Abstract
Background
Sepsis is a systemic inflammatory response syndrome caused by severe infection in children, but cases of sepsis associated with human parainfluenza virus (HPIV) have been rarely reported in newborns.
Case presentation
We report a case of HPIV-3 positive full-term newborn admitted to the Neonatal Intensive Care Unit of Bei**g Children’s Hospital due to hematuria, gloomy spirit, inactivity and loss of appetite for 6 h. He had septic shock when he arrived the Accident & Emergency Department requiring immediate intubation and mechanical ventilation. Intravenous antibiotics were started. He had completely negative response to all anti-shock treatments including fluid resuscitation and vasopressor supports, and died 14 h later. Viral nucleic acid detection and metagenomic next-generation sequencing (mNGS) analyses of nasopharyngeal aspirate and blood specimens verified an HPIV-3 infection, with negative bacterial culture results. The HPIV-3 strain detected in this patient was subtyped as HPIV C3a, and two unreported amino acid mutations were found in the HN protein region.
Conclusion
The patient had a severe infection associated with HPIV-3, which was the cause of sepsis and septic shock. This study showed the diagnostic value of mNGS in etiological diagnosis, especially in severe neonatal case.
Similar content being viewed by others
Background
Sepsis is a systemic inflammatory syndrome triggered by a variety of pathogens (such as bacteria, viruses, and fungi), which can lead to severe sepsis, septic shock and even multiple organ dysfunctions [1]. Neonatal sepsis (NS) remains the third leading cause of mortality among neonates despite continuous improvements in neonatal medicine [2]. Although bacterial infections are the major etiological factors of sepsis, specimen detection turned out to be negative for bacteria in almost 42% sepsis patients [3]. In fact, viral sepsis was previously under-recognized due to limited diagnostic techniques for the detection and identification. Recently, a wide variety of viruses have been confirmed to cause sepsis with the application of different technology, including PCR and metagenomics next-generation sequencing (mNGS). Sepsis has been reported in children and adults caused by dengue fever virus, enterovirus, influenza virus, and herpes simplex virus [4]. A few studies have proposed that critically ill patients with severe pneumonia, acute respiratory distress syndrome (ARDS), sepsis, or myocarditis should be tested for influenza virus regardless of respiratory symptoms and epidemiology [5].
HPIVs are divided into types 1 to 4, which belong to two genera of HPIV, Respirovirus (HPIV-1 and HPIV-3) and Rubulavirus (HPIV-2 and HPIV-4) of the Paramyxoviridae family. Hospitalized children with acute respiratory tract infections had a high detection rate of HPIVs, ranging from 9 to 30%, only below the rate of respiratory syncytial virus (RSV) [6, 7]. Infection with HPIVs can present as asymptomatic, fever, cough, runny nose, wheezing, or apnea, with an incubation period ranging from 1 to 7 days [8, 9]. HPIVs can cause outbreaks of nosocomial infections in neonates and immunocompromised children, mainly characterized by respiratory symptoms and even respiratory failure in severe cases, and mechanical ventilation (MV) is an irreplaceable therapeutic measure for some critically ill children [10]. However, there is little evidence of HPIVs associated with neonatal sepsis.
In this paper, we report a neonatal case that showed a weak response to external stimuli, poor appetite and hematuria at onset, then rapidly progressed to septic shock and death. The etiological agent in this case was identified as HPIV-3 by mNGS method. The study suggested a potential link of neonatal sepsis with HPIV-3 and should be considered by clinicians. Meanwhile, mNGS has shown the value of clinical applications for infants with neonatal sepsis with unknown etiology.
Case presentation
This was a G2P2 (mother: gravida 2, para 2) baby boy with 8 days of age who was born by caesarean section at full term with birth weight 3660 g. His perinatal history was un-eventful. His mother had herpes zoster at 4–5 gestational weeks and common cold at 32 gestational weeks. No family history of genetic diseases was found in his families. Neither the mother nor the infant was found any lab evidence of vertically transmitted diseases, including HIV and CMV. The boy had a healthy elder brother (3 years old).
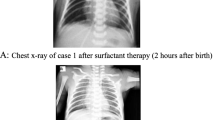
He started to have jaundice on his day of life 4 (DOL 04). The jaundice was slightly progressed but his condition was generally well, and no medical intervention was required. He suddenly presented gross hematuria on DOL 08 with unknown reason. Then he progressively presented gloomy spirit, inactivity and loss of appetite. There was no fever, hematochezia, ecchymosis, or petechiae. After 6 h (17:50, October 06), his parents took him to the Accident & Emergency (A&E) Department of Bei**g Children’s Hospital, Capital Medical University, Bei**g. He had septic shock when he arrived the A&E Department requiring immediate intubation and mechanical ventilation. Fluid resuscitation and intravenous antibiotics were then started. He was emergently transferred to the neonatal intensive care unit (NICU). His first hemoglobin (Hb) was 122 g/L when he arrived the A&E Department (18:00, October 06), but dropped to 45 g/L in 2 h (Ret 2.57%), requiring repeated packed red blood cell (pRBC) transfusion. His abdominal and chest X-ray found no specific abnormalities (shown in Fig. 1). During his stay in the NICU, he was given 2 times of pRBC (Table 1) due to refractory drop of Hb. No blood loss was found in brain, lungs, abdominal organs and intestine by bed-side ultrasound examination. A urethral catheter was inserted in the A&E Department, but no further urine was collected except for 3ml blood-look urine. His blood gas was severe refractory mixed acidosis. The coagulation test was prothrombin time (PT) 38.4s, fibrinogen (FIB) 0.5 g/L, activated partial prothrombin time (APTT) > 180s(normal range: 11–14 s, 2–4 g/L and 25–37 s, respectively), blood ammonia was 352 µmol/L (normal range: 18–72 µmol/L). He had completely negative response to all anti-shock treatments including fluid resuscitation and vasopressor supports, and died 14 h later.
The anterior-posterior abdominal and chest X-ray in NICU. The first anterior-posterior abdominal and chest X-ray after the patient was admitted to the NICU when he was intubated with low settings of mechanical ventilation. Lungs were normal. Gastric dilatation and slight sausage-like colon were observed. No chest fluid nor abdominal fluid sign was observed. Abbreviation: NICU, neonatal intensive care unit
Five days after death, his blood cultures were reported negative for bacteria, but pharyngeal swab and blood were positive for HPIV-3 virus nucleic acid. Whole exome sequencing did not discover any potential single gene disease or chromosome disorders, such as coagulation abnormalities, congenital metabolic diseases and immunodeficiency diseases. There was no abnormality of copy number variation sequencing (CNV-seq).
Nucleic acid detection by PCR
Nucleic acid testing was performed on pharyngeal swabs and serum samples from the patients. The nucleic acid was extracted using a nucleic acid extraction kit (Da An Gene, Guangzhou, China, Cat. DA0630) and automatic nucleic acid extractor instrument provided by Da An Gene Co., Ltd. The multiplex real-time PCR assay (XABT, Bei**g, China) was performed by LightCycler®480 PCR instrument (Roche, Basel, Switzerland). The multiplex PCR kit for 6 respiratory pathogens, including respiratory syncytial virus (RSV), adenovirus (ADV), influenza A virus (Flu A), influenza B virus (Flu B), human parainfluenza virus 1(HPIV-1) and human parainfluenza virus 3(HPIV-3). Both pharyngeal swab and serum sample of the patient showed a positive result in the fluorescent channel of HPIV-3 (Ct values were 17.31 and 35.76, respectively), with CT value less than 36.
Metagenomics next-generation sequencing (mNGS) for pathogen detection
Pharyngeal swabs and serum samples (collected on the day of admission) from the patients were sent to Tian** Novogene Medical Laboratory for mNGS. The nucleic acid was extracted using QIAamp Viral RNA Mini Kit (52,906, Qiagen Biotech, Germany). RNA libraires were constructed using TIANSeq Fast RNA Library Kit (NR102, Tiangen Biotech, Bei**g, China). After the removal of rRNA, RNA fragments of size 150–200 bp were obtained using the enzyme. The first strand cDNA was synthesized, and then the second strand cDNA was synthesized, followed by terminal repair and A-tailing reactions. Adapters were ligated onto the A-tailed fragments. Fragments with adapters were purified and amplified using PCR. After purifying the PCR product, the libraries were pooled and sequenced for 50 bp single ends on an Illumina NovaSeq 6000 machine. The data output of 40 M reads was obtained. Reads were then mapped against the human reference genome using Bowtie2 v2.3.5.1 [11]. After alignment, we removed human reads, the remaining reads were aligned with PD-seq™ database v1.0, which is a self-built database of 9,295 bacteria, 7,210 viruses, 412 fungi and 104 parasites. Kraken2 v2.1.2 [12] and Bracken software v2.6 [13] were used to further analyzed and annotated.
Due to the short time between onset and death, we did not have enough blood samples to detect mNGS, but HIPV-3 was the causative agent of this patient’ death according to the positive result by mNGS in pharyngeal swab and PCR in blood. Additionally, Acinetobacter baumannii (295 reads) and Trichosporon asahii (15 reads) were detected in pharyngeal swab, however bacterial culture of blood specimens was negative, so they are not considered to be the causal pathogens.
Viral molecular analysis
The resultant strain belongs to HPIV-3 and was named HPIV3/BCH/NM (shown in Fig. 2). The whole genome sequence is retrieved by mNGS method and has been deposited in GenBank (accession number: OQ785280). Clean Data were assembled with SOAP denovo software [14, 15]. Phylogenetic analysis of nucleotide sequence based on partial polyprotein gene (HN) or complete genomes of HPIV3/BCH/NM used Neighbor-Joining method with 1000 bootstrap replicates in MEGA 7.0 program. It showed that the HPIV3/BCH/NM strain belongs to Cluster C3a, which is the most common subtype in Bei**g. Nucleotide homologies analysis showed that the HPIV3/BCH/NM strain was closely related to the Human respirovirus 3 isolate C256 and HPIV3/06/ZJ/CHN/2017 from China. Comparing with the Wash/47,885/57 prototype strain of HPIV-3, Fourteen amino acid changes were identified in the HN protein region for the strain found in this study. Two unreported amino acid mutations (N86S and S512A) were found in the HN protein region. It is worth noting that S512A lie within a beta − 5 sheet of a key region for the HN protein and cell receptor binding [16].
Discussion
Infection with HPIV-3 is initiated by viral glycoprotein-mediated fusion between viral and host cell membranes. The envelope of HPIV-3 contains two viral glycoproteins: the hemagglutinin-neuraminidase (HN) and the fusion protein (F) [17]. In addition to its receptor-attaching and fusion-promoting functions, the HN protein is also a neuraminidase that promotes the release of virions from cells and prevent them from self-agglutinating. These functions have made it a key molecule when HPIV-3 infects a host cell. Further analysis of the amino acid substitutions in the HN protein region is crucial for virus infectivity and host cell tropism.
HN is a type II transmembrane protein, which perform a number of functions by coordination with its cytoplasmic domain, membrane-spanning region, stalk region, and a globular head. The receptor binding site is located at the center of the beta-propeller in a globular head for HPIV-3. The seven highly conserved amino acid residues (R192, D216, E409, R424, R502, Y530 and E549) is crucial for combination with N -acetylneuraminic acid (Neu5Ac) of sialic acid [18]. The HPIV-3 found in this study was sequenced and found no mutations at any of these sites. N86S and S512A, previously unreported amino acid mutations, were discovered in this study. The clinical impact of this mutation in HPIV-3 needs to be confirmed by long-term follow-up outcome studies.
The yearly Infection rates of HPIV ranged from 1.9 to 12 per 1000 children younger than 1 year [19], in which most of the illnesses were upper respiratory tract infections (40-60%) and some were lower respiratory tract infection (< 20%) including pneumonia and acute bronchitis [20, 21], Human parainfluenza virus 3 (HPIV-3) is the common pathogen causing respiratory disorders in infants and young children compared to other parainfluenza viruses [22]. Newborns and young infants are particularly susceptible to HPIV infection, numerous outbreaks of HPIV have been reported in the NICU [23, 24]. After the procedure of respiratory treatment, the majority of children with HPIV-3 have a favorable prognosis, with rare fatalities [23, 25].
HPIV infection has caused other systemic diseases in some cases. HPIV-1 and HPIV-3 can cause mumps-like disease reported by Bloom et al. [25]. It was previously reported that HPIV-3 and HPIV-4 were responsible for 62% and 10% of febrile seizures, respectively, and HPIV infections were associated with meningitis, encephalitis and ventriculitis [26,27,28]. MacDonald et al. have reported that HPIV infection can aggravate primary nephrotic syndrome [29]. The nucleocapsid-like structures of HPIV were detected in adults and children with hepatitis [30]. Meanwhile, HPIV infections have been reported to associate with myocarditis and pericarditis [31]. Kazuhiro et al. reported a fatal case of rhabdomyolysis in a child infected with HPIV-3 [32], and James et al. reported that HIPV-2 causes myoglobinuria in an adult [33]. However, there is no report of neonatal sepsis caused by HPIV-3.
Currently, there is no effective antiviral drug available for this pathogen. Symptomatic support is the main treatment for patients with HPIV-3 infection. Some studies suggest that patients can be treated with intravenous immunoglobulin (IVIG) and glucocorticoids [34]. Notably, DAS181 is originally used as a drug to treat seasonal influenza virus infections, which can block viral binding to receptors on host cells and prevent entry [35]. In immunodeficient patients with HPIV-3 infection, it exhibits good curative effects [36, 37]. Other antiviral drugs under development include HN inhibitors, bcx2798 and bcx2855. They act by competing for the binding site of virus invasion into host cells, and were shown to be highly effective in a humanized mouse model of infection [38, 39].
Most people with HPIV infection have a good prognosis, but in immunocompromised patients, such as those with blood system diseases or those who received hematopoietic stem cell transplantation, can lead to viremia, disseminated infection and even death. Hematopoietic stem cell transplants have been reported to have a mortality rate of 10 to 33% infected with HPIV [40,41,42].
Conclusion
We reported a rare neonatal death case of sepsis and septic shock caused by HPIV-3. mNGS was needed to make a rapid diagnosis of critical cases, especially in neonate. Further research is needed to understand the pathophysiology and risk factors of neonatal sepsis after HPIV infection, as well as the management of HPIV-3 mutation S512A.
Data availability
All data that support the findings of this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.
Abbreviations
- HPIV:
-
Human parainfluenza virus
- HPIV-3:
-
Human parainfluenza virus type-3
- MNGS:
-
Metagenomic next-generation sequencing
- NS:
-
Neonatal sepsis
- ARDS:
-
Acute respiratory distress syndrome
- HPIV-1:
-
Human parainfluenza virus type-1
- HPIV-2:
-
Human parainfluenza virus type-2
- HPIV-4:
-
Human parainfluenza virus type-4
- RSV:
-
Respiratory syncytial virus
- MV:
-
Mechanical ventilation
- DOL:
-
Day of life
- A&E:
-
Accident & Emergency
- NICU:
-
Neonatal intensive care unit
- Hb:
-
Hemoglobin
- pRBC:
-
Packed red blood cell
- PT:
-
Prothrombin time
- FIB:
-
Fibrinogen
- APTT:
-
Activated partial prothrombin time
- CNV-seq:
-
Copy number variation sequencing
- ADV:
-
Adenovirus
- Flu A:
-
Influenza A virus
- Flu B:
-
Influenza B virus
- HN:
-
Hemagglutinin-neuraminidase protein
- F:
-
Fusion protein
- Neu5Ac:
-
N -acetylneuraminic acid
- IVIG:
-
Intravenous immunoglobulin
References
Xu L, Gao H, Zeng J, Liu J, Lu C, Guan X, et al. A fatal case associated with respiratory syncytial virus infection in a young child. BMC Infect Dis. 2018;18(1):217. https://doi.org/10.1186/s12879-018-3123-8.
Fanaroff AA, Fanaroff JM. Advances in neonatal infections. Am J Perinatol. 2020;37(02):5. https://doi.org/10.1055/s-0040-1715584.
Lin GL, McGinley JP, Drysdale SB, Pollard AJ. Epidemiology and Immune Pathogenesis of viral Sepsis. Front Immunol. 2018;9:2147. https://doi.org/10.3389/fimmu.2018.02147.
Network SAIDCR. Causes and outcomes of sepsis in southeast Asia: a multinational multicentre cross-sectional study. Lancet Glob Health. 2017;5(2):e157–e67. https://doi.org/10.1016/s2214-109x(17)30007-4.
Kalil AC, Thomas PG. Influenza virus-related critical illness: pathophysiology and epidemiology. Crit Care. 2019;23(1). https://doi.org/10.1186/s13054-019-2539-x.
Singh-Naz N, Willy M, Riggs N. Outbreak of parainfluenza virus type 3 in a neonatal nursery. Pediatr Infect Dis J. 1990;9(1):31–3. https://doi.org/10.1097/00006454-199001000-00007.
Wang Y, Ji W. Relationship between common and new respiratory viruses and respiratory tract infection in children. Chin J Prev Med. 2011;45(03):266–9. https://doi.org/10.3760/cma.j.issn.0253-9624.2011.03.016.
Abedi GR, Prill MM, Langley GE, Wikswo ME, Weinberg GA, Curns AT, et al. Estimates of Parainfluenza Virus-Associated Hospitalizations and cost among children aged Less Than 5 years in the United States, 1998–2010. J Pediatr Infect Dis Soc. 2016;5(1):7–13. https://doi.org/10.1093/jpids/piu047.
Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344(25):1917–28. https://doi.org/10.1056/nejm200106213442507.
Harvala H, Gaunt E, McIntyre C, Roddie H, Labonte S, Curran E, et al. Epidemiology and clinical characteristics of parainfluenza virus 3 outbreak in a Haemato-oncology unit. J Infect. 2012;65(3):246–54. https://doi.org/10.1016/j.**f.2012.04.011.
Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357–9. https://doi.org/10.1038/nmeth.1923.
Wood DE, Lu J, Langmead B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019;20(1). https://doi.org/10.1186/s13059-019-1891-0.
Lu J, Breitwieser FP, Thielen P, Salzberg SL. Bracken: estimating species abundance in metagenomics data. PeerJ Comput Sci. 2017;3:e104. https://doi.org/10.7717/peerj-cs.104.
Li R, Li Y, Kristiansen K, Wang J. SOAP: short oligonucleotide alignment program. Bioinformatics. 2008;24(5):713–4. https://doi.org/10.1093/bioinformatics/btn025.
Li R, Zhu H, Ruan J, Qian W, Fang X, Shi Z, et al. De novo assembly of human genomes with massively parallel short read sequencing. Genome Res. 2010;20(2):265–72. https://doi.org/10.1101/gr.097261.109.
Aso J, Kimura H, Ishii H, Saraya T, Kurai D, Nagasawa K, et al. Molecular evolution of the hemagglutinin-neuraminidase (HN) gene in human respirovirus 3. Virus Res. 2020;277:197824. https://doi.org/10.1016/j.virusres.2019.197824.
Iorio RM, Field GM, Sauvron JM, Mirza AM, Deng R, Mahon PJ, et al. Structural and functional relationship between the receptor recognition and neuraminidase activities of the Newcastle disease virus hemagglutinin-neuraminidase protein: receptor recognition is dependent on neuraminidase activity. J Virol. 2001;75(4):1918–27. https://doi.org/10.1128/jvi.75.4.1918-1927.2001.
Chu FL, Wen HL, Hou GH, Lin B, Zhang WQ, Song YY, et al. Roles of conserved residues in the receptor binding sites of human parainfluenza virus type 3 HN protein. Microb Pathog. 2021;158:105053. https://doi.org/10.1016/j.micpath.2021.105053.
Counihan ME, Shay DK, Holman RC, Lowther SA, Anderson LJ. Human parainfluenza virus-associated hospitalizations among children less than five years of age in the United States. Pediatr Infect Dis J. 2001;20(7):646–53. https://doi.org/10.1097/00006454-200107000-00003.
Reed G, Jewett PH, Thompson J, Tollefson S, Wright PF. Epidemiology and clinical impact of parainfluenza virus infections in otherwise healthy infants and young children < 5 years old. J Infect Dis. 1997;175(4):807–13. https://doi.org/10.1086/513975.
Vesa S, Kleemola M, Blomqvist S, Takala A, Kilpi T, Hovi T. Epidemiology of documented viral respiratory infections and acute otitis media in a cohort of children followed from two to twenty-four months of age. Pediatr Infect Dis J. 2001;20(6):574–81. https://doi.org/10.1097/00006454-200106000-00006.
Fiore AE, Iverson C, Messmer T, Erdman D, Lett SM, Talkington DF, et al. Outbreak of pneumonia in a long-term care facility: antecedent human parainfluenza virus 1 infection may predispose to bacterial pneumonia. J Am Geriatr Soc. 1998;46(9):1112–7. https://doi.org/10.1111/j.1532-5415.1998.tb06649.x.
Ben-Shimol S, Landau D, Zilber S, Greenberg D. Parainfluenza virus type 3 outbreak in a neonatal nursery. Clin Pediatr (Phila). 2013;52(9):866–70. https://doi.org/10.1177/0009922812441674.
Maeda H, Haneda K, Honda Y. Parainfluenza virus type 3 outbreak in a neonatal intensive care unit. Pediatr Int. 2017;59(11):1219–22. https://doi.org/10.1111/ped.13389.
Bloom HJK, Jacobsen R. Recovery of parainfluenza viruses from adults with upper respiratory tract illnesses. Am J Epi. 1961;74:50–9.
Bitnun A, Ford-Jones EL, Petric M, MacGregor D, Heurter H, Nelson S, et al. Acute childhood encephalitis and Mycoplasma pneumoniae. Clin Infect Dis. 2001;32(12):1674–84. https://doi.org/10.1086/320748.
Downham MA, McQuillin J, Gardner PS. Diagnosis and clinical significance of parainfluenza virus infections in children. Arch Dis Child. 1974;49(1):8–15. https://doi.org/10.1136/adc.49.1.8.
Seidman DS, Nass D, Mendelson E, Shehtman I, Mashiach S, Achiron R. Prenatal ultrasonographic diagnosis of fetal hydrocephalus due to infection with parainfluenza virus type 3. Ultrasound Obstet Gynecol. 1996;7(1):52–4. https://doi.org/10.1046/j.1469-0705.1996.07010052.x.
MacDonald NE, Wolfish N, McLaine P, Phipps P, Rossier E. Role of respiratory viruses in exacerbations of primary nephrotic syndrome. J Pediatr. 1986;108(3):378–82. https://doi.org/10.1016/s0022-3476(86)80876-9.
Phillips MJ, Blendis LM, Poucell S, offterson J, Petric M, Roberts E, et al. Syncytial giant-cell hepatitis. Sporadic hepatitis with distinctive pathological features, a severe clinical course, and paramyxoviral features. N Engl J Med. 1991;324(7):455–60. https://doi.org/10.1056/nejm199102143240705.
Wilks D, Burns SM. Myopericarditis associated with parainfluenza virus type 3 infection. Eur J Clin Microbiol Infect Dis. 1998;17(5):363–5. https://doi.org/10.1007/bf01709464.
Ueda K, Robbins DA, Iitaka K, Linnemann CC. Jr. Fatal rhabdomyolysis associated with parainfluenza type 3 infection. Hiroshima J Med Sci. 1978;27(2):99–103.
O’Connor JV, Iyer SK. Myoglobinuria associated with parainfluenza type 2 infection. N Y State J Med. 1982;82(10):1469–70.
Branche AR, Falsey AR. Parainfluenza Virus infection. Semin Respir Crit Care Med. 2016;37(4):538–54. https://doi.org/10.1055/s-0036-1584798.
Triana-Baltzer GB, Sanders RL, Hedlund M, Jensen KA, Aschenbrenner LM, Larson JL, et al. Phenotypic and genotypic characterization of influenza virus mutants selected with the sialidase fusion protein DAS181. J Antimicrob Chemother. 2011;66(1):15–28. https://doi.org/10.1093/jac/dkq387.
Chen YB, Driscoll JP, McAfee SL, Spitzer TR, Rosenberg ES, Sanders R, et al. Treatment of parainfluenza 3 infection with DAS181 in a patient after allogeneic stem cell transplantation. Clin Infect Dis. 2011;53(7):e77–80. https://doi.org/10.1093/cid/cir501.
Drozd DR, Limaye AP, Moss RB, Sanders RL, Hansen C, Edelman JD, et al. DAS181 treatment of severe parainfluenza type 3 pneumonia in a lung transplant recipient. Transpl Infect Dis. 2013;15(1):E28–32. https://doi.org/10.1111/tid.12045.
Watanabe M, Mishin VP, Brown SA, Russell CJ, Boyd K, Babu YS, et al. Effect of hemagglutinin-neuraminidase inhibitors BCX 2798 and BCX 2855 on growth and pathogenicity of Sendai/human parainfluenza type 3 chimera virus in mice. Antimicrob Agents Chemother. 2009;53(9):3942–51. https://doi.org/10.1128/aac.00220-09.
Alymova IV, Watanabe M, Boyd KL, Chand P, Babu YS, Portner A. Efficacy of the novel parainfluenza virus haemagglutinin-neuraminidase inhibitor BCX 2798 in mice - further evaluation. Antivir Ther. 2009;14(7):891–8. https://doi.org/10.3851/imp1420.
Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome. Blood. 2001;98(3):573–8. https://doi.org/10.1182/blood.v98.3.573.
Wendt CH, Weisdorf DJ, Jordan MC, Balfour HH Jr, Hertz MI. Parainfluenza virus respiratory infection after bone marrow transplantation. N Engl J Med. 1992;326(14):921–6. https://doi.org/10.1056/nejm199204023261404.
Elizaga J, Olavarria E, Apperley J, Goldman J, Ward K. Parainfluenza virus 3 infection after stem cell transplant: relevance to outcome of rapid diagnosis and ribavirin treatment. Clin Infect Dis. 2001;32(3):413–8. https://doi.org/10.1086/318498.
Acknowledgements
The authors highly appreciate the parents of this baby boy for giving their consents to publish this manuscript.
Funding
This study was supported by the National Science and Technology Major Projects (grant number 2017ZX10104001-005-010), CAMS Innovation Fund for Medical Sciences (CIFMS) (Grant Number 2019–I2M–5–026), National Key Research and Development Program of China (Grant Number 2022YFC2704805), and Capital’s Funds for Health Improvement and Research (Grant Number CFH 222-2-2095).
Author information
Authors and Affiliations
Contributions
XPC and HW conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. QL, YJQ, FL and WWH collected data, carried out the initial analyses, and reviewed and revised the manuscript. QSW, FJ, YQG collected data and carried out the initial analyses. MYH and ZDX designed the data collection instruments, coordinated and supervised data collection, and critically reviewed the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethical Review Committee of Bei**g Children’s Hospital. Written informed consent for the further analysis that followed treatment of the patient and the sequencing that followed the routine care was obtained on the patient’s behalf from her parents.
Consent for publication
Written consent was obtained from the patient’s parents to publish the case report.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, X., Wang, H., Li, Q. et al. A fatal case of neonatal viral sepsis caused by human parainfluenza virus type 3. Virol J 20, 248 (2023). https://doi.org/10.1186/s12985-023-02141-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12985-023-02141-9