Abstract
Background
Thrombosis in the pulmonary vein stump (PVS) is not a well-known complication after pulmonary lobectomy, but it has the potential to cause embolism to vital organs. The aim of this study was to evaluate the risk factors for thrombosis in the PVS after pulmonary lobectomy.
Methods
A total of 439 patients who underwent pulmonary lobectomy from 2008 to 2017 were retrospectively reviewed, and 412 patients were further analyzed. The state of the PVS was evaluated by chest contrast-enhanced computed tomography (CECT). Univariate analysis was performed to evaluate the potential risk factors for thrombosis in the PVS.
Results
Thrombosis in the PVS was detected in 6 of 412 (1.5%) patients, and 5 of them underwent left upper lobectomy (LUL) (5/100, 5.0%) (P = 0.004). In the analyses of the LUL group, postoperative chest radiotherapy was identified as a risk factor for thrombosis in the PVS (P = 0.024), and postoperative atrial fibrillation showed a tendency to be a risk factor for thrombosis (P = 0.058).
Conclusions
Chest radiotherapy after LUL is a possible risk factor for thrombosis in the PVS. Periodic chest CECT is recommended after postoperative chest radiotherapy for patients after LUL.
Similar content being viewed by others
Background
Thrombosis in the pulmonary vein stump (PVS) is not a well-known complication after pulmonary lobectomy, but it has the potential to cause embolism to vital organs. Left upper lobectomy (LUL) has been considered to have a higher risk of thrombus formation in the PVS than other lobectomies [1,2,3]. Previous studies showed that the length of the left superior pulmonary vein (LSPV) stump was significantly longer than that of other PVSs due to the anatomical aspect of the resected pulmonary vein [1, 4]. The long LSPV stump resulted in stasis of blood flow and subsequent thrombus formation [2, 3]. Although LUL is considered to be a risk factor for thrombus formation in the PVS, relevant research reports are still rare, and the risk factors for thrombosis in the LSPV stump remain unclear. In the present study, the risk factors for thrombus formation in the PVS after pulmonary lobectomy were evaluated, with special focus on the risk factors in the LUL group.
Methods
Selection of patients and management of clinical data
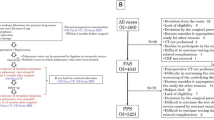
A total of 439 patients who underwent pulmonary lobectomy in Hirosaki University Hospital from January 2008 to December 2017 were retrospectively reviewed. Patients who underwent partial resection, segmentectomy, multiple lobectomy, and pneumonectomy were not included, and 27 patients who did not undergo chest contrast-enhanced computed tomography (CECT) at least once within 2 years after surgery were excluded. Thus, 412 patients remained for the further analyses.
Evaluation of thrombosis in the PVS
Chest CECT images were retrospectively interpreted to check for PVS thrombus by three doctors, including two thoracic and cardiovascular surgeons and one radiologist.
Operative policy
Anatomical lobectomy with systematic regional lymph node dissection was performed for primary lung cancer (N = 396). The extent of the lymph node dissection was determined according to the criteria of the Japan Lung Cancer Society [5]. Anatomical lobectomy was performed for metastatic carcinoma of the lung (N = 14) and for benign disease (N = 2) in the present study. The pulmonary vein was dissected in the extrapericardial space by ligation or linear stapler as a routine procedure.
Statistical analysis
Statistical analyses were carried out using the Statistical Package for the Social Sciences (SPSS) (version 25, IBM, Armonk, NY, USA). Univariate analysis was used to predict the risk factors for thrombosis in the PVS. A significant difference was accepted as a P value less than 0.05 for all analyses.
Results
Patients’ characteristics
A total of 412 patients (261 males, 151 females) were analyzed in the present study. The patients’ ages ranged from 22 to 84 years (median 68 years). The operative procedures consisted of 100 left upper lobectomies (LULs), 73 left lower lobectomies (LLLs), 146 right upper lobectomies (RULs), 22 right middle lobectomies (RMLs), and 71 right lower lobectomies (RLLs). The operative approaches were 318 video-assisted thoracic surgery (VATS) procedures and 94 open thoracotomies. The tumor pathologies included 394 cases of primary lung cancer, 14 cases of metastatic lung tumors, 2 cases of pulmonary sarcoma, and 2 cases of benign tumor. Overall, 138 cases received postoperative chemotherapy, and 61 received postoperative chest radiotherapy.
Thrombosis in the PVS after pulmonary lobectomy
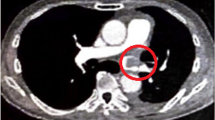
Thrombosis in the PVS was observed in 6/412 (1.5%) patients after pulmonary lobectomy, including 5 patients after LUL and 1 patient after RUL (Table 1, P = 0.004). Figure 1 shows the flow chart. Figure 2 presents the typical radiological findings of thrombosis in the PVS after pulmonary lobectomy.
Typical radiological findings of thrombosis in the pulmonary vein stump after pulmonary lobectomy. a Thrombosis in the left superior pulmonary vein stump was detected by postoperative chest contrast-enhanced axial computed tomography scans; b Thrombosis in the left superior pulmonary vein stump was detected by postoperative chest contrast-enhanced coronal computed tomography scans
Risk factors for thrombosis in the PVS after LUL
In the patients who underwent LUL, clinicopathological factors were compared between the patients with and without PVS thrombus. Table 2 shows the result of the univariable analysis to evaluate the possible risk factors associated with PVS thrombus formation after LUL. It was found that postoperative chest radiotherapy was significantly related to thrombus formation in the PVS (P = 0.024). Postoperative atrial fibrillation (AF) showed a tendency to be a risk factor for thrombosis (P = 0.058).
Main details of the cases with thrombosis in the LSPV
The 5 cases with thrombosis in the PVS after LUL are shown in Table 3. Thrombosis was detected on the first postoperative chest CECT in case 1 and case 2 at 12 days and 14 days after surgery, respectively. Thrombosis was not detected on the first postoperative chest CECT in cases 3, 4, and 5, but it was detected on chest CECT after postoperative chest radiotherapy (Fig. 3). Case 3 and case 5 received 60-Gy radiotherapy to the mediastinal region, and case 4 received 45-Gy radiotherapy to the right lung field. Postoperative AF was detected in case 4 and case 5. Case 1 and case 4 received anti-coagulant drug therapy, and the thrombus disappeared after treatment. However, the 3 other cases did not receive antithrombotic therapy; thrombus disappeared spontaneously in 2 cases and became smaller in 1 case. Acute renal infarction was detected in case 1 in the 12 days after surgery. The other 4 cases did not develop acute organ infarction.
Discussion
In this study, the risk factors for thrombosis in the PVS after lobectomy were investigated. It was confirmed that LUL was a risk factor for thrombosis in the PVS after lobectomy. More importantly, postoperative chest radiotherapy was found to be significantly associated with thrombosis in the LSPV stump in patients who underwent LUL.
Some researchers have reported LUL as a risk factor for thrombosis in the PVS [1,2,3]. The mechanism of the thrombosis in the LSPV stump was considered to be related to two main factors. One was the hemodynamic changes (stasis or turbulence) caused by a long LSPV stump [1, 2, 6], and the other was vascular endothelial injury of the PVS caused by surgery [6, 7]. Ohtaka [2] considered that thrombus was more likely to develop within a few days after LUL, meaning that short-term thrombosis was closely related to the operation.
In the present study, there were 3 cases with thrombosis in the PVS within 2 weeks of surgery, including 2 cases after LUL and 1 case after RUL. However, 3 cases with thrombosis in the PVS were observed 365–1127 days after LUL, and the PVS thrombus was not observed on the first postoperative chest CECT. All 3 cases with thrombosis in the LSPV stump received postoperative radiotherapy. We considered that the thrombus that developed a long time after the operation, especially thrombus that was not detected on the first chest CECT after surgery, might not be closely related to the operation. Radiotherapy was considered to be a risk factor for endothelial vascular injury [8,9,10]. Some researchers suggested that high-dose radiation to the chest wall may cause intimal injury, leading to endothelial disruption and activation of myofibroblasts and platelets. Endothelial injury results in the formation of cholesterol plaques containing infiltrates of macrophages and neutrophils, which have been associated with plaque hemorrhage and an increased risk of coronary thrombosis [11,12,13]. Chest radiotherapy might aggravate the injury of the vascular endothelium in the PVS, which might be an important reason for thrombus formation in the PVS. We consider that postoperative chest radiotherapy is a risk factor for thrombosis in the PVS. The pattern diagram was provided in Fig. 4.
Atrial fibrillation (AF) was considered to increase the risk of vital organ infarction by the formation of a thrombus in the left atrial appendage due to turbulent blood flow [14, 15]. In the present study, AF was found to be a potential risk factor for thrombus formation in the LSPV stump. AF might not only result in turbulent blood flow in the left atrial appendage, but it may also aggravate blood stasis in the LSPV stump after LUL, which might be the reason for the high risk with AF for thrombosis in the LSPV stump. However, this hypothesis needs further experimental verification.
Thrombus in the PVS was found to disappear spontaneously without any anticoagulant therapy (Table 3), which was also reported by Hattori et al. [16]. Therefore, some developed thrombus might have disappeared when the patient underwent chest CECT. We considered that the timing of chest CECT had an effect on the detection of thrombosis in the PVS. More thrombosis in the PVS might be observed if chest CECT is routinely performed shortly (within 2 weeks) after pulmonary lobectomy.
Conclusion
LUL was a risk factor for thrombosis in the PVS. Postoperative chest radiotherapy was a risk factor for thrombosis in the LSPV stump after LUL. Periodic chest CECT is recommended after postoperative chest radiotherapy for patients who underwent LUL.
Limitations
This study has several limitations: (I) potential selection bias, given the nature of a retrospective study; (II) potential bias caused by the nonuniform chest CECT time; and (III) potential bias caused by the limited number of cases.
Availability of data and materials
Not applicable.
Abbreviations
- PVS:
-
Pulmonary vein stump
- LUL:
-
Left upper lobectomy
- LLL:
-
Left lower lobectomy
- RUL:
-
Right upper lobectomy
- RML:
-
Right middle lobectomy
- RLL:
-
Right lower lobectomy
- LSPV:
-
Left superior pulmonary vein
- SPSS:
-
Statistical Package for the Social Sciences
- VATS:
-
Video-assisted thoracic surgery
- AF:
-
Atrial fibrillation
- CECT:
-
Contrast-enhanced computed tomography
References
Ohtaka K, Hida Y, Kaga K, Kato T, Muto J, Nakada-Kubota R, et al. Thrombosis in the pulmonary vein stump after left upper lobectomy as a possible cause of cerebral infarction. Ann Thorac Surg. 2013;95:1924–9.
Ohtaka K, Takahashi Y, Uemura S, Shoji Y, Hayama S, Ichimura T, et al. Blood stasis may cause thrombosis in the left superior pulmonary vein stump after left upper lobectomy. J Cardiothorac Surg. 2014;9:159.
Ohtaka K, Hida Y, Kaga K, Takahashi Y, Kawase H, Hayama S, et al. Left upper lobectomy can be a risk factor for thrombosis in the pulmonary vein stump. J Cardiothorac Surg. 2014;9:5.
Ohtaka K, Hida Y, Kaga K, Iimura Y, Shiina N, Muto J, et al. Pulmonary vein thrombosis after video-assisted thoracoscopic left upper lobectomy. J Thorac Cardiovasc Surg. 2012;143:E3–5.
Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P, et al. The IASLC lung cancer staging project a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4:568–77.
Hashimoto H, Usui G, Tsugeno Y, Sugita K, Amori G, Morikawa T, et al. Cerebral thromboembolism after lobectomy for lung cancer: pathological diagnosis and mechanism of thrombus formation. Cancers. 2019;11:488.
Brotman DJ, Deitcher SR, Lip GYH, Matzdorff AC. Virchow’s triad revisited. South Med J. 2004;97:213–4.
Venkatesulu BP, Mahadevan LS, Aliru ML, Yang X, Bodd MH, Singh PK, et al. Radiation-induced endothelial vascular injury: a review of possible mechanisms. JACC Basic Transl Sci. 2018;3:563–72.
Mendonca MS, Chin-Sinex H, Dhaemers R, Mead LE, Yoder MC, Ingram DA. Differential mechanisms of X-ray-induced cell death in human endothelial progenitor cells isolated from cord blood and adults. Radiat Res. 2011;176:208–16.
Milliat F, Francois A, Isoir M, Deutsch E, Tamarat R, Tarlet G, et al. Influence of endothelial cells on vascular smooth muscle cells phenotype after irradiation: implication in radiation-induced vascular damages. Am J Pathol. 2006;169:1484–95.
Hull MC, Morris CG, Pepine CJ, Mendenhall NP. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of hodgkin lymphoma treated with radiation therapy. JAMA. 2003;290:2831–7.
Amromin GD, Gildenhorn HL, Solomon RD, Nadkarni BB. The synergism of X-irradiation and cholesterol-fat feeding on the development of coronary artery lesions. J Atheroscler Res. 1964;4:325–34.
Stewart FA, Heeneman S, Te Poele J, Kruse J, Russell NS, Gijbels M, et al. Ionizing radiation accelerates the development of atherosclerotic lesions in ApoE-/- mice and predisposes to an inflammatory plaque phenotype prone to hemorrhage. Am J Pathol. 2006;168:649–58.
Friedman DJ, Piccini JP, Wang T, Zheng J, Malaisrie SC, Holmes DR, et al. Association between left atrial appendage occlusion and readmission for thromboembolism among patients with atrial fibrillation undergoing concomitant cardiac surgery. JAMA. 2018;319:365–74.
Yao X, Gersh BJ, Holmes DR Jr, Melduni RM, Johnsrud DO, Sangaralingham LR, et al. Association of surgical left atrial appendage occlusion with subsequent stroke and mortality among patients undergoing cardiac surgery. JAMA. 2018;319:2116–26.
Hattori A, Takamochi K, Kitamura Y, Matsunaga T, Suzuki K, Oh S, et al. Risk factor analysis of cerebral infarction and clinicopathological characteristics of left upper pulmonary vein stump thrombus after lobectomy. Gen Thorac Cardiovasc. 2019;67:247–53.
Acknowledgements
Not applicable.
Presented at the 72nd Annual Scientific Meeting of the Japanese Association for Thoracic Surgery, Kyoto, Japan, Oct 30–Nov 2, 2019.
Funding
No.
Author information
Authors and Affiliations
Contributions
C-YS, DK, IF, and FT designed the study. C-YS and DK collected the clinical data. C-YS, DK, and FT checked CECT to detect thrombosis in the PVS. C-YS analysed and interpreted the data. C-YS performed statistical analysis. C-YS drafted the manuscript. FT made critical revisions of the manuscript for important intellectual content. TT and TS made critical revisions of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by The Committee of Medical Ethics of Hirosaki University Graduate School of Medicine, Hirosaki, Japan (Approval Number 2019-1095), and the need for informed consent was waived.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Song, CY., Kimura, D., Fukuda, I. et al. Chest radiotherapy after left upper lobectomy may be a risk factor for thrombosis in the pulmonary vein stump. J Cardiothorac Surg 17, 154 (2022). https://doi.org/10.1186/s13019-022-01902-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-022-01902-x