Abstract
Background
Higher circulating levels of trimethylamine N-oxide (TMAO), which is a metabolite that can be produced by the gut microbiota from L-carnitine (LC), have been associated with bone mineral density (BMD). Because LC supplementation can improve bone density and microstructural properties in animal models, this study aimed to examine the effects of 12 weeks of LC supplementation on BMD and selected blood markers involved in bone metabolism of postmenopausal women participating in a resistance training (RT) program.
Methods
Twenty-seven postmenopausal women, who had not been treated for osteoporosis, with a total T-score above − 3.0 and no diet differences completed 12 weeks of RT. The participants’ diets were supplemented with either 1 g of LC-L-tartrate and 3 g of leucine per day (LC group) or 4 g of leucine per day as a placebo (PLA group), in a double-blind fashion.
Results
After the intervention in the LC group, plasma total carnitine and serum decorin levels were higher than the corresponding preintervention values (p = 0.040 and p = 0.042, respectively). Moreover, plasma TMAO and serum SPARC levels were higher in the LC group than the corresponding postintervention values in the PLA group (p < 0.001 and p = 0.030, respectively). No changes in the BMD were observed after 3 months of the intervention.
Conclusions
Twelve weeks of LC supplementation during RT program increased plasma TMAO levels and appeared to affect signaling molecules, as indicated by the increase in the resting SPARC and decorin levels, with no significant modification in the BMD.
Trial registration
Retrospectively registered at the ClinicalTrials.gov (NCT05120011).
Similar content being viewed by others
Introduction
Osteoporosis is a complex, multifactorial condition characterized by the loss of bone mineral density (BMD) leading to an increased susceptibility to fractures. The prevalence of osteoporosis increases with age and is higher among older women [1]. According to numerous studies, resistance training (RT) intervention may significantly preserve bone mass or avert bone loss in women [2]. These effects are not only dependent on mechanical load, because the skeletal muscle may serve as an endocrine organ that is capable of secreting cytokines to modulate bone metabolism [3, 4]. Even 3 months of an exercise training program can increase circulating markers of bone formation in postmenopausal women [5].
Changes in bone metabolism, BMD, a high risk of recurrent falls, or a combination of these factors are potentially related to nutritional factors [6]. Several studies, using an aging ovariectomized rat model of osteoporosis, have shown that L-carnitine (LC) supplementation can improve bone density and microstructural properties [7,8,9]. These findings indicated a reduction in bone resorption rate and a decrease in mineral turnover by LC [7,8,9]. In addition, an improvement in the levels of inflammatory biomarkers was demonstrated. The LC treatment led to a decrease in the serum levels of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) [8, 9].
Moreover, recent studies have shown a strong relationship between bone metabolism and intestinal microbiota, and their potential effect on the risk of develo** osteoporosis [10]. Furthermore, it is well known that LC is metabolized by the gut microbes to trimethylamine (TMA), which is converted to TMA N-oxide (TMAO). Thus, LC treatment increases plasma TMAO levels [11]. A tenfold increase in fasting plasma TMAO levels was noted following 3 months of oral LC supplementation in healthy older women [12]. Interestingly, TMAO affects bone homeostasis [13]. The decline in plasma TMAO levels was related to a greater reduction in BMD during a weight loss program in overweight and obese participants, suggesting that TMAO might protect against decreases in BMD [14]. In contrast, elevated TMAO levels were associated with hip fractures [15, 16].
Therefore, this study aimed to establish whether the increase in plasma TMAO levels induced by LC supplementation affects the BMD and selected blood markers of older women involved in an RT program. We hypothesized that changes in plasma TMAO levels related to LC supplementation affect bone metabolism.
Methods
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the Independent Bioethics Committee for Scientific Research at the Medical University of Gdansk (NKBBN/354 − 201/2017) and registered in the ClinicalTrials.gov Registry (NCT05120011). Before starting the experimental procedure, all participants were informed about the procedure, risks, and expected outcomes and they provided their written informed consent for participation.
Sample size
Previously reported results [12] were adopted in two-way ANOVA sample size calculation using Statistica 13.1 software (Dell Inc., Tulsa, OK, USA). Means and standard deviations of plasma TMAO in the supplemented and placebo groups, before and after three months of LC treatment, were applied with the alpha level and the power of the test set at 0.05 and 0.95, respectively. On the basis of these parameters, the required sample size has been obtained equal to 9.
Participants
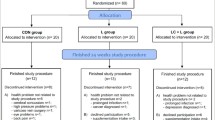
Postmenopausal women, with no cardiovascular disease, liver or kidney disease, gastrointestinal disorder, including stomach ulcer or erosions, cancer, diabetes, musculoskeletal disease, and other severe chronic diseases were recruited through local advertisements. All included participants presented a physician’s certificate indicating a lack of contradictions to strength training. Thirty-six women without a history of osteoporosis, low-energy fractures, or antiresorptive treatment were included in the study (Fig. 1).
Experimental design and study procedure
Participants were randomly assigned (1:1 ratio) to one of the two groups using the Random Sequence Generator (RANDOM.ORG, Dublin, Ireland). The randomization sequence was stored by a researcher who had no contact with the participants. Over the 12 weeks, the participants were supplemented with either 1 g of LC-L-tartrate and 3 g of leucine per day (LC group) or 4 g of leucine per day as a placebo (PLA group), in a double-blind fashion. The supplements were encapsulated in identical gelatin capsules, and the participants were instructed to consume the supplements once a day with their main meal.
RT protocol
During the study period, all subjects participated in the RT program, which was held in a commercial gym, twice a week. Each participant attended the program on Mondays and Wednesdays, or Tuesdays and Thursdays. Professional coaches conducted each training session. Over the 12 weeks, 24 training sessions, each lasting for 45–60 min were performed for each group. The RT protocol was based on a previously described procedure [17]. Each training session started with a 10-min warm-up on a treadmill (walking). The participants then performed three sets of the following four exercises: leg press, leg extension, shoulder press or horizontal row, and chest press or lateral pulldown. The leg press and extension were performed at every training session, but the shoulder press and lateral pulldown were performed only on Monday or Tuesday, and the horizontal row and chest press only on Wednesday or Thursday. Each session ended with a 10-min cooldown on a cycle ergometer.
Before starting the training protocol, a one-repetition maximum (RM) test was performed. During the first 2 weeks, the workload was set at 65% of RM for each exercise, and the exercise was performed in three sets of 10–12 repetitions. After 2 weeks, the workload was increased to 80% of RM, and each exercise was performed in three sets of 6–8 repetitions.
Blood collection and analysis
During the week before initiation of the experimental protocol and 12 weeks after the initiation, fasting blood samples were taken from the antecubital vein into BD Vacutainer tubes (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). After collection, the samples were centrifuged at 2000 g and 4 °C for 10 min, and aliquots were stored at -80 °C for later analyses. Plasma TMAO, and total carnitine (TC) levels were measured by the liquid chromatography-mass spectrometry system at the Mass Spectrometry Laboratory, Institute of Biochemistry and Biophysics, Polish Academy of Sciences (Warsaw, Poland) as described previously [18, 19]. Serum dickkopf-1 (DKK1), osteoprotegerin (OPG), osteopontin (OPN), sclerostin (SOST), and fibroblast growth factor-23 (FGF-23) levels were determined using the MILLIPLEX Human Bone Magnetic Bead Panel (catalog number [cat. #]: HBNMAG-51 K; Merck KGaA, Darmstadt, Germany). Insulin-like growth factor-1 (IGF-1), IL-6, TNF-α, decorin, and osteonectin/secreted protein acidic and rich in cysteine (SPARC) levels were measured using commercially available enzyme immunoassay kits (total IGF-1, cat. # DG100; IL-6, cat. # HS600B; TNF-α, cat. # HSTA00E; decorin, cat. # DY143 and # DY008; SPARC, cat. #DY941-05; R&D Systems, Minneapolis, MN, USA), and C-reactive protein (CRP) level using cat. # EIA-3954 kit (DRG Instruments GmbH, Marburg, Germany).
Dual-energy X-ray absorptiometry (DXA)
Areal BMDs of the lumbar spine (L1–L4), hip, and whole body were measured on separate days, at baseline and after 12-week RT. Measurements were performed using a Hologic Discovery Wi DXA scanner and analyzed using APEX software version 13.4. All DXA scans were performed by the same trained technician according to the manufacturer’s instructions, and analyzed by the same investigator who was blinded to the intervention. T-scores and Z-scores were calculated using the manufacturer’s reference ranges.
Diet
Three-day food records were self-reported for 2 weekdays and 1 weekend day at the beginning of the study. Participants were instructed to note the amounts of food and beverages consumed. The diet was analyzed in terms of the amount of energy, protein, carbohydrates, and fat consumed.
Statistical analysis
Participants included in the statistical analyses completed a minimum of 80% of the training sessions. All calculations were performed using Statistica 13.1 software (Dell Inc., Tulsa, OK, USA). Baseline characteristics and dietary composition of the participants were compared using Student’s t-test. To examine the treatment and time interaction in blood markers, BMD data, and strength variables two-way analysis of variance (ANOVA) for repeated measurements was performed. In case ANOVA yielded a significant effect, Tukey’s HSD test was used for post hoc comparisons. Correlations between the changes in absolute values from before to after the RT intervention were calculated using Pearson and Spearman correlation tests for normally and nonnormally distributed data, respectively. A probability level of p < 0.05 was considered significant. All data are expressed as mean ± standard deviation, unless otherwise stated.
Results
The study protocol was completed by 27 participants (Fig. 1); their characteristics are presented in Table 1.
After the intervention in the LC group, plasma TC (Fig. 2A) and serum decorin (Fig. 2C) levels were higher than the corresponding preintervention values (p = 0.040 and p = 0.042, respectively). Moreover, plasma TMAO (Fig. 2B) and serum SPARC (Fig. 2D) levels were higher in the LC group than the corresponding postintervention values in the PLA group (p < 0.001 and p = 0.030, respectively). No differences in circulating OPN, OPG, SOST, FGF-23, DKK1, IGF-1, IL-6, TNF-α, and CRP levels were noted (Table 2). Lumbar spine BMD tended to decrease in the LC group after the 3-month intervention (p = 0.069), but no other changes in BMD were noted (Table 3). Significant improvement in RM in time was observed with the seated leg press (p < 0.001), leg extension (p < 0.001), seated chest press (p < 0.001), shoulder press (p < 0.001), lateral pulldown (p < 0.001), cable row (p < 0.001), and total (p < 0.001), with no differences between the groups (Table 4).
We observed a positive correlation between the changes in serum decorin and SPARC levels (r = 0.543, p = 0.003; Fig. S1A), but only changes in the decorin level correlated with total BMD (r = 0.383, p = 0.049; Fig. S1B). Changes in the levels of both circulating biomarkers were correlated with the TMAO levels (rho = 0.568, p = 0.002; Fig. S2A and rho = 0.574, p = 0.002; Fig. S2B, for SPARC and decorin, respectively).
No differences were noted in the participants’ diets (Table S1).
Discussion
Plasma TMAO reached a level that was comparable with the previously reported level following 12 weeks of supplementation in older women [12], despite the administration of a lower LC dose. However, changes in the TMAO level were not associated with BMD. The combination of LC supplementation and RT increased circulating SPARC and decorin levels but did not affect the other studied markers. Interestingly, changes in SPARC and decorin levels correlated with the TMAO level.
Physical activity may alter the secretion of signaling proteins from skeletal muscles [20]. For example, the SPARC level increased in the skeletal muscles of young male subjects after 11 weeks of a strength-training program [21], and it transiently increased in the serum of young healthy men immediately after a single bout of cycling [22]. In addition, 4 weeks of training at 70% of maximal oxygen uptake significantly promoted the exercise-induced increase in serum SPARC level. In contrast, resting serum SPARC level was not modified between pre- and post-training [22]. Our findings suggest that LC supplementation may have augmented SPARC secretion in the postmenopausal women who participated in the RT program, because such an effect was not observed in the PLA group participants who completed the same RT protocol.
SPARC plays a critical role in maintaining bone mass and quality [23]. Plasma SPARC levels positively correlate with lumbar spine BMD, and 12 months of recombinant human parathyroid hormone (1–34) treatment in osteoporotic patients increases the circulating SPARC level, which is associated with changes in lumbar BMD at L2-L4 [24]. The mechanisms by which SPARC affects bone formation, maintenance, and repair might occur through multiple pathways, including the regulation of procollagen processing and assembly in the bone matrix, cross-linking, mineralization, and/or osteoblast/osteoclast differentiation and activity [25]. Changes in SPARC level in our study were correlated with changes in circulating levels of decorin, which is a collagen-associated extracellular matrix proteoglycan that promotes the formation of bone matrix and calcium deposition to regulate bone morphogenesis [26].
The positive effect of LC supplementation on bone mineral turnover has been indicated in animal studies [7,8,9]. Moreover, LC administration may prevent the loss of BMD in patients with chronic kidney disease [27], chronic liver disease [28], and pemphigus vulgaris [29]. The anti-inflammatory effect of LC has been suggested as a potential mechanism underlying these findings [8, 9, 28]. However, we observed no changes in the levels of inflammatory biomarkers, which is similar to the findings for a previously reported 6-month LC treatment in healthy older women [30]. Furthermore, levels of other well-known bone turnover markers such as DKK-1, SOST, OPG, and OPN [31,32,33,34] were not changed in the present study. In addition, higher circulating SPARC and decorin levels were not associated with changes in BMD. Despite the recently reported positive [14] and negative [15, 16] associations between BMD and TMAO levels, we observed no correlation between them. However, the intervention period in the present study was relatively short.
SPARC [35] and decorin [36] levels may also affect skeletal muscle metabolism via different pathways. Increased expression of SPARC mediates some of the exercise-induced benefits both in terms of metabolic functions, including enhancement of glucose usage and oxidative phosphorylation, as well as tissue remodeling [35]. In addition, increased muscle decorin expression, correlates with improvement in leg press performance [36]. Taken together, SPARC and decorin could enhance the metabolic ability and structural (mechanical) properties of skeletal muscles. However, we found no associations between the changes in circulating SPARC and decorin levels and those in RM. This finding may indicate that exercise increases muscle strength by predominately locally derived mediators rather than circulating factors.
Notably, we observed a high correlation between the increase in SPARC and decorin levels with the increase in plasma TMAO levels. The associations of TMAO with inflammation, endothelial dysfunction, type 2 diabetes, central adiposity, hypertension, and cancer [37,38,39] have led to a growing interest in this metabolite. SPARC or decorin secretion is related to health benefits. Thus, modulation of SPARC production may have potential implications for postinfarction healing [40], and decorin has a protective role in several cardiac diseases, including atherosclerosis, hypertrophy, and myocardial infarction [41]. Moreover, SPARC and decorin might exert an anticancer effect by decreasing cancer cell proliferation, limiting migration and increasing apoptosis [42].
Numerous studies have examined the effect of training on BMD in postmenopausal women [43,44,45,46]. The results of the meta-analyses show that resistance training programs increase BMD when applied for ≥ 6 months [46], combined with high-impact or weight-bearing exercises [43], or in patients with osteoporosis and osteopenia [47]. In the present study, no clinically significant changes were found in the BMDs of the spine, hip, and total skeleton. Because the study protocol consisted of both exercise and supplementation, some favorable changes in the BMD could be expected. However, it was not possible to detect them in the short study period [46]. This finding is consistent with the lack of concurrent changes in the biochemical bone metabolism parameters in the study participants.
Limitations of the present study include the relatively short duration of the observation. Furthermore, no control group undergoing sham exercise was included. Finally, self-reported data were used to monitor dietary intake only once. Given the increase in the physical activity levels of the participants, their energy intake may have also increased, which could have resulted in changes in the dietary macronutrient composition.
Conclusions
The administration of LC, as a dietary supplement during 12 weeks of RT program, increased the plasma TMAO levels and appeared to affect signaling molecules, as indicated by the increase in the resting SPARC and decorin levels. No significant modification in BMD and no differences between groups in RM could be due to the relatively short duration of the RT intervention. It is also not excluded that the same supplementation protocol without training program may induce different response in SPARC and decorin levels. Therefore, further studies are needed for a better definition of the effects of LC on SPARC, decorin, and TMAO levels in vivo in humans. Furthermore, it remains to be assessed whether LC supplementation, even that of short duration, may induce modifications, that can be preserved in the long term. Progress in understanding the mechanism of TMAO involvement in pathogenic processes may provide important information for antiaging strategies.
Data Availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BMD:
-
Bone mineral density
- CRP:
-
C-reactive protein
- DKK1:
-
Dickkopf-1
- DXA:
-
Dual-energy X-ray absorptiometry
- FGF-23:
-
Fibroblast growth factor-23
- IGF-1:
-
Insulin-like growth factor-1
- IL-6:
-
Interleukin-6
- LC:
-
L-carnitine
- OPG:
-
Osteoprotegerin
- OPN:
-
Osteopontin
- RM:
-
One-repetition maximum
- RT:
-
Resistance training
- SOST:
-
Sclerostin
- SPARC:
-
Secreted protein acidic and rich in cysteine/osteonectin
- TC:
-
Total carnitine
- TMA:
-
Trimethylamine
- TMAO:
-
Trimethylamine N-oxide
- TNF-α:
-
Tumor necrosis factor-α
References
Kanis JA, Cooper C, Rizzoli R, Reginster JY, Scientific Advisory Board of the European Society for C. Economic aspects of O, the Committees of Scientific A, National Societies of the international osteoporosis F: european guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2019;30:3–44.
Xu J, Lombardi G, Jiao W, Banfi G. Effects of Exercise on Bone Status in female subjects, from Young Girls to Postmenopausal Women: an overview of systematic reviews and Meta-analyses. Sports Med. 2016;46:1165–82.
Guo B, Zhang ZK, Liang C, Li J, Liu J, Lu A, Zhang BT, Zhang G. Molecular communication from skeletal muscle to bone: a review for muscle-derived Myokines regulating bone metabolism. Calcif Tissue Int. 2017;100:184–92.
Gomarasca M, Banfi G, Lombardi G. Myokines: the endocrine coupling of skeletal muscle and bone. Adv Clin Chem. 2020;94:155–218.
Pasqualini L, Ministrini S, Lombardini R, Bagaglia F, Paltriccia R, Pippi R, Collebrusco L, Reginato E, Sbroma Tomaro E, Marini E, et al. Effects of a 3-month weight-bearing and resistance exercise training on circulating osteogenic cells and bone formation markers in postmenopausal women with low bone mass. Osteoporos Int. 2019;30:797–806.
Rizzoli R. Nutritional aspects of bone health. Best Pract Res Clin Endocrinol Metab. 2014;28:795–808.
Hooshmand S, Balakrishnan A, Clark RM, Owen KQ, Koo SI, Arjmandi BH. Dietary l-carnitine supplementation improves bone mineral density by suppressing bone turnover in aged ovariectomized rats. Phytomedicine. 2008;15:595–601.
Orsal E, Halici Z, Bayir Y, Cadirci E, Bilen H, Ferah I, Aydin A, Ozkanlar S, Ayan AK, Seven B, Ozaltin S. The role of carnitine on ovariectomy and inflammation-induced osteoporosis in rats. Exp Biol Med (Maywood). 2013;238:1406–12.
Aydin A, Halici Z, Albayrak A, Polat B, Karakus E, Yildirim OS, Bayir Y, Cadirci E, Ayan AK, Aksakal AM. Treatment with Carnitine enhances Bone Fracture Healing under Osteoporotic and/or inflammatory conditions. Basic Clin Pharmacol Toxicol. 2015;117:173–9.
Li C, Huang Q, Yang R, Dai Y, Zeng Y, Tao L, Li X, Zeng J, Wang Q. Gut microbiota composition and bone mineral loss-epidemiologic evidence from individuals in Wuhan, China. Osteoporos Int. 2019;30:1003–13.
Rebouche CJ. Quantitative estimation of absorption and degradation of a carnitine supplement by human adults. Metabolism. 1991;40:1305–10.
Samulak JJ, Sawicka AK, Hartmane D, Grinberga S, Pugovics O, Lysiak-Szydlowska W, Olek RA. L-Carnitine supplementation increases Trimethylamine-N-Oxide but not markers of atherosclerosis in healthy aged women. Ann Nutr Metab. 2019;74:11–7.
Zhong X, Zhang F, Yin X, Cao H, Wang X, Liu D, Chen J, Chen X. Bone homeostasis and gut microbial-dependent signaling pathways. J Microbiol Biotechnol. 2021;31:765–74.
Zhou T, Heianza Y, Chen Y, Li X, Sun D, DiDonato JA, Pei X, LeBoff MS, Bray GA, Sacks FM, Qi L. Circulating gut microbiota metabolite trimethylamine N-Oxide (TMAO) and changes in bone density in response to weight loss diets: the POUNDS Lost Trial. Diabetes Care. 2019;42:1365–71.
Liu Y, Guo YL, Meng S, Gao H, Sui LJ, ** S, Li Y, Fan SG. Gut microbiota-dependent trimethylamine N-Oxide are related with hip fracture in postmenopausal women: a matched case-control study. Aging. 2020;12:10633–41.
Elam RE, Buzkova P, Barzilay JI, Wang Z, Nemet I, Budoff MJ, Cauley JA, Fink HA, Lee Y, Robbins JA et al. Trimethylamine N-oxide and hip fracture and bone mineral density in older adults: the cardiovascular health study. Bone 2022:116431.
Bell KE, Snijders T, Zulyniak M, Kumbhare D, Parise G, Chabowski A, Phillips SM. A whey protein-based multi-ingredient nutritional supplement stimulates gains in lean body mass and strength in healthy older men: a randomized controlled trial. PLoS ONE. 2017;12:e0181387.
Jaworska K, Huc T, Samborowska E, Dobrowolski L, Bielinska K, Gawlak M, Ufnal M. Hypertension in rats is associated with an increased permeability of the colon to TMA, a gut bacteria metabolite. PLoS ONE. 2017;12:e0189310.
Samborowska E, Olek RA. Twenty-four weeks of L-carnitine combined with leucine supplementation does not increase the muscle carnitine content in healthy active subjects. Ann Nutr Metab. 2023;79:51–9.
Murphy RM, Watt MJ, Febbraio MA. Metabolic communication during exercise. Nat Metab. 2020;2:805–16.
Norheim F, Raastad T, Thiede B, Rustan AC, Drevon CA, Haugen F. Proteomic identification of secreted proteins from human skeletal muscle cells and expression in response to strength training. Am J Physiol Endocrinol Metab. 2011;301:E1013–1021.
Aoi W, Naito Y, Takagi T, Tanimura Y, Takanami Y, Kawai Y, Sakuma K, Hang LP, Mizushima K, Hirai Y, et al. A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut. 2013;62:882–9.
Termine JD, Kleinman HK, Whitson SW, Conn KM, McGarvey ML, Martin GR. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981;26:99–105.
Zhang L, Li L, Yang M, Xu K, Boden G, Yang G. The rhPTH treatment elevates plasma secreted protein acidic and rich in cysteine levels in patients with osteoporosis. Osteoporos Int. 2013;24:1107–12.
Rosset EM, Bradshaw AD. SPARC/osteonectin in mineralized tissue. Matrix Biol. 2016;52–54:78–87.
Han XG, Wang DK, Gao F, Liu RH, Bi ZG. Bone morphogenetic protein 2 and decorin expression in old fracture fragments and surrounding tissues. Genet Mol Res. 2015;14:11063–72.
Mercadal L, Tezenas du Montcel S, Chonchol MB, Debure A, Depreneuf H, Servais A, Bassilios N, Assogba U, Allouache M, Prie D. Effects of L-Carnitine on Mineral metabolism in the Multicentre, Randomized, double blind, placebo-controlled CARNIDIAL Trial. Am J Nephrol. 2018;48:349–56.
Ohashi K, Ishikawa T, Hoshii A, Hokari T, Suzuki M, Noguchi H, Hirosawa H, Koyama F, Kobayashi M, Hirosawa S, et al. Effect of levocarnitine administration in patients with chronic liver disease. Exp Ther Med. 2020;20:94.
Yaghubi E, Daneshpazhooh M, Mohammadi MDJ, Sepandar H, Fakhri F, Ghaedi Z, Keshavarz E, Balighi SA, Mahmoudi K. Effects of l-carnitine supplementation on cardiovascular and bone turnover markers in patients with pemphigus vulgaris under corticosteroids treatment: a randomized, double-blind, controlled trial. Dermatol Ther. 2019;32:e13049.
Sawicka AK, Hartmane D, Lipinska P, Wojtowicz E, Lysiak-Szydlowska W, Olek RA. l-Carnitine Supplementation in Older Women. A Pilot Study on Aging Skeletal Muscle Mass and Function. Nutrients 2018, 10.
Chang IC, Chiang TI, Yeh KT, Lee H, Cheng YW. Increased serum osteopontin is a risk factor for osteoporosis in menopausal women. Osteoporos Int. 2010;21:1401–9.
Amrein K, Amrein S, Drexler C, Dimai HP, Dobnig H, Pfeifer K, Tomaschitz A, Pieber TR, Fahrleitner-Pammer A. Sclerostin and its association with physical activity, age, gender, body composition, and bone mineral content in healthy adults. J Clin Endocrinol Metab. 2012;97:148–54.
Garnero P, Sornay-Rendu E, Munoz F, Borel O, Chapurlat RD. Association of serum sclerostin with bone mineral density, bone turnover, steroid and parathyroid hormones, and fracture risk in postmenopausal women: the OFELY study. Osteoporos Int. 2013;24:489–94.
Dovjak P, Dorfer S, Foger-Samwald U, Kudlacek S, Marculescu R, Pietschmann P. Serum levels of sclerostin and dickkopf-1: effects of age, gender and fracture status. Gerontology. 2014;60:493–501.
Ghanemi A, Melouane A, Yoshioka M, St-Amand J. Secreted protein acidic and rich in cysteine and bioenergetics: extracellular matrix, adipocytes remodeling and skeletal muscle metabolism. Int J Biochem Cell Biol. 2019;117:105627.
Kanzleiter T, Rath M, Gorgens SW, Jensen J, Tangen DS, Kolnes AJ, Kolnes KJ, Lee S, Eckel J, Schurmann A, Eckardt K. The myokine decorin is regulated by contraction and involved in muscle hypertrophy. Biochem Biophys Res Commun. 2014;450:1089–94.
Sawicka AK, Renzi G, Olek RA. The bright and the dark sides of L-carnitine supplementation: a systematic review. J Int Soc Sports Nutr. 2020;17:49.
Thomas MS, Fernandez ML. Trimethylamine N-Oxide (TMAO), Diet and Cardiovascular Disease. Curr Atheroscler Rep. 2021;23:12.
Jalandra R, Dalal N, Yadav AK, Verma D, Sharma M, Singh R, Khosla A, Kumar A, Solanki PR. Emerging role of trimethylamine-N-oxide (TMAO) in colorectal cancer. Appl Microbiol Biotechnol. 2021;105:7651–60.
Avolio E, Mangialardi G, Slater SC, Alvino VV, Gu Y, Cathery W, Beltrami AP, Katare R, Heesom K, Caputo M, Madeddu P. Secreted protein acidic and Cysteine Rich Matricellular protein is enriched in the Bioactive Fraction of the human vascular pericyte Secretome. Antioxid Redox Signal. 2021;34:1151–64.
Vu TT, Marquez J, Le LT, Nguyen ATT, Kim HK, Han J. The role of decorin in cardiovascular diseases: more than just a decoration. Free Radic Res. 2018;52:1210–9.
Kim JS, Galvao DA, Newton RU, Gray E, Taaffe DR. Exercise-induced myokines and their effect on prostate cancer. Nat Rev Urol. 2021;18:519–42.
Zhao R, Zhao M, Xu Z. The effects of differing resistance training modes on the preservation of bone mineral density in postmenopausal women: a meta-analysis. Osteoporos Int. 2015;26:1605–18.
Mohammad Rahimi GR, Smart NA, Liang MTC, Bijeh N, Albanaqi AL, Fathi M, Niyazi A, Mohammad Rahimi N. The impact of different modes of Exercise Training on Bone Mineral density in older Postmenopausal Women: a systematic review and Meta-analysis research. Calcif Tissue Int. 2020;106:577–90.
Shojaa M, von Stengel S, Kohl M, Schoene D, Kemmler W. Effects of dynamic resistance exercise on bone mineral density in postmenopausal women: a systematic review and meta-analysis with special emphasis on exercise parameters. Osteoporos Int. 2020;31:1427–44.
Kemmler W, Shojaa M, Kohl M, von Stengel S. Effects of different types of Exercise on Bone Mineral density in Postmenopausal Women: a systematic review and Meta-analysis. Calcif Tissue Int. 2020;107:409–39.
Kitsuda Y, Wada T, Noma H, Osaki M, Hagino H. Impact of high-load resistance training on bone mineral density in osteoporosis and osteopenia: a meta-analysis. J Bone Miner Metab. 2021;39:787–803.
Acknowledgements
The authors are grateful to Angelika Sawicka, and Joanna Jaworska, for their technical assistance.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Robert A. Olek: the study conception; samples collection, samples analysis, statistical analysis, manuscript drafting; Emilia Samborowska: samples analysis; manuscript revision; Piotr Wisniewski: data analysis; manuscript revision; Pawel Wojtkiewicz: data analysis; manuscript rveision; Krystian Wochna data analysis; manuscript revision; Jacek Zielinski: data interpretation, manuscript revision. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Independent Bioethics Committee for Scientific Research at the Medical University of Gdansk (NKBBN/354 − 201/2017). Before starting the experimental procedure, all participants were informed about the procedure, risks, and expected outcomes and they provided their written informed consent for participation.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Olek, R.A., Samborowska, E., Wisniewski, P. et al. Effect of a 3-month L-carnitine supplementation and resistance training program on circulating markers and bone mineral density in postmenopausal women: a randomized controlled trial. Nutr Metab (Lond) 20, 32 (2023). https://doi.org/10.1186/s12986-023-00752-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12986-023-00752-1