Abstract
Influenza is an acute viral respiratory illness with high morbidity rates worldwide. Excessive pulmonary inflammation is the main characteristic of lethal influenza A virus (IAV) infections. Therapeutic options for managing influenza are limited to vaccines and some antiviral medications. Phillyrin is one of the major bioactive components of the Chinese herbal medicine Forsythia suspensa, which has the functions of sterilization, heat clearing and detoxification. In this work, the effect and mechanism of phillyrin on H1N1 influenza (PR8)-induced pneumonia were investigated. We reported that phillyrin (15 mg/kg) treatment after viral challenge significantly improved the weight loss, ameliorated pulmonary inflammation and inhibited the accumulation of multiple cytokines and chemokines in bronchoalveolar lavage fluid on 7 days post infection (dpi). In vitro, phillyrin suppressed influenza viral replication (Matrixprotein and nucleoprotein messenger RNA level) and reduced influenza virus-induced cytopathic effect (CPE). Furthermore,chemokine receptor CXCR2 was confirmed to be markedly inhibited by phillyrin. Surface plasmon resonance results reveal that phillyrin exhibits binding affinity to CXCR2, having a binding affinity constant (KD) value of 1.858e-5 M, suggesting that CXCR2 is a potential therapeutic target for phillyrin. Moreover, phillyrin inhibited the mRNA and protein expression levels of Caspase1, ASC and NLRP3 in the lungs of mice with H1N1-induced pneumonia.This study reveals that phillyrin ameliorates IAV-induced pulmonary inflammation by antagonizing CXCR2 and inhibiting NLRP3 inflammasome activation partly.
Similar content being viewed by others
Background
Influenza is a fatal respiratory infectious disease. The excessive immune response caused by influenza infection may cause irreversible damage to the body [1, 2]. Influenza virus infection can lead to apoptosis or necrosis of tissue cells, which activates the immune system. The infected respiratory epithelial cells and vascular endothelial cells secrete cytokines and chemokines to recruit and activate various immune cells. Immune cells perform their respective functions to phagocytose pathogens and infected cells or secrete more cytokines, resulting in an expanded immune response [3]. An excessive immune response can bring serious consequences, such as immune cell infiltration and cytokine storm, which can result in pulmonary edema and may even cause death [4]. Excessive pulmonary inflammation is the main characteristic of lethal IAV infections.
Therapeutic actions for influenza are limited to vaccines and a few anti-viral drugs. However, the frequent mutation of influenza virus has limited the current treatment methods, and some studies have found that the cause of severe influenza is often an excessive immune response [5,6,7]. Therefore, antiviral therapy has been unable to meet our requirements, and a new way to resist influenza is urgently needed. Suppressing excessive immune responses to alleviate tissue damage caused by influenza virus infection could become an effective anti-influenza therapeutic strategy.
Phillyrin is one of the major bioactive components of the Chinese herbal medicine Forsythia suspensa, which has the functions of sterilization, heat clearing and detoxification [4b). These DEGs might be the important therapeutic effector molecules of phillyrin treatment on IAV-induced pneumonia.
The cytokine-cytokine receptor interaction is responsible for the suppressive effect of phillyrin on IAV-induced pneumonia. (a) Hierarchical cluster analysis of DEGs among CON group, virus group and phillyrin group. Red and blue hues indicate upregulated and downregulated expression, respectively. (b) The quantity of DEGs in virus-vs.-CON and phillyrin-vs.-virus groups, and comparison of DEGs between virus-vs.-CON and phillyrin-vs.-virus. (c) KEGG enrichment bubble diagram with common DEGs. The mRNA expression of CXCR2 (d), CCR2 (e), CCR1 (f), CCR5 (g). Mean ± SD (n = 6/group). One-way ANOVA, # p < 0.05 vs. CON, * p < 0.05 vs. IAV.
Furthermore, pathway enrichment analysis of the 163 DEGs was performed through the KEGG database. The results showed that 15 pathways were markedly enriched for these DEGs (P < 0.05; Table 1), including the calcium signaling pathway, cytokine-cytokine receptor interaction, viral protein interaction with cytokine and cytokine receptor, chemokine signaling pathway, focal adhesion and cAMP signaling pathway (Fig. 4c). It is worth noting that there are three classic chemokine signaling pathways (ko04060, ko04061, and ko04062). These results suggest that the cytokine-cytokine receptor interaction plays a crucial role in enhancing the therapeutic effect of phillyrin on IAV-induced pneumonia.
Moreover, the levels of 5 chemokines such as CCL11/Eotaxin, CXCL1/KC, CCL5/RANTES, CCL2/MCP-1 and CCL3/MIP-1α were dramatically reduced by phillyrin in BALF of IAV-infected mice as mentioned previously (Fig. 3). These inflammatory mediators normally recruit cells of the innate immune system through selectively binding to the corresponding receptors. CCL11 is mainly involved in the recruitment of eosinophils into the lung, and its receptor is CCR3 [14]. CCL3 and CCL2 play a role in inflammatory responses through binding to the receptors CCR1, CCR2 and CCR5 [15]. As one of the natural ligands of CCR5, CCL5/CCR5 can mediate the recruitment and activation of neutrophils and monocytes during influenza, and CXCL1 can chemotactic T cells, monocytes, neutrophils and other immune cells when combined with its specific CXCR2 receptor [16,17,18]. Previous findings showed that activation of cytokine receptors at the initial stage of influenza infection could ensure proper recruitment of white blood cells and activation of antiviral pathway in epithelial cells. However, continuous or excessive activation of cytokine receptors during severe influenza infection may aggravate inflammatory reaction, leading to increased lung injury [19, 20]. One possible strategy for enhancing anti-inflammatory effects is to selectively bind and neutralize the chemokines and chemokine receptors. In view of the important role of chemokine receptors, we further detected the mRNA expression levels of CCR3, CXCR2, CCR2, CCR1 and CCR5 in the lungs. The findings demonstrated that the mRNA levels of CCR5, CCR2, CCR1 and CXCR2 genes were increased after influenza infection. Phillyrin treatment could significantly reduce the expression of CCR2 and CXCR2, among which the latter was more significantly regulated by phillyrin (Fig. 4d-g). CCR3 gene was undetermined due to its low expression in the lungs (Results not listed).
Phillyrin has good binding ability to CXCR2
CXCR2 is a critical target in the efforts to suppress neutrophilic inflammation. Excessive neutrophil influx is related to greater disease severity in patients with influenza infection. A number of studies on the experimental inhibition of CXCR2 have reported its ameliorative effects on inflammatory responses and lung injury in the sublethal influenza murine models [21,22,23]. We verified the affinity of phillyrin to CXCR2 based on molecular docking. Generally, the molecular docking score of less than 0 kcal mol− 1 indicates that ligands can spontaneously bind to receptors, and the score less than − 5 kcal mol− 1 indicates that they have good binding ability [24, 25]. Our results showed that binding affinity was − 8.9 kcal mol− 1 between phillyrin and CXCR2, and the drug-target had good binding ability. In addition, their bonding types were hydrogen bonds (from residues LYS320, LYS327 and VAL72) and electrostatic force (Fig. 5.). As a special intermolecular force, hydrogen bond is a critical index to measure the affinity between protein and small molecules. Therefore, CXCR2 may be an important drug-target of phillyrin against IAV-induced pneumonia.
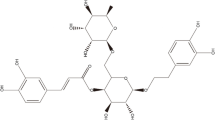
Phillyrin has good binding ability to CXCR2. (a) Protein structure of CXCR2. (b) Molecular structure of phillyrin. (c) 3D diagrams showed the distribution of hydrogen donors and receptors in active pockets. (d) 2D diagrams showed the bond types and amino acid residues. Green and orange dashed lines indicate hydrogen bonds and Pi-Anion, respectively
To further confirm whether phillyrin is the direct binding to CXCR2. An SPR assay was then performed to evaluate the binding affinity of phillyrin and CXCR2. As shown in Fig. 6a, phillyrin bound to CXCR2 in a dose-dependent manner. The response units at equilibrium were plotted against phillyrin concentrations (Fig. 6b), and the binding affinity constant (KD) value was calculated by non-linear regression. The results showed that phillyrin bound to CXCR2 with a KD value of 18.58µM, indicating that phillyrin presented strong affinity for CXCR2.
The binding affinity between phillyrin and CXCR2. Interactions of CXCR2 with phillyrin measured by SPR. The CXCR2 with a his-Tag was fixed on the NTA sensor chip via capture coupling and serial dilutions of phillyrin (6.25µM, 12.5µM,25µM,50µM) were used as analytes. Changes in plasmon resonance are shown as response units. (a) Multicycle kinetics using CXCR2 as ligand and phillyrin as analyte. Different colored lines correspond to different concentrations of Phillyrin; (b)The KD value of phillyrin and CXCR2 was calculated by non-linear regression analysis
Phillyrin inhibits overactivation of NLRP3 inflammasome
Previous studies have reported that CXCL1-CXCR2 axis could regulate NLRP3 inflammasome activation in some inflammatory scenarios [26]. IAV can induce excessive secretion of cytokines by triggering NLRP3 activation, thus resulting in lung damage and death [27, 28]. Specifically, hyperinflammatory responses, such as the secretion of NLRP3-dependent IL-1β, are characteristic features of severe IAV infection [29, 30]. In our study, phillyrin downregulated IL-1β production and inflammatory responses in BALF from H1N1-induced pneumonia mice (Fig. 3a). To further elucidate the anti-inflammatory mechanisms of phillyrin, the expression levels of NLRP3 inflammasomes and the Caspase1 p20 subunit (representing activated Caspase1)/Caspase1 were analyzed. Notably, the mRNA and protein expression levels of Caspase1, ASC and NLRP3 were all elevated in the lungs of mice with H1N1-induced pneumonia, but these trends were suppressed in the phillyrin group (Figs. 7a-c and 8a-d). Besides, in the virus group, the proportion of Caspase1 p20 to Caspase1 and the mRNA expression of IL-1β were higher than those in CON group, but were obviously reduced in phillyrin-treated mice (Figs. 7d and 8a and e). These findings suggest that phillyrin can suppress the overactivation of NLRP3 inflammasomes in mice with IAV-induced pneumonia.
Phillyrin inhibits protein expression of NLRP3 inflammasome. The protein levels of NLRP3 (b), ASC (c) and Caspase 1 (d), and Caspase1 p20 subunit/Caspase1 (e) in the lungs on 7 dpi were detected by Western blotting. Mean ± SD(n = 3 independent experiments) One-way ANOVA, # p < 0.05 vs. CON, * p < 0.05 vs. IAV.
Phillyrin has the similar effect as CXCR2 antagonist
I repeated an in vivo experiment in the context of CXCR2 antagonist SB225002 (a potent, selective and non-peptide CXCR2 antagonist, MedChemExpress). The body weights of mice in different treatment groups are demonstrated in Fig. 9. The result showed that infection of PR8 significantly reduced the body weights of virus-infected mice compared to the control mice, while treatment with phillyrin-only, CXCR2 antagonist SB225002-only, phillyrin and SB225002 synergistically significantly alleviated this loss. Oseltamivir phosphate was employed as a positive control. Compared to phillyrin-only group or SB225002-only group, we found that phillyrin and SB225002 synergistically enhanced weight regain. This data indicated that phillyrin has the similar effect as CXCR2 antagonist in improving virus-induced weight loss. However, further testing is needed to determine what changes have occurred in the CXCR2-NLRP3-inflammasome pathway.
Phillyrin and CXCR2 antagonist SB225002 treatment alleviates percentage weight loss caused by IAV. Briefly, BALB/c mice were randomized into several groups: Control group (n = 6, Saline-treated), Phillyrin group (n = 6, intraperitoneal injection (i.p.) of 15 mg/kg Phillyrin once a day from 0 day to 4 days without infection ), IAV group (n = 6, intranasally challenged with PR8), IAV + Phillyrin group (n = 6, i.p. of 15 mg/kg Phillyrin once a day from 0 day to 4 days postinfection ), IAV + SB225002 group (n = 6, i.p. of 20 μm SB225002 once a day from 0 day to 4 days postinfection ), IAV + Phillyrin + SB225002 group (n = 6, daily i.p. of 20 μm SB225002 from 1 days pre- to 4 days post-infection and i.p. of 15 mg/kg Phillyrin once a day from 0 day to 4 days postinfection), IAV + oseltamivir group (n = 6, orally administrated with 10 mg/kg oseltamivir once a day from 0 day to 4 days postinfection). Phillyrin and SB225002 was suspended in 0.4% carboxymethylcellulose sodium. The body weight change of each group were observed daily following PR8 challenge for a total of 8 days. Mean ± SD (n = 6/group). One-way ANOVA, # p < 0.05 vs. Control, * p < 0.05 vs. IAV.
Discussion
The influenza virus causes seasonal influenza and can even lead to outbreak and epidemic worldwide [3, 31]. Viral pneumonia from IAV infection is a common complication and leading cause of death [32]. As a lack of drugs used to control viral acute lung injury continues to persist, the development of new therapeutic agents is essential. This study found that phillyrin, a monomer TCM ingredient with anti-inflammatory activity, exerted therapeutic effects on the pneumonia induced by IAV infection. The results showed that phillyrin treatment alleviated pneumonia induced by virus infection and significantly ameliorated the upregulation of multiple pro-inflammatory cytokines and chemokines (e.g., IL-1β, CXCL1, CCL3, CCL2, CCL5 and so on) in BALF. More importantly, the results of RNA sequencing analysis revealed that the cytokine-cytokine receptor interaction plays an essential role in regulating the therapeutic effect of phillyrin on influenza-induced pneumonia, and the mRNA expression level of CXCR2 was confirmed to be markedly inhibited by phillyrin. We performed computerized molecular docking of phillyrin with CXCR2 and verified good binding activity. Furthermore, SPR analysis, the gold standard method for studying protein-protein interactions, confirmed that phillyrin direct bound to CXCR2 with high affinity activity. All of these results indicate that CXCR2 is a potential therapeutic target for phillyrin. We also showed that phillyrin treatment could affect the mRNA and protein expression levels of Caspase1, ASC and NLRP3 in the lung homogenates of mice infected with H1N1. The ameliorative effect of phillyrin on influenza-induced pulmonary pathological damage may be partly related to the antagonization of CXCR2 and inhibition of NLRP3 inflammasome activation.
Influenza patients are often afflicted with severe pneumonia that is characterized by excessive infiltration of leukocytes and secretion of proinflammatory cytokines [33, 34]. Despite the inflammatory response produced by the immune host to remove the virus, the elevated production of different inflammatory cytokines typically contributes to the pathogenesis of IAV-induced acute lung injury [35,36,37]. In the present study, phillyrin (15 mg/kg) remarkably decreased the lung index, relieved the extensive degeneration and necrosis of the bronchial and bronchiolar epithelium after IAV infection and inhibited the accumulation of multiple pro-inflammatory cytokines and chemokines, revealing that phillyrin can alleviate pulmonary inflammation in mice with virus-induced pneumonia.
-
Our study also found that oseltamivir, as a positive control, led to similar reductions in pathology, suggesting that a decrease in viral replication alone can alleviate the pathological and inflammatory responses.To determine the effect of phillyrin on influenza viral replication, the relative levels of M and NP mRNA expression and virus-induced CPE at indicated time points in vitro as well as the contents of M mRNA and the levels of HA protein of lungs at 7 dpi in vivo were examined. The cell experiments verified the inhibitory effect of phillyrin on influenza viral multiplication and the results were similar to the previous report [38], however, the inhibitory trend was not significant in vivo. Unlike a single cellular environment, lung tissue is composed of a variety of cells such as respiratory epithelial cells and macrophages [39]. The higher levels of viral genomic replication, transcription, and translation in alveolar epithelial cells probably makes IAV maintain a certain amount in lung tissue to partially “counteract” the inhibition of phillyrin on viral multiplication. Therefore, in the present study, although the research in vitro have confirmed the inhibitory effect of phillyrin on influenza viral multiplication, this inhibition was not significant in vivo. The amelioration of phillyrin on pulmonary inflammation in influenza viral pneumonia mice is not mainly dependent on its antiviral activity.
The pathogenesis of pneumonia caused by influenza is not only directly associated with viral cytopathology but also forms an overzealous host immune response. Neutrophils have been recognized as an important cell type that leads to deterioration in lung functions [40]. When the virus reaches the lung epithelium, neutrophils are recruited by the immune/non-immune cells, leading to the production of chemokines (e.g., CXCL1/CXCL2) in mice [1, 41]. These chemokines act through their receptor CXCR2 in different types of cells. Previous studies have shown that the chemokine receptor CXCR2 has an essential role in regulating pulmonary inflammation and damage during severe IAV infection. The downregulation of CXCR2 could prevent such aggravated response, even after sequential IAV infections, without affecting the virus-specific adaptive immune responses [22]. In the present work, DEGs and potential pathways in the lung tissue of the virus group and phillyrin group were evaluated using RNA-seq and qRT-PCR. The data suggest that the cytokine-cytokine receptor interaction plays a vital role in regulating the inhibitory effect of phillyrin on IAV-induced pneumonia. It was found that the expression of CXCL1 and CXCR2 was markedly upregulated in the BALF and lungs of mice with IAV infection. Interestingly, phillyrin treatment could remarkably decrease the expression of CXCR2 and the secretion of CXCL1. Furthermore, we performed the computerized molecular docking of phillyrin with CXCR2 and confirmed good binding activity between them, indicating that phillyrin is a potential CXCR2 inhibitor.
The CXCL1-CXCR2 axis has been reported to be involved in NLRP3 inflammasome activation and functions in some inflammatory scenarios. Tang and co-workers showed that this axis might induce inflammatory response in diabetic nephropathy by promoting NLRP3 inflammasome overactivation [26]. The activation of NLRP3 inflammasomes in LPS-primed macrophages was observed to be specific for CXCL1 and CXCL2 but not other chemokines such as CCL2 and CCL5 [42,43,44]. The inflammatory response is an integral part of IAV infection. Recently, the NLRP3 inflammasome complex has been revealed to have essential roles in both detrimental and protective immune responses during IAV infection. NLRP3 inflammasomes are oligomeric signaling platforms that induce Caspase1-associated pyroptotic cell death and mature the prototypic inflammatory cytokines IL-18 and IL-1β [45]. Recent studies have highlighted that inhibition of NLRP3 inflammasome activation can effectively treat influenza viral pulmonary inflammation. MCC950, an inhibitor specific to NLRP3 inflammasome, has been shown to improve the survival and alleviate pulmonary inflammation in IAV-infected mice by downregulating the expression of Caspase1 and NLRP3 [46]. In this study, phillyrin downregulated the expression levels of Caspase1, ASC and NLRP3 in the lungs of mice with virus-induced pneumonia and reduced the protein ratio of Caspase1p20/Caspase1, thereby inhibiting NLRP3 inflammasome overactivation. However, the previous report [42] shows that ILK and PKCµare the linkage to CXCL1 or 2/CXCR2 and NLRP3 inflammasome activation. The detection of phosphor-ILK and phosphor-PKCµ level will help to further clarify if phillyrin affect inflammasome activity upon influenza A virus infection in the furture.
Conclusion
This study demonstrates that phillyrin can ameliorate pulmonary inflammation in mice with virus-induced pneumonia, and its anti-inflammatory mechanisms may be through antagonizing CXCR2 and suppressing NLRP3 inflammasome overactivation in the lung of infected mice. Although we observed this phenomenon and the possible mechanism, lung tissue–specific CXCR2 knockout mouse models should be constructed in future experiments in order to evaluate this pathway in vivo. In addition, some key questions also remain to be resolved. For instance, is it specific for CXCL1 or CXCR2 that phillyrin inhibits NLRP3 inflammasome activation in the lungs of IAV-infected mice? If so, what is the precise molecular mechanism by which phillyrin ameliorates influenza viral pneumonia through depressing NLRP3 inflammasome activation via declining CXCR2 expression? Still, although significant work remains to be conducted, our findings provide novel insights into the treatment of IAV-induced pulmonary inflammation.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- IAV:
-
Influenza A virus
- dpi:
-
Days post infection
- BALF:
-
Bronchoalveolar lavage fluid
- RNA-Seq:
-
RNA Sequencing
- CXCR2:
-
C-X-C motif chemokine receptor 2
- NLRP3:
-
NLR family pyrin domain containing 3
- IL-1β:
-
Interleukin 1 beta
- LD50:
-
50% Lethal dose
- PBS:
-
Phosphate buffered saline
- p.o.:
-
Oral gavage
- i.p.:
-
Intraperitoneally injected
- CON:
-
Control
- qRT-PCR:
-
Quantitative real-time PCR
- H&E:
-
Hematoxylin-eosin
- FPKM:
-
Fragments per kilobase millon mapped reads
- DEGs:
-
Differentially expressed genes
- SPR:
-
Surface plasmon resonance
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- ASC:
-
Apoptosis-associated speck-like protein containing a CARD
- HA:
-
Hemagglutinin
- IL-2:
-
Interleukin 2
- IL-3:
-
Interleukin 3
- IL-5:
-
Interleukin 5
- IL-9:
-
Interleukin 9
- IL-12:
-
Interleukin 12
- IL-13:
-
Interleukin 13
- IL-17:
-
Interleukin 17
- GM-CSF:
-
Colony stimulating factor 2
- G-CSF:
-
Colony Stimulating Factor 3
- CCL11:
-
C-C motif chemokine ligand 11
- CXCL1:
-
C-X-C motif chemokine ligand 1
- CCL3:
-
C-C motif chemokine ligand 3
- CCL4:
-
C-C motif chemokine ligand 4
- CCL5:
-
C-C motif chemokine ligand 5
- CCR3:
-
C-C motif chemokine receptor 3
- CCR2:
-
C-C motif chemokine receptor 2
- CCR5:
-
C-C motif chemokine receptor 5
- KD:
-
Affinity constant
References
Washburn ML, Crosby R, Remlinger K, Wang F, Creech D. Therapeutically attenuating Neutrophil Recruitment with a CXCR2 antagonist in Combination with Oseltamivir ameliorates Influenza-Induced Lung Injury and Disease. Open Forum Infect Dis. 2019;6(4):ofz106.
Rojas-Quintero J, Wan X, Tipper J, Burkett PR, Zuñiga J, Ashtekar AR, Polverino F, Rout A, Yambayev I, Hernández C, Jimenez L, Ramírez G, Harrod KS, Owen CA. Matrix metalloproteinase-9 deficiency protects mice from severe Influenza a viral Infection. Jci Insight. 2018;3(24).
hen X, Liu S, Goraya MU, Maarouf M, Huang S, Chen JL. Host Immune Response to Influenza A Virus Infection. Front Immunol. 2018;9:320.
Denney L, Ho LP. The role of respiratory epithelium in host defence against Influenza virus Infection. Biomed J. 2018;41(4):218–33.
Yang J, Huang YN, Liu SW. Investigational antiviral therapies for the treatment of Influenza. Expert Opin Investig Drugs. 2019;28(5):481–8.
Shie JJ, Fang JM. Development of effective anti-influenza Drugs: congeners and conjugates - a review. J Biomed Sci. 2019;26(1).
Yamayoshi S, Kawaoka Y. Current and future Influenza vaccines. Nat Med. 2019;25(2):212–20.
Zhong WT, Wu YC, **e XX, Zhou X, Wei MM, Soromou LW, Ci XX, Wang DC. Phillyrin attenuates LPS-induced pulmonary inflammation via suppression of MAPK and NF-kappaB activation in acute lung injury mice. Fitoterapia. 2013;90:32–9.
Jiang Q, Chen J, Long X, Yao X, Zou X, Yang Y, Huang G, Zhang H. Phillyrin protects mice from traumatic brain injury by inhibiting the inflammation of microglia via PPARgamma signaling pathway. Int Immunopharmacol. 2020;79:106083.
Zhang Y, Ding Y, Zhao H, Wang Z, Zeng F, Qian Z, Li J, Ma T, Huang C. Downregulating PDPK1 and taking phillyrin as PDPK1-targeting drug protect hepatocytes from alcoholic steatohepatitis by promoting autophagy. Cell Death Dis. 2022;13(11):991.
Qu XY, Li QJ, Zhang HM, Zhang XJ, Shi PH, Zhang XJ, Yang J, Zhou Z, Wang SQ. Protective effects of phillyrin against Influenza a virus in vivo. Arch Pharm Res. 2016;39(7):998–1005.
Khalil H, Abd El Maksoud AI, Roshdey T, El-Masry S. Guava flavonoid glycosides prevent Influenza a virus Infection via rescue of P53 activity. J Med Virol. 2019;91(1):45–55.
Maruyama H, Kimura T, Liu H, Ohtsuki S, Miyake Y, Isogai M, Arai F, Honda A. Influenza virus replication raises the temperature of cells. Virus Res. 2018;257:94–101.
Welleman V, Benhassou HA, Fuselier E, Bellesort F, Dury S, Lebargy F, Dormoy V, Fichel C, Naour RL, Gounni AS, Lamkhioued B. Role of CCR3 in respiratory syncytial virus Infection of airway epithelial cells. iScience. 2021;24(12):103433.
Zhao X, Gu M, Xu X, Wen X, Yang G, Li L, Sheng P, Meng F. CCL3/CCR1 mediates CD14(+)CD16(-) circulating monocyte recruitment in knee osteoarthritis progression. Osteoarthritis Cartilage. 2020;28(5):613–25.
Zeng Z, Lan T, Wei Y, Wei X. CCL5/CCR5 axis in human Diseases and related treatments. Genes Dis. 2022;9(1):12–27.
Wang L, Zhang YL, Lin QY, Liu Y, Guan XM, Ma XL, Cao HJ, Liu Y, Bai J, **a YL, Du J, Li HH. CXCL1-CXCR2 axis mediates angiotensin II-induced cardiac hypertrophy and remodelling through regulation of monocyte infiltration. Eur Heart J. 2018;39(20):1818–31.
Korbecki J, Kojder K, Kapczuk P, Kupnicka P, Gawrońska-Szklarz B, Gutowska I, Chlubek D, Baranowska-Bosiacka I. The Effect of Hypoxia on the expression of CXC Chemokines and CXC Chemokine Receptors-A Review of Literature. Int J Mol Sci. 2021;22(2).
Rudd JM, Pulavendran S, Ashar HK, Ritchey JW, Snider TA, Malayer JR, Marie M, Chow VTK, Narasaraju T. Neutrophils induce a Novel Chemokine receptors Repertoire during Influenza Pneumonia. Front Cell Infect Microbiol. 2019;9:108.
Major J, Crotta S, Llorian M, McCabe TM, Gad HH, Priestnall SL, Hartmann R, Wack A. Type I and III interferons disrupt lung epithelial repair during recovery from viral Infection. Science. 2020;369(6504):712–7.
Ashar HK, Pulavendran S, Rudd JM, Maram P, Achanta M, Chow VTK, Malayer JR, Snider TA, Teluguakula N. Administration of a CXC chemokine receptor 2 (CXCR2) antagonist, SCH527123, together with Oseltamivir suppresses NETosis and protects mice from Lethal Influenza and piglets from Swine-Influenza Infection. Am J Pathol. 2021;191(4):669–85.
Tavares LP, Garcia CC, Machado MG, Queiroz-Junior CM, Barthelemy A, Trottein F, Siqueira MM, Brandolini L, Allegretti M, Machado AM, de Sousa LP, Teixeira MM. CXCR1/2 antagonism is protective during Influenza and Post-influenza Pneumococcal Infection. Front Immunol. 2017;8:1799.
Peiris JS, Yu WC, Leung CW, Cheung CY, Ng WF, Nicholls JM, Ng TK, Chan KH, Lai ST, Lim WL, Yuen KY, Guan Y. Re-emergence of fatal human Influenza a subtype H5N1 Disease. Lancet. 2004;363(9409):617–9.
Qi JH, Dong FX, Wang K, Zhang SY, Liu ZM, Wang WJ, Sun FZ, Zhang HM, Wang XL. Feasibility analysis and mechanism exploration of Rhei Radix et Rhizome-Schisandrae Sphenantherae Fructus (RS) against COVID-19. J Med Microbiol. 2022;71(5).
Bai YL, Zhang JF, Sha ZJ, Zhu N, Huang XL, Li ZY. Study on potential molecular mechanism of Mongolian medicine Bawei Sanxiang San in treatment of chronic Heart Failure based on network pharmacology and molecular docking. Zhongguo Zhong Yao Za Zhi. 2021;46(10):2392–402.
Tang H, Yang M, Liu Y, Liu H, Sun L, Song P. The CXCL1-CXCR2 Axis mediates tubular Injury in Diabetic Nephropathy through the regulation of the inflammatory response. Front Physiol. 2021;12:782677.
McAuley JL, Tate MD, MacKenzie-Kludas CJ, Pinar A, Zeng W, Stutz A, Latz E, Brown LE, Mansell A. Activation of the NLRP3 inflammasome by IAV virulence protein PB1-F2 contributes to severe pathophysiology and Disease. PLoS Pathog. 2013;9(5):e1003392.
Tate MD, Ong JDH, Dowling JK, McAuley JL, Robertson AB, Latz E, Drummond GR, Cooper MA, Hertzog PJ, Mansell A. Reassessing the role of the NLRP3 inflammasome during pathogenic Influenza a virus Infection via temporal inhibition. Sci Rep. 2016;6:27912.
Ji S, Dai MY, Huang Y, Ren XC, Jiang ML, Qiao JP, Zhang WY, Xu YH, Shen JL, Zhang RQ, Fei GH. Influenza a virus triggers acute exacerbation of Chronic Obstructive Pulmonary Disease by increasing proinflammatory cytokines secretion via NLRP3 inflammasome activation. J Inflamm (Lond). 2022;19(1):8.
Bawazeer AO, Rosli S, Harpur CM, Docherty CA, Mansell A, Tate MD. Interleukin-1β exacerbates Disease and is a potential therapeutic target to reduce pulmonary inflammation during severe Influenza a virus Infection. Immunol Cell Biol. 2021;99(7):737–48.
Qiu LN, Tan YR, Luo YJ, Chen XJ. Leonurine protects against Influenza a virus infection-induced Pneumonia in mice. Pathog Dis. 2021;79(7).
Hui DS, Lee N, Chan PK, Beigel JH. The role of adjuvant immunomodulatory agents for treatment of severe Influenza. Antiviral Res. 2018;150:202–16.
Wan X, Li J, Wang Y, Yu X, He X, Shi J, Deng G, Zeng X, Tian G, Li Y, Jiang Y, Guan Y, Li C, Shao F, Chen H. H7N9 virus Infection triggers lethal cytokine Storm by activating gasdermin E-mediated pyroptosis of lung alveolar epithelial cells. Natl Sci Rev. 2022;9(1):nwab137.
Janssens Y, Joye J, Waerlop G, Clement F, Leroux-Roels G, Leroux-Roels I. The role of cell-mediated immunity against Influenza and its implications for vaccine evaluation. Front Immunol. 2022;13:959379.
Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine Storm. Microbiol Mol Biol Rev. 2012;76(1):16–32.
Wang Y, Sharma P, Jefferson M, Zhang W, Bone B, Kipar A, Bitto D, Coombes JL, Pearson T, Man A, Zhekova A, Bao Y, Tripp RA, Carding SR, Yamauchi Y, Mayer U, Powell PP, Stewart JP, Wileman T. Non-canonical autophagy functions of ATG16L1 in epithelial cells limit lethal Infection by Influenza a virus. Embo j. 2021;40(6):e105543.
Kataoka M, Ishida K, Ogasawara K, Nozaki T, Satoh YI, Sata T, Sato Y, Hasegawa H, Nakajima N. Serial Section Array Scanning Electron Microscopy Analysis of Cells from lung autopsy specimens following fatal A/H1N1 2009 pandemic Influenza virus Infection. J Virol. 2019;93:19.
Chen Y, Wu C, Li H, Powell H, Chen A, Zhu G, Cong W, Fu L, Pekosz A, Leng S. Antiviral effect and mechanism of Phillyrin and its reformulated FS21 against Influenza. Influenza Other Respir Viruses. 2023;17(3):e13112.
An C, Wu Y, Wu J, Liu H, Zhou S, Ge D, Dong R, You L, Hao Y. Berberine ameliorates pulmonary inflammation in mice with Influenza viral Pneumonia by inhibiting NLRP3 inflammasome activation and gasdermin D-mediated pyroptosis. Drug Dev Res. 2022;83(7):1707–21.
Radermecker C, Sabatel C, Vanwinge C, Ruscitti C, Maréchal P, Perin F, Schyns J, Rocks N, Toussaint M, Cataldo D, Johnston SL, Bureau F, Marichal T. Locally instructed CXCR4(hi) neutrophils trigger environment-driven allergic Asthma through the release of neutrophil extracellular traps. Nat Immunol. 2019;20(11):1444–55.
Mattos MS, Ferrero MR, Kraemer L, Lopes GAO, Reis DC, Cassali GD, Oliveira FMS, Brandolini L, Allegretti M, Garcia CC, Martins MA, Teixeira MM, Russo RC. CXCR1 and CXCR2 inhibition by Ladarixin improves neutrophil-dependent airway inflammation in mice. Front Immuno. 2020;11:566953.
Boro M, Balaji KN. CXCL1 and CXCL2 regulate NLRP3 inflammasome activation via G-Protein-coupled receptor CXCR2. J Immunol. 2017;199(5):1660–71.
Sierra-Filardi E, Nieto C, Domínguez-Soto A, Barroso R, Sánchez-Mateos P, Puig-Kroger A, López-Bravo M, Joven J, Ardavín C, Rodríguez-Fernández JL, Sánchez-Torres C, Mellado M, Corbí AL. CCL2 shapes macrophage polarization by GM-CSF and M-CSF: identification of CCL2/CCR2-dependent gene expression profile. J Immunol. 2014;192(8):3858–67.
Tyner JW, Uchida O, Kajiwara N, Kim EY, Patel AC, O’Sullivan MP, Walter MJ, Schwendener RA, Cook DN, Danoff TM, Holtzman MJ. CCL5-CCR5 interaction provides antiapoptotic signals for macrophage survival during viral Infection. Nat Med. 2005;11(11):1180–7.
Tate MD, Mansell A. An update on the NLRP3 inflammasome and Influenza: the road to redemption or perdition? Curr Opin Immunol. 2018;54:80–5.
Coates BM, Staricha KL, Ravindran N, Koch CM, Cheng Y, Davis JM, Shumaker DK, Ridge KM. Inhibition of the NOD-Like receptor protein 3 inflammasome is protective in Juvenile Influenza A Virus Infection. Front Immunol. 2017;8:782.
Acknowledgements
Thanks to the Experimental Center of Shandong University of Traditional Chinese Medicine, Shandong Provincial Collaborative Innovation Center for Antiviral Traditional Chinese Medicine and Shandong University of Traditional Chinese Medicine Postdoctoral Programme for their support and help. We thank LetPub (www.letpub.com) and EditSprings (https://www.editsprings.cn) for its linguistic assistance during the preparation of this manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82003715), the Shandong Provincial Natural Science Foundation of China (ZR2021LZY017) and the Postdoctoral Program of Shandong University of Traditional Chinese Medicine.
Author information
Authors and Affiliations
Contributions
X.W. and S.Z. designed the study, analyzed the data, interpreted the results, and drafted the manuscript. S.Z., W.W., Z.L., W.L., C.L., X.L., N.W. and X.S. performed in vivo experiments and all of assays; F.S., J.Z. and J.Q. performed molecular docking; X.W., N.W. and X.S. performed SPR assay. F.S. contributed to the data collection and manuscript revision; X.W., D.Q. and D.Z. was involved in the study design, data interpretation, and manuscript revision. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
All experiments were conducted under biosafety level 2 conditions. All experiments were conducted in compliance with the Regulations for the Administration of Affairs Concerning Experimental Animals in China, and were approved by the Animal Ethics Committee of the Animal Experiment Center of Shandong University of TCM following the regulations of the **an Administration Office of Laboratory Animals in **an, China.
Consent for publication
All the authors approved the publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, S., Sun, F., Zhu, J. et al. Phillyrin ameliorates influenza a virus-induced pulmonary inflammation by antagonizing CXCR2 and inhibiting NLRP3 inflammasome activation. Virol J 20, 262 (2023). https://doi.org/10.1186/s12985-023-02219-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12985-023-02219-4