothelial growth factor secretion and angiogenesis in lung adenocarcinoma through STAT3 signaling. J Translational Med. 2022;20(1):72.
Lv X, Zhao Y, Wu Y. Effects of the training of aerobic function on clinical symptoms and quality of life in patients with medium and advanced Lung Cancer. J Healthc Eng. 2022;2022:6753959.
Li G, Guo X. LncRNA STARD13-AS blocks lung squamous carcinoma cells growth and movement by targeting miR-1248/C3A. Pulm Pharmacol Ther. 2020;64:101949.
Yin YZ, Yao SH, Li CG, Ma YS, Kang ZJ, Zhang JJ, et al. Systematic analysis using a bioinformatics strategy identifies SFTA1P and LINC00519 as potential prognostic biomarkers for lung squamous cell carcinoma. Am J Translational Res. 2021;13(1):168–82.
Wan Y, Yao D, Fang F, Wang Y, Wu G, Qian Y. LncRNA WT1-AS downregulates lncRNA UCA1 to suppress non-small cell lung cancer and predicts poor survival. BMC Cancer. 2021;21(1):104.
Qian K, Li Q, Deng W, **ang X. Multiple-scales integrative analysis of MicroRNAs unveils biomarkers and Key Regulatory connections for Hepatocellular Carcinoma. Crit Rev Eukaryot Gene Expr. 2019;29(3):189–241.
He L, Chang H, Qi Y, Zhang B, Shao Q. ceRNA networks: the Backbone Role in Neoadjuvant Chemoradiotherapy Resistance/Sensitivity of locally advanced rectal Cancer. Technol Cancer Res Treat. 2021;20:15330338211062313.
Yi X, Cheng X. Understanding competitive endogenous RNA network mechanism in type 1 diabetes Mellitus using computational and Bioinformatics approaches. Diabetes Metabolic Syndrome Obesity: Targets Therapy. 2021;14:3865–945.
Liang G, Meng W, Huang X, Zhu W, Yin C, Wang C, et al. miR-196b-5p-mediated downregulation of TSPAN12 and GATA6 promotes tumor progression in non-small cell lung cancer. Proc Natl Acad Sci USA. 2020;117(8):4347–57.
Xu Q, Xu Z. miR-196b-5p promotes Proliferation, Migration and Invasion of Lung Adenocarcinoma cells via Targeting RSPO2. Cancer Manage Res. 2020;12:13393–402.
Zhu W, Yu Y, Ye Y, Tu X, Zhang Y, Wu T, et al. MiR-196b-5p activates NF-κB signaling in non-small cell lung cancer by directly targeting NFKBIA. Translational Oncol. 2023;37:101755.
Wang QZ, Zhao ZL, Liu C, Zheng JW. Exosome-derived miR-196b-5p facilitates intercellular interaction in infantile hemangioma via down-regulating CDKN1B. Annals Translational Med. 2021;9(5):394.
Yang J, Deng P, Qi Y, Feng X, Wen H, Chen F. NEAT1 Knockdown inhibits keloid fibroblast progression by miR-196b-5p/FGF2 Axis. J Surg Res. 2021;259:261–70.
Visani M, Marucci G, Biase D, Giangaspero F, Buttarelli FR, Brandes AA et al. miR-196B-5P and miR-200B-3P Are Differentially Expressed in Medulloblastomas of Adults and Children. Diagnostics (Basel, Switzerland). 2020;10(5).
Acknowledgements
Not applicable.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Study conception and design: Z. Zhao, H. Li; data collection: J. Li, Y. Rong, M. Hao; Analysis and interpretation of results: Z. Zhao, L. Zhao, F. Tian; Draft manuscript preparation: Z. Zhao, H. Li. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of KaiLuan General Hospital approved this study, and all participating patients signed an informed consent form as required.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhao, Z., Li, H., Li, J. et al. Expression of lncRNA LINC00943 in lung squamous cell carcinoma and its relationship with tumor progression. J Cardiothorac Surg 19, 222 (2024). https://doi.org/10.1186/s13019-024-02771-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-024-02771-2