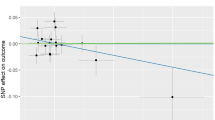
Abstract
Background
Observational studies can suggest potential associations between variables but cannot establish a causal effect on their own. This study explored the causal associations between body mass index (BMI), physical activity (PA), and joint sports injuries.
Methods
We conducted two-sample Mendelian randomization (MR) using publicly accessed genome-wide association studies (GWAS) datasets to investigate the causal effects of BMI and PA on joint sports injury risk. The inverse-variance weighted method was believed to be the primary MR analysis. Subsequently, sensitivity, pleiotropy, and heterogeneity analyses were employed to estimate the reliability of the results of the current research.
Results
Genetically predicted increased BMI was causally related to the higher sports injury risk of the ankle–foot (OR 1.23, 95% CI 1.09–1.37, p = 4.20E−04), knee (OR 1.32, 95% CI 1.21–1.43, p = 1.57E−11), and shoulder (OR 1.23, 95% CI 1.08–1.40, p = 1.28E−03). Further, the mentioned effects were validated using another set of GWAS data on BMI. Similar causal linkages were exhibited between increased BMI and the growing risk of sports injuries of the ankle–foot (OR 1.34, 95% CI 1.13–1.60, p = 9.51E−04), knee (OR 1.26, 95% CI 1.09–1.45, p = 1.63E−03), and shoulder (OR 1.35, 95% CI 1.09–1.67, p = 5.66E−03). Additionally, accelerometer-based PA measurement (overall average acceleration) (AccAve) was negatively related to sports injuries of the ankle–foot (OR 0.93, 95% CI 0.87–0.99, p = 0.046) and lumbar spine (OR 0.68, 95% CI 0.51–0.92, p = 0.012). Furthermore, we verified that the effect of AccAve on the risk of injury at the ankle–foot still had statistical significance after adjusting BMI. Results were verified as reliable under all sensitive analyses.
Conclusions
This research determined that a higher BMI could raise the sports injury risk of the ankle–foot, knee, and shoulder, while an overall average acceleration PA could reduce the injury risk of the ankle–foot and lumbar spine. These conclusions contribute to a greater knowledge of the roles of BMI and PA in the mechanism of joint sports injuries and offer several suggestions for patients and clinicians.
Similar content being viewed by others
Introduction
People all around the world love doing sports as hobbies, exercises, and ways to stay healthy. However, compared to transportation-related injuries, home and recreational accidents, and work-related damages, sports are among the leading causes of joint injuries [1,2,3]. Minor sports-related joint injuries, including dislocation, sprain, and strain, are the most commonly reported [4]. Some factors, including intrinsic factors like BMI, age, and gender, and extrinsic risk factors, such as the type of sport practiced and physical activity, could affect the risk of a joint injury [5]. Determining the risk factors for sports-related joint injuries might assist patients and caregivers in better understanding the etiology and develo** care and treatment recommendations.
Body mass index (BMI), which serves as a surrogate indicator for obesity, has been a remarkable risk factor for sports injuries of the joints, such as ankle sprains and strains. Observational studies have reported that an increased BMI is correlated with a greater risk of sports injury [6,7,8,9]. Despite this, it should be recognized that the constraints of conventional study approaches, notably underlying confounders or reverse causalities, prevented rigorous confirmation of the correlations [10].
Mendelian randomization (MR) analyses can be an ideal strategy to address these constraints [11]. This method takes advantage of instrumental variants (IVs) as proxies for exposures (e.g., disease, lifestyle), which can be conducive to overcoming the constraints of observational studies. Therefore, MR analyses are an effective approach for enhancing causal inference. PA is an essential factor that should not be ignored in joint sports injuries [5]. The occurrence rate of sports injuries to the ankle varied depending on the intensity of PA [12,13,14]. Nevertheless, the role of BMI and PA in sports injuries of other joints and whether PA mediates the correlation between BMI and joint injury have not been fully illustrated.
To address this issue, we first performed a two-sample Mendelian randomization analysis to explore the causal effect of BMI and PA on the risk of 13 different types of joint injury in sports. Then, multivariate MR was implemented to verify the causal effect of physical activity on the susceptibility to joint injury, adjusting for potential pleiotropy. With robust IVs, the MR method is less vulnerable to confounders and reverse causalities, which could interfere with the findings compared to conventional research.
Methods
Study design
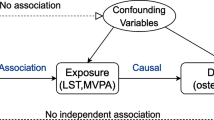
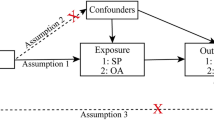
This research employed a two-sample MR analysis to explore the causal effect of BMI on joint sports injuries. Figure 1 presents a graphical diagram of the study design strategy; that is, the analysis process must meet three basic requirements: (A) IVs must be robustly linked to the exposure (BMI, PA); (B) IVs are supposed to be isolated from any potential confounders; and (C) SNPs must be linked to the risk of outcomes (joint sports injuries) only via exposure (BMI, PA). All datasets utilized for analysis are summary-level GWAS and publicly available. Ethical approvals and informed consent are also fully qualified by their corresponding institutions. Ultimately, this research report complies with the STROBE-MR guideline, which is beneficial for peer evaluation and result interpretation [15].
Three basic presumptions of the MR study. A The IVs must be correlated with the exposures (BMI or PA). B IVs must be entirely unconnected with confounders, and C IVs should not be directly linked to the outcomes (joint sports injuries). MR Mendelian randomization, IVs instrumental variables, BMI body mass index, PA physical activity
Data sources and selection of IVs
For exploring the causal relationship between BMI and joint injuries, data available for adult BMI was acquired from the currently largest GWAS for BMI from the Genetic Investigation of Anthropometric Traits (GIANT) consortium (https://portals.broadinstitute.org/collaboration/giant/index.php/GIANT_consortium), which contains samples of 681,275 European populations [16]. Outcome data for various body parts of sports injuries, such as dislocation, sprain, and strain, were extracted from the FinnGen [17], which can be easily accessed via the IEU Open GWAS Project (https://gwas.mrcieu.ac.uk/). Further details regarding the exposure and outcomes were provided in Additional file 1: Table S1.
We selected two sets of BMI-associated IVs: one contained 656 primary genome-wide significant (p < 5 × 10−8) genetic variants, and the other group contained 77 IVs that did not overlap with the first one based on previously published studies [16, 18] (Additional file 1: Tables S2 and S3).
A large GWAS involving 377,234 individuals from the UK Biobank yielded exposure data on physical activities [19]. In their research, an accelerometer worn on the wrist or a questionnaire was employed for estimating PA intensity [20]. Four phenotypes of PA from their study were investigated, including moderate-to-vigorous physical activity (MVPA), vigorous physical activity (VPA), accelerometer-based physical activity measurement (overall average acceleration) (AccAve), and 2–3 days/week or more in doing strenuous sports or other exercises for 15–30 min or greater (SSOE) [19]. Detailed information such as overall sample size, resource link, and number of SNPs was exhibited in Additional file 1: Table S1.
Then, these IVs associated with four PA phenotypes at genome-wide significance (p < 5E−08) throughout the genome were clumped and harmonized in R (version 4.2.1) utilizing the TwoSampleMR package (version 0.5.6) [21]. SNPs were removed during analysis when their linkage disequilibrium (LD) was consistent with r2 > 0.01 and clum** distance < 10,000. Beyond that, proxy SNPs serve as substitutes for those SNPs that are palindromic with intermediate allele frequencies [22]. To avoid weak instrumental bias, the F statistic was used to determine the strength of each IV. If F > 10, it can be considered that the association between IVs and exposure is effective, and the MR results are not affected by weak instrumental bias [23]. Finally, details of the SNPs selected as IVs for BMI and PA were provided in Additional file 1: Tables S2–S3, S16, respectively.
MR analysis
Following harmonization of the effect alleles across the GWAS data of BMI, PA, and joint injuries, we conducted five methods of MR analysis to identify the causal effect of BMI and PA on joint injuries, which are inverse variance weight (IVW), weighted median, MR-Egger, simple mode, and weighted mode. The IVW was considered the main outcome because this method assumes that IVs affect the outcome only via exposure and not in any other way [24]. Moreover, the MR-Egger and weighted median methods provide more modest estimated values under a more permissive assumption but with low precision (broader CIs). In this study, it was considered that there was a causal effect when all those MR approaches were consistent in direction. To address multiple testing, a Bonferroni-corrected p value of 0.00384 (i.e., 0.05/13 putative outcomes) was regarded as significant, and p values between 0.00384 and 0.05 were defined as nominal significance.
Sensitivity analysis
Complying with the MR study design strategy, the IVs affect outcomes only via exposure. The estimated values may be inaccurate if the SNPs used as IVs have horizontal pleiotropy. To detect this, the intercept contained in the MR-Egger method must be markedly different from 0, as well as the visual observation of the funnel plot, in which asymmetry represents horizontal pleiotropy [24, 25]. Simultaneously, the MR Pleiotropy Residual Sum and Outlier (MR-PRESSO) method was also conducted to check and rectify horizontal pleiotropy [26]. Eventually, Cochran’s Q test was implemented to determine whether there was heterogeneity in each IV [27].
Results
Genetically predicted increased BMI and joint sports injuries
Additional file 1: Table S13 exhibited the selected IVs strongly associated with an increased BMI, which were derived from the recent study by Yengo et al. [16]. Results showed that increased BMI was significantly correlated with dislocation, sprain, and strain of joints and ligaments (called together “sports injury,” the same below) at ankle–foot level (IVW: OR 1.23, 95% CI 1.09–1.37, p = 4.20E−04), the knee (IVW: OR 1.32, 95% CI 1.21–1.43, p = 1.57E−11), and shoulder girdle (IVW: OR 1.23, 95% CI 1.08–1.40, p = 1.28E−03). Additionally, a nominal significance was revealed after analyzing the correlation between BMI and sports injury at the neck level (IVW: OR 1.22, 95% CI 1.02–1.47, p = 0.030) (Fig. 2). Detailed information on each MR analysis was provided in Additional file 1: Tables S4–S7. No evidence was found of directional pleiotropy through the MR-Egger intercept scatter plot or the funnel plot (Additional file 2: Figs. S1–S4). In the MR-PRESSO global test, p = 0.021, when analyzing the association between BMI and ankle injury, two SNPs were identified as outliers (rs2230590 and rs12364470). MR-PRESSO outlier-corrected estimates stayed in accordance with the original analysis (Additional file 1: Table S14).
A forest plot depicting the results of five different MR estimating methods on the associations between BMI (IVs derived from the Yengo et al. study) and the joint sports injury risk of the ankle–foot, knee, shoulder girdle, and neck. MR Mendelian randomization, IVs instrumental variables, BMI body mass index
For further confirmation of the results, another BMI GWAS obtained from the study by Locke et al. [18] was implemented to validate the causal effect of BMI on the risk of joint injuries. Significant effects were revealed for increased BMI on sports injury risk of ankle–foot (IVW: OR 1.34, 95% CI 1.13–1.60, p = 9.51E−04), knee (IVW: OR 1.26, 95% CI 1.09–1.45, p = 1.63E−03). Additionally, a nominal significant causal effect was identified between BMI and shoulder girdle injury (IVW: OR 1.35, 95% CI 1.09–1.67, p = 5.66E−03) (Fig. 3, Additional file 1: Table S15). Detailed information on each MR analysis was exhibited in Additional file 1: Tables S8–S10. Furthermore, sensitivity analysis suggested no heterogeneity or horizontal pleiotropy through Cochran’s Q test and the MR-Egger intercept. Meanwhile, the MR-PRESSO test revealed no potential outliers (Additional file 1: Table S15). The leave-one-out results indicated that the causal effect was not actuated by a single IV (Additional file 3: Figs. S5–S7).
A forest plot depicting the results of five different MR estimating methods on the associations between BMI (IVs derived from the Locke et al. study) and the joint sports injury risk of the ankle–foot, knee, and shoulder girdle. MR Mendelian randomization, IVs instrumental variables, BMI body mass index
Genetically predicted physical activities and joint sports injuries
Additional file 1: Table S16 displayed the IVs we used in analyzing the causal effect of four kinds of physical activities on joint sports injuries. No causal associations were observed when analyzing the effect of physical activities on joint sports injuries except for accelerometer-based physical activity measurement (average acceleration) (AccAve) (Additional file 1: Tables S17–S20). Evidence of a protective causal correlation was discovered between AccAve and injury at the ankle and foot level (IVW: OR 0.93, 95% CI 0.87–0.99, p = 0.046) and injury of the lumbar spine (IVW: OR 0.68, 95% CI 0.51–0.92, p = 0.012) (Fig. 4). Information on MR analyses between AccAve and sports injuries at the ankle–foot and lumbar spine was provided in Additional file 1: Tables S11–S12. Sensitive analysis revealed that no heterogeneity or horizontal pleiotropy existed in this analysis via Cochran’s Q test, the egger intercept, MR-PRESSO (Additional file 1: Table S17), as well as the scatter plot, funnel plot, and forest plot of the leave-one-out analysis (Additional file 4: Figs. S8–S9).
Effect of genetically predicted BMI on the risk of injury at ankle and foot level by adjusting physical activity
Ultimately, the PhenoScanner online website tool (http://www.phenoscanner.medschl.cam.ac.uk/) was conducted to confirm whether the SNPs served as IVs in connection with other phenotypes. Several SNPs related to BMI were reported to have an impact on the risk of injury at the ankle and foot level [28, 29] (Additional file 1: Table S21). Accordingly, multivariate MR (MVMR) was implemented to illustrate the causal associations between physical activity and the risk of injury at the ankle and foot level, adjusting potential pleiotropy related to BMI. The BMI summary data are exhibited in Additional file 1: Table S1. Results showed that the effect of AccAve on the risk of injury at the ankle–foot still had a nominal statistical significance after adjusting BMI (Fig. 5).
Forest plot of MVMR estimates from the IVW method of BMI and PA with the joint sports injury risk of the ankle–foot. MVMR multivariate Mendelian randomization, IVW inverse-variance weighted, BMI body mass index, PA physical activity, AccAve accelerometer-based physical activity measurement (average acceleration), MVPA moderate-to-vigorous physical activity, VPA vigorous physical activity, SSOE strenuous sports or other exercises Accelerometer-based PA (acceleration fraction > 425 mg)
Discussion
The previous studies provided evidence that participation in certain sports and BMI were risk factors that heightened the risk of suffering joint sports injuries such as sprains and strains. However, such conclusions remained debatable across studies. In the meantime, uncertain confounding factors in observational research might be influential on the correlation results. Studies on the epidemiology of sports joint injuries indicated that increased BMI and greater physical activity were risk factors for sports joint injuries [12, 30,31,32,33]. Nevertheless, the sample size in these studies was insufficient, and only BMI or a single physical activity was analyzed without correcting for potential bias. In this study, we systematically explored the causal effect of BMI and different intensities of physical activity on 13 types of joint injuries in sports by employing two-sample and MVMR methods. Significant positive causal correlations were identified between BMI and sports injury risk of the ankle, knee, and shoulder girdle. Further investigation showed that AccAve was negatively correlated with the injury risk of the ankle and lumbar spine. These findings offered a genetic perspective on the causal relationships between BMI, physical activity, and the risk of joint sports injury, which might have clinical value for clinicians and patients.
Increased body mass index was reported to be a significant risk factor for knee, shoulder, and ankle–foot injuries, including meniscal tears, rotator cuff disease, plantar fasciitis, and ankle sprains [12, 32,33,34]. Despite almost all epidemiological investigations supporting a higher BMI as a risk factor for joint sports injuries, the mechanism behind it is still controversial. One hypothesis holds that a strong mass moment of inertia acting around the ankle leads to ankle sprains and other lower extremity injuries [35, 36]. Other theories believe BMI is a valid risk factor as obesity is connected with chronic inflammation [37], which may contribute to degenerated tendons and pain. Obesity is also linked to other diseases like dyslipidemia and high blood pressure [38], which may also raise the risk of shoulder injury [39, 40]. Using the MR method, we identified increased BMI as a risk factor for injuries to the ankle–foot, knee, and shoulder. Further, overall average acceleration (AccAve) was demonstrated as a protective factor against injury to the ankle–foot and lumbar spine. Previous studies suggested men who had better scores in push-ups and sit-ups (number of push-ups or sit-ups completed in 2 min) and better performance in a 2-mile run had a higher incidence of ankle sprains than their counterparts [12]. Basketball, football, and soccer account for over half of all ankle sprains sustained while participating in physical activity, which accounts for almost half of all ankle sprains [31]. Ankle sprains are thought to occur more likely in physical activities involving frequent contact with others, as well as repeated running, jum**, and sharp cutting actions that subject the ankle to greater angular and rotational pressure [13, 41,42,43]. Compared to lower extremity injuries, the incidence of lumbar spine injuries was uncommon [44]. Despite this, lumbar spine injuries are still a non-negligible reason athletes are absent from competition [45]. Moreover, studies found that athletic competition is more likely to result in a lumbar spine injury than routine training [44], which proved that injuries are more likely to be caused by enhanced athletic effort in sports competitions [46]. In contrast to these perspectives, our results suggested a protective role for overall average acceleration (AccAve) against ankle–foot and lumbar spine injuries. Although the precise mechanism of this protection is yet unidentified, one possible interpretation is that moderate physical activity, such as slow walking, can strengthen multi-muscular coordination for smooth motion, like neuromuscular training [47, 48]. It is worth noting that vigorous physical activity has no causal relationship with the risk of ankle–foot and lumbar spine injuries, according to the current results. The inconsistency between the results and the previous epidemiologic study may be because the definition of vigorous physical activity in the original GWAS data needed to be clarified. People were asked to answer a questionnaire or wear an accelerometer; fraction accelerations greater than 425 milligravities (approximately 4.2 m/s2) were considered high intensity [19], which may be affected by cognitive bias or not reach the condition of causing joint injuries.
However, the study is subject to some limitations. Despite the statistical significance of the results, they do not indicate clinical relevance. The case number of some sports injury sites is still small; more clinical traits and epidemiological research with larger sample sizes are urgently needed to confirm the conclusions. Second, self-reported measures of physical activity may be influenced by mental states and cognitive bias. Even though this does not weaken the reliability of self-reported evaluations [49], objective evaluations are still needed to confirm their results. Meanwhile, accelerometer-based assessments of physical activity have their limitations. Movement posture, intensity of muscles and ligaments, and nonambulatory activity (e.g., bicycle) are difficult to record. Therefore, further investigation is required into the causal links between more classified physical activities and joint injuries. Third, the outcome data extracted from the FinnGen database concerning different joint injuries need to be updated due to insufficient cases.
In summary, this research conducted a genetic approach and evidently found that BMI is causally connected with the injury risk of the ankle–foot, knee, and shoulder. Additionally, overall average acceleration (AccAve) could causally decrease the injury risk of the ankle–foot and lumbar spine. These conclusions supported a genetic perspective about the causal relationships between BMI, physical activity, and the risk of joint sports injury and offered clinical advice for patients and caregivers.
Availability of data and materials
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Additional file 1.
References
Michaud PA, Renaud A, Narring F. Sports activities related to injuries? A survey among 9–19 year olds in Switzerland. Inj Prev J Int Soc Child Adolesc Inj Prev. 2001;7(1):41–5. https://doi.org/10.1136/ip.7.1.41.
Hølmer P, Søndergaard L, Konradsen L, Nielsen PT, Jørgensen LN. Epidemiology of sprains in the lateral ankle and foot. Foot Ankle Int. 1994;15(2):72–4. https://doi.org/10.1177/107110079401500204.
Dekker R, Kingma J, Groothoff JW, Eisma WH, Ten Duis HJ. Measurement of severity of sports injuries: an epidemiological study. Clin Rehabil. 2000;14(6):651–6. https://doi.org/10.1191/0269215500cr374oa.
Farfel M, DiGrande L, Brackbill R, Prann A, Cone J, Friedman S, et al. An overview of 9/11 experiences and respiratory and mental health conditions among World Trade Center Health Registry enrollees. J Urban Health Bull N Y Acad Med. 2008;85(6):880–909. https://doi.org/10.1007/s11524-008-9317-4.
Vuurberg G, Hoorntje A, Wink LM, van der Doelen BFW, van den Bekerom MP, Dekker R, et al. Diagnosis, treatment and prevention of ankle sprains: update of an evidence-based clinical guideline. Br J Sports Med. 2018;52(15):956. https://doi.org/10.1136/bjsports-2017-098106.
Hartley EM, Hoch MC, Boling MC. Y-balance test performance and BMI are associated with ankle sprain injury in collegiate male athletes. J Sci Med Sport. 2018;21(7):676–80. https://doi.org/10.1016/j.jsams.2017.10.014.
Mansori AE, Lording T, Schneider A, Dumas R, Servien E, Lustig S. Incidence and patterns of meniscal tears accompanying the anterior cruciate ligament injury: possible local and generalized risk factors. Int Orthop. 2018;42(9):2113–21. https://doi.org/10.1007/s00264-018-3992-x.
Chassé M, Fergusson DA, Chen Y. Body mass index and the risk of injury in adults: a cross-sectional study. Int J Obes. 2014;38(11):1403–9. https://doi.org/10.1038/ijo.2014.28.
Richmond SA, Kang J, Emery CA. Is body mass index a risk factor for sport injury in adolescents? J Sci Med Sport. 2013;16(5):401–5. https://doi.org/10.1016/j.jsams.2012.11.898.
Carreras-Torres R, Johansson M, Haycock PC, Relton CL, Davey Smith G, Brennan P, et al. Role of obesity in smoking behaviour: Mendelian randomisation study in UK Biobank. BMJ (Clin Res Ed). 2018;361:k1767. https://doi.org/10.1136/bmj.k1767.
Smith GD, Ebrahim S. “Mendelian randomization”: Can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32(1):1–22. https://doi.org/10.1093/ije/dyg070.
Waterman BR, Belmont PJ Jr, Cameron KL, Deberardino TM, Owens BD. Epidemiology of ankle sprain at the United States Military Academy. Am J Sports Med. 2010;38(4):797–803. https://doi.org/10.1177/0363546509350757.
Fong DT, Hong Y, Chan LK, Yung PS, Chan KM. A systematic review on ankle injury and ankle sprain in sports. Sports Med (Auckl NZ). 2007;37(1):73–94. https://doi.org/10.2165/00007256-200737010-00006.
Verhagen EA, Van der Beek AJ, Bouter LM, Bahr RM, Van Mechelen W. A one season prospective cohort study of volleyball injuries. Br J Sports Med. 2004;38(4):477–81. https://doi.org/10.1136/bjsm.2003.005785.
Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: the STROBE-MR statement. JAMA. 2021;326(16):1614–21. https://doi.org/10.1001/jama.2021.18236.
Yengo L, Sidorenko J, Kemper KE, Zheng Z, Wood AR, Weedon MN, et al. Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European ancestry. Hum Mol Genet. 2018;27(20):3641–9. https://doi.org/10.1093/hmg/ddy271.
Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner KM, et al. Finngen provides genetic insights from a well-phenotyped isolated population. Nature. 2023;613(7944):508–18. https://doi.org/10.1038/s41586-022-05473-8.
Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197–206. https://doi.org/10.1038/nature14177.
Klimentidis YC, Raichlen DA, Bea J, Garcia DO, Wineinger NE, Mandarino LJ, et al. Genome-wide association study of habitual physical activity in over 377,000 UK Biobank participants identifies multiple variants including Cadm2 and Apoe. Int J Obes. 2018;42(6):1161–76. https://doi.org/10.1038/s41366-018-0120-3.
Doherty A, Jackson D, Hammerla N, Plötz T, Olivier P, Granat MH, et al. Large scale population assessment of physical activity using wrist worn accelerometers: the UK Biobank study. PLoS ONE. 2017;12(2):e0169649. https://doi.org/10.1371/journal.pone.0169649.
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The Mr-Base platform supports systematic causal inference across the human phenome. Elife. 2018. https://doi.org/10.7554/eLife.34408.
Vandebergh M, Becelaere S, Dubois B, Goris A. Body mass index, interleukin-6 signaling and multiple sclerosis: a Mendelian randomization study. Front Immunol. 2022;13:834644. https://doi.org/10.3389/fimmu.2022.834644.
Burgess S, Thompson SG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 2011;40(3):755–64. https://doi.org/10.1093/ije/dyr036.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. 2015;44(2):512–25. https://doi.org/10.1093/ije/dyv080.
Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the Mr-Egger method. Eur J Epidemiol. 2017;32(5):377–89. https://doi.org/10.1007/s10654-017-0255-x.
Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–8. https://doi.org/10.1038/s41588-018-0099-7.
Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using Mr-Egger regression: the role of the I2 statistic. Int J Epidemiol. 2016;45(6):1961–74. https://doi.org/10.1093/ije/dyw220.
Kobayashi T, Tanaka M, Shida M. Intrinsic risk factors of lateral ankle sprain: a systematic review and meta-analysis. Sports Health. 2016;8(2):190–3. https://doi.org/10.1177/1941738115623775.
Gribble PA, Terada M, Beard MQ, Kosik KB, Lepley AS, McCann RS, et al. Prediction of lateral ankle sprains in football players based on clinical tests and body mass index. Am J Sports Med. 2016;44(2):460–7. https://doi.org/10.1177/0363546515614585.
Doherty C, Delahunt E, Caulfield B, Hertel J, Ryan J, Bleakley C. The incidence and prevalence of ankle sprain injury: a systematic review and meta-analysis of prospective epidemiological studies. Sports Med (Auckl NZ). 2014;44(1):123–40. https://doi.org/10.1007/s40279-013-0102-5.
Waterman BR, Owens BD, Davey S, Zacchilli MA, Belmont PJ Jr. The epidemiology of ankle sprains in the United States. J Bone Jt Surg Am. 2010;92(13):2279–84. https://doi.org/10.2106/jbjs.I.01537.
Yanik EL, Colditz GA, Wright RW, Saccone NL, Evanoff BA, Jain NB, et al. Risk factors for surgery due to rotator cuff disease in a population-based cohort. Bone Jt J. 2020;102-b(3):352–9. https://doi.org/10.1302/0301-620x.102b3.Bjj-2019-0875.R1.
Yeh PC, Starkey C, Lombardo S, Vitti G, Kharrazi FD. Epidemiology of isolated meniscal injury and its effect on performance in athletes from the National Basketball Association. Am J Sports Med. 2012;40(3):589–94. https://doi.org/10.1177/0363546511428601.
van Leeuwen KD, Rogers J, Winzenberg T, van Middelkoop M. Higher body mass index is associated with plantar fasciopathy/’plantar fasciitis’: systematic review and meta-analysis of various clinical and imaging risk factors. Br J Sports Med. 2016;50(16):972–81. https://doi.org/10.1136/bjsports-2015-094695.
Tyler TF, McHugh MP, Mirabella MR, Mullaney MJ, Nicholas SJ. Risk factors for noncontact ankle sprains in high school football players: the role of previous ankle sprains and body mass index. Am J Sports Med. 2006;34(3):471–5. https://doi.org/10.1177/0363546505280429.
Jones BH, Knapik JJ. Physical training and exercise-related injuries. Surveillance, research and injury prevention in military populations. Sports Med (Auckl NZ). 1999;27(2):111–25. https://doi.org/10.2165/00007256-199927020-00004.
Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13(11):633–43. https://doi.org/10.1038/nrendo.2017.90.
O’Neill S, O’Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev Off J Int Assoc Study Obes. 2015;16(1):1–12. https://doi.org/10.1111/obr.12229.
Djerbi I, Chammas M, Mirous MP, Lazerges C, Coulet B. Impact of cardiovascular risk factor on the prevalence and severity of symptomatic full-thickness rotator cuff tears. Orthop Traumatol Surg Res OTSR. 2015;101(6 Suppl):S269–73. https://doi.org/10.1016/j.otsr.2015.06.011.
Gumina S, Arceri V, Carbone S, Albino P, Passaretti D, Campagna V, et al. The association between arterial hypertension and rotator cuff tear: the influence on rotator cuff tear sizes. J Shoulder Elbow Surg. 2013;22(2):229–32. https://doi.org/10.1016/j.jse.2012.05.023.
Beynnon BD, Vacek PM, Murphy D, Alosa D, Paller D. First-time inversion ankle ligament trauma: the effects of sex, level of competition, and sport on the incidence of injury. Am J Sports Med. 2005;33(10):1485–91. https://doi.org/10.1177/0363546505275490.
Giza E, Fuller C, Junge A, Dvorak J. Mechanisms of foot and ankle injuries in soccer. Am J Sports Med. 2003;31(4):550–4. https://doi.org/10.1177/03635465030310041201.
Kofotolis ND, Kellis E, Vlachopoulos SP. Ankle sprain injuries and risk factors in amateur soccer players during a 2-year period. Am J Sports Med. 2007;35(3):458–66. https://doi.org/10.1177/0363546506294857.
Makovicka JL, Deckey DG, Patel KA, Hassebrock JD, Chung AS, Tummala SV, et al. Epidemiology of lumbar spine injuries in men’s and women’s National Collegiate Athletic Association basketball athletes. Orthop J Sports Med. 2019;7(10):2325967119879104. https://doi.org/10.1177/2325967119879104.
Drakos MC, Domb B, Starkey C, Callahan L, Allen AA. Injury in the National Basketball Association: a 17-year overview. Sports Health. 2010;2(4):284–90. https://doi.org/10.1177/1941738109357303.
Dick R, Hertel J, Agel J, Grossman J, Marshall SW. Descriptive epidemiology of collegiate men’s basketball injuries: National Collegiate Athletic Association injury surveillance system, 1988–1989 through 2003–2004. J Athl Train. 2007;42(2):194–201.
Zech A, Hübscher M, Vogt L, Banzer W, Hänsel F, Pfeifer K. Balance training for neuromuscular control and performance enhancement: a systematic review. J Athl Train. 2010;45(4):392–403. https://doi.org/10.4085/1062-6050-45.4.392.
Fukuchi CA, Fukuchi RK, Duarte M. Effects of walking speed on gait biomechanics in healthy participants: a systematic review and meta-analysis. Syst Rev. 2019;8(1):153. https://doi.org/10.1186/s13643-019-1063-z.
Choi KW, Chen CY, Stein MB, Klimentidis YC, Wang MJ, Koenen KC, et al. Assessment of bidirectional relationships between physical activity and depression among adults: a 2-sample Mendelian randomization study. JAMA Psychiat. 2019;76(4):399–408. https://doi.org/10.1001/jamapsychiatry.2018.4175.
Acknowledgements
We want to acknowledge the participants and investigators of the FinnGen study and the GIANT consortium and their open-access datasets.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
Conception and design were performed by WB and MY; development of methodology was analyzed by WB; acquisition of data (acquired and managed patients, provided facilities, etc.) was collected by WB and MY; analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis) were provided by WB; writing of the manuscript was done by WB; supervision was conducted by Changqing Jiang. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that no potential competing interests existed in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary Tables S1 to S21.
Additional file 2.
Supplementary Figures 1 to 4.
Additional file 3.
Supplementary Figures 5 to 7.
Additional file 4.
Supplementary Figures 8 to 9.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bi, W., Yang, M. & Jiang, C. Causal effect of body mass index and physical activity on the risk of joint sports injuries: Mendelian randomization analysis in the European population. J Orthop Surg Res 18, 676 (2023). https://doi.org/10.1186/s13018-023-04172-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-023-04172-y