Abstract
Background
Surgical site infection (SSI) is a common and serious complication of elective clean orthopedic surgery that can lead to severe adverse outcomes. However, the prognostic efficacy of the current staging systems remains uncertain for patients undergoing elective aseptic orthopedic procedures. This study aimed to identify high-risk factors independently associated with SSI and develop a nomogram prediction model to accurately predict the occurrence of SSI.
Methods
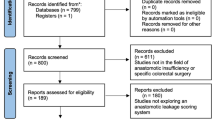
A total of 20,960 patients underwent elective clean orthopedic surgery in our hospital between January 2020 and December 2021, of whom 39 developed SSI; we selected all 39 patients with a postoperative diagnosis of SSI and 305 patients who did not develop postoperative SSI for the final analysis. The patients were randomly divided into training and validation cohorts in a 7:3 ratio. Univariate and multivariate logistic regression analyses were conducted in the training cohort to screen for independent risk factors of SSI, and a nomogram prediction model was developed. The predictive performance of the nomogram was compared with that of the National Nosocomial Infections Surveillance (NNIS) system. Decision curve analysis (DCA) was used to assess the clinical decision-making value of the nomogram.
Results
The SSI incidence was 0.186%. Univariate and multivariate logistic regression analysis identified the American Society of Anesthesiology (ASA) class (odds ratio [OR] 1.564 [95% confidence interval (CI) 1.029–5.99, P = 0.046]), operative time (OR 1.003 [95% CI 1.006–1.019, P < 0.001]), and D-dimer level (OR 1.055 [95% CI 1.022–1.29, P = 0.046]) as risk factors for postoperative SSI. We constructed a nomogram prediction model based on these independent risk factors. In the training and validation cohorts, our predictive model had concordance indices (C-indices) of 0.777 (95% CI 0.672–0.882) and 0.732 (95% CI 0.603–0.861), respectively, both of which were superior to the C-indices of the NNIS system (0.668 and 0.543, respectively). Calibration curves and DCA confirmed that our nomogram model had good consistency and clinical predictive value, respectively.
Conclusions
Operative time, ASA class, and D-dimer levels are important clinical predictive indicators of postoperative SSI in patients undergoing elective clean orthopedic surgery. The nomogram predictive model based on the three clinical features demonstrated strong predictive performance, calibration capabilities, and clinical decision-making abilities for SSI.
Similar content being viewed by others
Background
Surgical site infections (SSIs) are postoperative infections encompassing the superficial, deep, and interstitial layers [1,2,3]. SSI is a common nosocomial infection, leading to extended patient hospitalization and imposing substantial burdens on patients [1, 4, 5]. According to a U.S. Centers for Disease Control and Prevention health care-associated infection (HAI) prevalence survey, nearly 600,000 cases of SSI occurred in the USA in 2011, making it the most common HAI [6]. It is estimated that approximately 5% of patients develop SSI during the perioperative period, which prolongs the average length of stay by more than 9 days and increases the risk of death by 11 times [1].
Notably, orthopedic patients have heightened susceptibility to SSI relative to other patients owing to the enduring presence of internal fixation and implant apparatus within the body [7, 8]. These components create conducive niches and substrates for pathogenic proliferation, consequently significantly elevating the risk of postoperative wound infections [9, 10]. When SSI occurs during joint implant surgery, the cost per treatment may exceed $90,000 [2, 11, 12]. However, approximately 55% of SSIs are preventable through proper implementation of evidence-based strategies, so timely preoperative detection of high-risk SSI patients is critical [13].
The National Nosocomial Infections Surveillance (NNIS) risk index [14, 15] is the prevailing clinical prognostic instrument for predicting overall SSI risk. The NNIS system employs three autonomous and equitably significant variables—the American Society of Anesthesiology (ASA) classification [16], surgical incision type, and operative duration—to predict SSI risk. However, the prognostic efficacy of the NNIS system remains uncertain with respect to the prediction of SSI risk in patients undergoing elective aseptic orthopedic procedures [17, 18]. Consequently, the formulation of a composite predictive model based on multiple preoperative clinical parameters is imperative to aid orthopedic practitioners in identifying candidates at high risk of SSI.
A nomogram is a straightforward instrument for clinical prognostication and is used to predict clinical outcomes [19]. Nomograms have extensive applications across domains, such as oncology [20], cardiovascular ailments [3A).
Predictive value of the nomogram model for SSI in the validation cohort
The nomogram also showed higher performance in predicting SSI in the validation cohort, with a C-index of 0.732 (95% CI 0.603–0.861), compared with the C-index of 0.543 (95% CI 0.410–0.677) for the NNIS system (Fig. 2B). In addition, the calibration curve of the SSI forecast showed that the nomogram agreed well with the observed development of SSI. This demonstrates that our nomogram-based prediction model had good predictive performance for the occurrence of SSI in the validation cohort (Fig. 3B).
Comparison of the nomogram and NNIS system
In the training cohort, the nomogram showed favorable predictive performance for SSI detection, with an NPV, PPV, specificity, sensitivity, accuracy, precision, and recall of 0.946, 0.444, 0.906, 0.593, 0.871, 0.444, and 0.593, respectively (Table 3). The nomogram had a higher predictive performance for SSI than the NNIS system (Table 3). The nomogram also showed good performance for SSI in the validation cohort (Table 4). The NPV, PPV, specificity, sensitivity, accuracy, precision, and recall for the nomogram and NNIS system in the validation cohort are summarized in Table 4.
DCA showed that the nomogram for predicting SSI was more valuable than the NNIS system in the training cohort (Fig. 4A) and in the validation cohort (Fig. 4B). Therefore, our nomogram outperforms the existing models.
Discussion
Despite advances in the management of perioperative nosocomial infections in recent years, SSIs remain a common cause of increased mortality, length of stay, and cost in surgical patients [1, 2]. Our investigation devised a model aimed at predicting the incidence of SSI in individuals undergoing clean orthopedic surgery, thereby proficiently evaluating the risk of SSI among elective aseptic orthopedic patients. Using univariate and multivariate logistic regression analyses, we established that operation time, ASA class, and D-dimer level were independently correlated with a heightened risk of postoperative SSI. Subsequently, the logistic regression model was translated into a visual representation a nomogram. Our nomogram model not only exhibited robust predictive capability and impeccable calibration but also had substantial clinical utility in facilitating informed decision-making for patients within both the training and validation cohorts. Additionally, we extended our efforts to develop an easy-to-use and free-to-access online calculator based on the nomogram model (https://jitao.shinyapps.io/dynnomapp/), an accessible tool designed to enable clinicians and researchers to readily ascertain the probability of postoperative SSI in the special patient populations.
The American College of Surgeons incision grading system stratifies incisions into four distinct grades: grade I, grade II, grade III, and grade IV. Grade I incisions, characterized as clean surgeries, exhibit a propensity for swift and comprehensive healing within a condensed timeframe. Directives formulated by the US Centers for Disease Control and Prevention state that clean surgeries, including of drainage procedures, require no supplementary antibiotic prophylaxis after closure of the surgical incision [2]. Although, compared with other types of surgery, the risk of SSI in patients undergoing clean orthopedic surgery is relatively low, once SSI occurs, it may lead to serious clinical outcome [29, 30].
The NNIS grading system is currently the most widely used clinical tool for predicting the occurrence of SSI and includes three independent and equally important variables: ASA class, surgical incision type, and surgical duration. Through the qualitative classification of these variables, the NNIS system divides the surgical risk into four levels, namely, NNIS level 0, NNIS level 1, NNIS level 2, and NNIS level 3 [14, 15]. However, because all surgical incision types in clean surgery are the same, the NNIS system lacks specificity for clean surgery. Compared to the NNIS system, our nomogram model integrates qualitative and quantitative clinical variables. By assigning values to each clinical variable and intuitively obtaining the occurrence probability of SSI with a 95% CI, the nomogram is more convenient for orthopedic surgeons. More importantly, our predictive model had a higher predictive ability and is more suitable than traditional NNIS system for patients undergoing clean orthopedic surgery.
Similar to the NNIS system, our nomogram included the ASA class and operation time, as they were independent risk variables for SSI. ASA classification is a clinical tool used to assess the risk of develo** SSI and severity of potential disease in patients undergoing preoperative anesthesia. Many studies have confirmed that ASA classification can be used for SSI risk stratification [31,32,33,34]. A study of 310 patients who underwent general surgery and were classified as clean or clean-contaminated confirmed that the rate of SSI was significantly higher in patients with ASA class II-III than in patients with ASA class I (P = 0.003). An ASA class > 2 is independently associated with SSI [33]. The duration of surgery is another widely recognized clinical index closely related to the occurrence of SSI. In a study of 825 patients undergoing spinal surgery, operative time (P = 0.0019) and ASA class III (P = 0.0132) were independent risk factors for SSI [32]. Higher ASA classes are associated with more comorbidities and poorer immunity, whereas longer operation time usually indicates higher surgical difficulty and longer incision exposure time, all of which increase the risk of pathogen invasion [32, 35, 36]. Therefore, shortening the operation time, especially in patients with higher ASA classes, can effectively prevent SSI.
Our predictive model also incorporates another laboratory measure, the D-dimer level, which is not included in the NNIS system. Owing to the close relationship between the coagulation system, inflammation, and endothelial injury, an increase in D-dimer levels is also often observed in some non-thrombotic diseases, such as infection, surgery, trauma, heart failure, and malignant tumors [37,38,39]. A multicenter study of patients undergoing revision total joint arthroplasty examined elevated serum C-reactive protein (CRP > 1 mg/dL), D-dimer (> 860 ng/mL), and erythrocyte sedimentation rate (> 30 mm/h), which were assigned 2, 2, and 1 points, respectively, and jointly constructed a new standard for the diagnosis of periprosthetic infection (PJI) with other laboratory indicators; its sensitivity and specificity were significantly higher than those of the Musculoskeletal Infection Association and International Consensus Conference Definition [40]. Another study demonstrated that a serum D-dimer threshold of 0.75 mg/L predicted shoulder PJI with a sensitivity of 86%, specificity of 56%, and area under the curve of 0.74. When serum D-dimer and CRP above thresholds of 0.75 mg/L and 10 mg/L, respectively, were used to predict PJI, the sensitivity and specificity were 57% and 100%, respectively [41]. Therefore, it is necessary to maintain D-dimer levels in patients at normal or even slightly decreased levels to reduce the incidence of SSI [41,42,43,44].
This study had some limitations. First, owing to the retrospective nature of the study, it only included a small number of patients who did not develop SSI, and selection bias was inevitable. Second, some inflammatory indicators that may be related to SSI, such as C-reactive protein and procalcitonin, were missing from our study; the inclusion of these indicators may help improve the predictive accuracy of the model. Third, this was a single-center study. To verify the prediction model, we randomly divided the total cohort into training and internal validation cohorts; however, we still lacked an external validation cohort. In the future, another prospective multicenter study with a larger sample size is needed to further confirm the predictive performance of this model. Finally, models based on more advanced machine learning algorithms or radiomics may be more helpful in providing predictive model accuracy [45,46,47]. Further development of SSI models based on other artificial intelligence is still needed to further improve prediction capabilities.
Conclusions
In conclusion, operation time, ASA class, and D-dimer level are important clinical indicators of postoperative SSI in patients undergoing elective clean orthopedic surgery. The nomogram prediction model based on these clinical characteristics showed strong SSI prediction performance, calibration, and clinical decision-making utility. In addition, we created an online calculator using the nomogram so that orthopedic surgeons and researchers can easily and quickly predict the risk of postoperative SSI and identify patients at high risk as early as possible to reduce the risk of infection.
Availability of data and materials
The data of this study are available from the corresponding author upon request.
Abbreviations
- ASA:
-
American society of anesthesiology
- CI:
-
Confidence interval
- C-index:
-
Concordance-index
- CRP:
-
C-reactive protein
- DCA:
-
Decision curve analysis
- NNIS:
-
National nosocomial infections surveillance
- NPV:
-
Negative predictive value
- OR:
-
Odds ratio
- PJI:
-
Periprosthetic infection
- PPV:
-
Positive predictive value
- SSI:
-
Surgical site infection
References
Ban KA, Minei JP, Laronga C, Harbrecht BG, Jensen EH, Fry DE, et al. American college of surgeons and surgical infection society: surgical site infection guidelines, 2016 update. J Am Coll Surg. 2017;224(1):59–74. https://doi.org/10.1016/j.jamcollsurg.2016.10.029.
Berríos-Torres SI, Umscheid CA, Bratzler DW, Leas B, Stone EC, Kelz RR, et al. Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 2017;152(8):784–91. https://doi.org/10.1001/jamasurg.2017.0904.
Patel M, Kumar RA, Stamm AM, Hoesley CJ, Moser SA, Waites KB. USA300 genotype community-associated methicillin-resistant Staphylococcus aureus as a cause of surgical site infections. J Clin Microbiol. 2007;45(10):3431–3. https://doi.org/10.1128/jcm.00902-07.
Graf K, Ott E, Vonberg RP, Kuehn C, Schilling T, Haverich A, et al. Surgical site infections–economic consequences for the health care system. Langenbecks Arch Surg. 2011;396(4):453–9. https://doi.org/10.1007/s00423-011-0772-0.
Thakore RV, Greenberg SE, Shi H, Foxx AM, Francois EL, Prablek MA, et al. Surgical site infection in orthopedic trauma: A case-control study evaluating risk factors and cost. J Clin Orthop Trauma. 2015;6(4):220–6. https://doi.org/10.1016/j.jcot.2015.04.004.
Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–208. https://doi.org/10.1056/NEJMoa1306801.
Greene LR. Guide to the elimination of orthopedic surgery surgical site infections: an executive summary of the association for professionals in infection control and epidemiology elimination guide. Am J Infect Control. 2012;40(4):384–6. https://doi.org/10.1016/j.ajic.2011.05.011.
Onyekwelu I, Yakkanti R, Protzer L, Pinkston CM, Tucker C, Seligson D. Surgical wound classification and surgical site infections in the orthopaedic patient. J Am Acad Orthop Surg Glob Res Rev. 2017;1(3):e022. https://doi.org/10.5435/JAAOSGlobal-D-17-00022.
Razavi SM, Ibrahimpoor M, Sabouri Kashani A, Jafarian A. Abdominal surgical site infections: incidence and risk factors at an Iranian teaching hospital. BMC Surg. 2005. https://doi.org/10.1186/1471-2482-5-2.
van Kasteren ME, Manniën J, Ott A, Kullberg BJ, de Boer AS, Gyssens IC. Antibiotic prophylaxis and the risk of surgical site infections following total hip arthroplasty: timely administration is the most important factor. Clin Infect Dis. 2007;44(7):921–7. https://doi.org/10.1086/512192.
Bozic KJ, Ries MD. The impact of infection after total hip arthroplasty on hospital and surgeon resource utilization. J Bone Jt Surg Am. 2005;87(8):1746–51. https://doi.org/10.2106/jbjs.D.02937.
Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8 Suppl):61–5. https://doi.org/10.1016/j.arth.2012.02.022.
Umscheid CA, Mitchell MD, Doshi JA, Agarwal R, Williams K, Brennan PJ. Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect Control Hosp Epidemiol. 2011;32(2):101–14. https://doi.org/10.1086/657912.
National Nosocomial Infections Surveillance (NNIS) System Report. Data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32(8):470–85. https://doi.org/10.1016/s0196655304005425.
Emori TG, Culver DH, Horan TC, Jarvis WR, White JW, Olson DR, et al. National nosocomial infections surveillance system (NNIS): description of surveillance methods. Am J Infect Control. 1991;19(1):19–35. https://doi.org/10.1016/0196-6553(91)90157-8.
Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992;13(10):606–8.
Ercole FF, Chianca TC, Duarte D, Starling CE, Carneiro M. Surgical site infection in patients submitted to orthopedic surgery: the NNIS risk index and risk prediction. Rev Lat Am Enfermagem. 2011;19(2):269–76. https://doi.org/10.1590/s0104-11692011000200007.
Paryavi E, Stall A, Gupta R, Scharfstein DO, Castillo RC, Zadnik M, et al. Predictive model for surgical site infection risk after surgery for high-energy lower-extremity fractures: development of the risk of infection in orthopedic trauma surgery score. J Trauma Acute Care Surg. 2013;74(6):1521–7. https://doi.org/10.1097/TA.0b013e318292158d.
Wu J, Zhang H, Li L, Hu M, Chen L, Xu B, et al. A nomogram for predicting overall survival in patients with low-grade endometrial stromal sarcoma: a population-based analysis. Cancer Commun (Lond). 2020;40(7):301–12. https://doi.org/10.1002/cac2.12067.
Wang J, Zhang Z, Shang D, Liao Y, Yu P, Li J, et al. A novel nomogram for prediction of post-hepatectomy liver failure in patients with resectable hepatocellular carcinoma: a multicenter study. J Hepatocell Carcinoma. 2022;27(9):901–12. https://doi.org/10.2147/JHC.S366937.
Zhou Y, Shi W, Zhao D, **ao S, Wang K, Wang J. Identification of immune-associated genes in diagnosing aortic valve calcification with metabolic syndrome by integrated bioinformatics analysis and machine Learning. Front Immunol. 2022;4(13):937886. https://doi.org/10.3389/fimmu.2022.937886.
Wang J, Li Z, Liao Y, Li J, Dong H, Peng H, et al. Prediction of survival and analysis of prognostic factors for patients with combined hepatocellular carcinoma and cholangiocarcinoma: a population-based study. Front Oncol. 2021;16(11):686972. https://doi.org/10.3389/fonc.2021.686972.
Li J, Li Z, Hao S, Wang J, Chen W, Dai S, et al. Inversed albumin-to-globulin ratio and underlying liver disease severity as a prognostic factor for survival in hepatocellular carcinoma patients undergoing transarterial chemoembolization. Diagn Interv Radiol. 2023;29(3):520–8. https://doi.org/10.5152/dir.2022.211166.
Ma R, He J, Xu B, Zhao C, Zhang Y, Li X, et al. Nomogram prediction of surgical site infection of HIV-infected patients following orthopedic surgery: a retrospective study. BMC Infect Dis. 2020;20:896. https://doi.org/10.1186/s12879-020-05613-3.
Hara H, Kanayama M, Oha F, Shimamura Y, Watanabe T, Hashimoto T, et al. Effect of pre-operative HbA1c and blood glucose level on the surgical site infection after lumbar instrumentation surgery. J Orthop Sci. 2023. https://doi.org/10.1016/j.jos.2023.08.015.
Hameed D, Bains SS, Dubin JA, Chen Z, Nace J, Delanois RE, et al. Staged approach to tibial nail removal poses increased risk for infection in the setting of total knee arthroplasty. J Arthroplasty. 2023. https://doi.org/10.1016/j.arth.2023.10.022.
Wang J, Chang Y, Suo M, Huang H, Liu X, Li Z, et al. Incidence and risk factors of surgical site infection following cervical laminoplasty: a retrospective clinical study. Int Wound J. 2023. https://doi.org/10.1111/iwj.14450.
McLaren AC, Lundy DW. AAOS systematic literature review: summary on the management of surgical site infections. J Am Acad Orthop Surg. 2019;27(16):e717–20. https://doi.org/10.5435/jaaos-d-18-00653.
Meredith DS, Kepler CK, Huang RC, Brause BD, Boachie-Adjei O. Postoperative infections of the lumbar spine: presentation and management. Int Orthop. 2012;36(2):439–44. https://doi.org/10.1007/s00264-011-1427-z.
Heinz NR, Clement ND, Young RN, Duckworth AD, White TO, Molyneux SG. Rate and factors associated with surgical site infection following aseptic revision fixation of orthopaedic trauma injuries. Eur J Orthop Surg Traumatol. 2023. https://doi.org/10.1007/s00590-023-03573-3.
Kong L, Cao J, Zhang Y, Ding W, Shen Y. Risk factors for periprosthetic joint infection following primary total hip or knee arthroplasty: a meta-analysis. Int Wound J. 2017;14(3):529–36. https://doi.org/10.1111/iwj.12640.
Tominaga H, Setoguchi T, Ishidou Y, Nagano S, Yamamoto T, Komiya S. Risk factors for surgical site infection and urinary tract infection after spine surgery. Eur Spine J. 2016;25(12):3908–15. https://doi.org/10.1007/s00586-016-4674-2.
Khan M, ul Rooh M, Zarin M, Khalil J, Salman M. Influence of ASA score and charlson comorbidity index on the surgical site infection rates. J Coll Physicians Surg Pak. 2010;20(8):506–9.
Sebaaly A, Shedid D, Boubez G, Zairi F, Kanhonou M, Yuh SJ, et al. Surgical site infection in spinal metastasis: incidence and risk factors. Spine J. 2018;18(8):1382–7. https://doi.org/10.1016/j.spinee.2018.01.002.
**ng D, Ma JX, Ma XL, Song DH, Wang J, Chen Y, et al. A methodological, systematic review of evidence-based independent risk factors for surgical site infections after spinal surgery. Eur Spine J. 2013;22(3):605–15. https://doi.org/10.1007/s00586-012-2514-6.
Koutsoumbelis S, Hughes AP, Girardi FP, Cammisa FP Jr, Finerty EA, Nguyen JT, et al. Risk factors for postoperative infection following posterior lumbar instrumented arthrodesis. J Bone Jt Surg Am. 2011;93(17):1627–33. https://doi.org/10.2106/jbjs.J.00039.
Hansrani V, Khanbhai M, McCollum C. The diagnosis and management of early deep vein thrombosis. Adv Exp Med Biol. 2017;906:23–31. https://doi.org/10.1007/5584_2016_103.
Ribera T, Monreal L, Armengou L, Ríos J, Prades M. Synovial fluid D-dimer concentration in foals with septic joint disease. J Vet Intern Med. 2011;25(5):1113–7. https://doi.org/10.1111/j.1939-1676.2011.0758.x.
Azboy I, Çatal B, Başarır K, Mutlu M, Bilgen ÖF, Parvizi J. The natural course of serum D-dimer, C-reactive protein, and erythrocyte sedimentation rate levels after uneventful primary total joint arthroplasty. J Arthroplasty. 2021;36(9):3118–22. https://doi.org/10.1016/j.arth.2021.04.031.
Parvizi J, Tan TL, Goswami K, Higuera C, Della Valle C, Chen AF, et al. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty. 2018;33(5):1309–14. https://doi.org/10.1016/j.arth.2018.02.078.
Akgün D, Al-Muhtaresh F, Paksoy A, Lacheta L, Minkus M, Karczewski D, et al. The role of serum D-dimer for the diagnosis of periprosthetic shoulder infection. Arch Orthop Trauma Surg. 2023;143(4):1855–60. https://doi.org/10.1007/s00402-022-04385-6.
Zhang H, Sun X, **n P, Zhu X, Jie K, Cao H, et al. Diagnostic accuracy of D-dimer in periprosthetic joint infection: a diagnostic meta-analysis. J Orthop Surg Res. 2020;15(1):334. https://doi.org/10.1186/s13018-020-01853-w.
Wixted CM, Charalambous LT, Kim BI, Case A, Hendershot EF, Seidelman JL, et al. D-dimer, erythrocyte sedimentation rate, and C-reactive protein sensitivities for periprosthetic joint infection diagnosis. J Arthroplasty. 2023;38(5):914–7. https://doi.org/10.1016/j.arth.2022.12.010.
Shahi A, Kheir MM, Tarabichi M, Hosseinzadeh HRS, Tan TL, Parvizi J. Serum D-dimer test is promising for the diagnosis of periprosthetic joint infection and timing of reimplantation. J Bone Jt Surg Am. 2017;99(17):1419–27. https://doi.org/10.2106/jbjs.16.01395.
Hosseinzadeh M, Gorji A, Fathi JA, Rezaeijo SM, Rahmim A, Salmanpour MR. Prediction of cognitive decline in Parkinson’s disease using clinical and DAT SPECT imaging features, and hybrid machine learning systems. Diagnostics (Basel). 2023;13(10):1691. https://doi.org/10.3390/diagnostics13101691.
Salmanpour MR, Hosseinzadeh M, Rezaeijo SM, Rahmim A. Fusion-based tensor radiomics using reproducible features: application to survival prediction in head and neck cancer. Comput Methods Programs Biomed. 2023;240:107714. https://doi.org/10.1016/j.cmpb.2023.107714.
Rezaeijo SM, Chegeni N, Baghaei NF, Makris D, Bakas S. Within-modality synthesis and novel radiomic evaluation of brain MRI scans. Cancers (Basel). 2023;15(14):3565. https://doi.org/10.3390/cancers15143565.
Acknowledgements
None.
Funding
This research was supported by **ngtai City Key R&D Project Funding (No. 2021ZC068).
Author information
Authors and Affiliations
Contributions
ZL, JW and CL contributed to the idea and design. LS, BQ, KL, YS, HW, HW, and NM contributed to the data acquisition and analysis. ZL JW and CL contributed to the manuscript writing and revision. All authors contributed to data acquisition and analysis and to manuscript writing and revision, and agreed to all aspects of the work.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study followed the guidelines of the “Declaration of Helsinki” and was approved by the ethics committee of our hospital (The North China Healthcare Group **ngtai General Hospital No.ZCKT-2021-0025). All data is analyzed anonymously, and personal identifers are completely deleted.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplement Table 1. Characteristics of patients in the Non-SSI group and the SSI group.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, Z., Song, L., Qin, B. et al. A predictive nomogram for surgical site infection in patients who received clean orthopedic surgery: a retrospective study. J Orthop Surg Res 19, 38 (2024). https://doi.org/10.1186/s13018-023-04473-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-023-04473-2