Abstract
Objectives
Enhanced neuroinflammation is an important mechanism underlying perioperative neurocognitive disorders. Regulatory T cells (Tregs) play a crucial role in regulating systemic immune responses. The present study was aimed to investigate the participation of Tregs in the development of postoperative cognitive dysfunction (POCD).
Methods
Surgery-associated neurocognitive disorder was induced in 18-month-old mice subjected to internal fixation of tibial fracture. Morris water maze was used to examine mice cognitive function. Splenic Tregs were collected for RNA sequencing and flow cytometry. Levels of inflammatory factors in the circulation and hippocampus were measured by enzyme-linked immunosorbent assay. Protein presences of tight junction proteins were detected by immunofluorescence.
Results
Surgery of internal fixation of tibial fracture induced cognitive impairment in aged mice, accompanied by elevated plasma levels of inflammatory factors and increased circulating Tregs. Transfusion of Tregs from young mice partially restored the structure of the blood–brain barrier and alleviated POCD in aged mice. Compared with young Tregs, differentially expressed genes in aged Tregs were enriched in tumor necrosis factor (TNF) signaling pathway and cytokine–cytokine receptor interaction. Flow cytometry revealed that aged Tregs had blunted functions under basal and stimulated conditions. Blockade of the CD25 epitope protected the blood–brain barrier structure, reduced TNF-α levels in the hippocampus, and improved surgery-associated cognition in aged mice.
Conclusions
Blocking peripheral regulatory T cells improves surgery-induced cognitive function in aged mice. Therefore, aged Tregs play an essential role in the occurrence of POCD.
Similar content being viewed by others
Introduction
Postoperative cognitive dysfunction (POCD) is presented with impaired learning capacity, memory loss, confusion, anxiety, and personality changes. It is reported that 25.8% of patients exhibit some cognitive disorder in the 1st week after surgeries, of which 9.9% of senile patients remain cognitive impairment longer than 3 months [1]. Aging is a critical risk factor for the occurrence of POCD, as suggested by its features of cognitive impairment which are comparable to those in disease conditions, including neurodegenerative disease [2, 3].
The blood–brain barrier (BBB) provides primary protection for the central nervous system (CNS) against pathological stimuli [4]. Tight junction proteins in vascular endothelial cells of the BBB, including claudin1 and claudin5, is crucial for preventing harmful solutes from passing to the central nervous system [5]. Reduced expressions of junction proteins alter the permeability of the blood–brain barrier, resulting in overspills of plasma proteins and invasion of peripheral cells as well as pathogens [2, 6, 7]. The presence of lymphocytes in the brains of Alzheimer’s patients [8] and aged mice [9] is associated with a disruption of the blood–brain barriers, leading to sterile neuroinflammation in the central nervous system. Of importance, elevated levels of interleukin-6 (IL-6), C-reactive protein, and chitinase 3-like protein in cerebrospinal fluid of aged patients [10] are positively correlated with their cognitive disorders [8], suggesting that chronic neuroinflammation takes part in cognitive impairment [11,12,13].
Regulatory T cells (Tregs), characterized by high expressions of cluster of differentiation 4 (CD4), CD25 (also known as interleukin 2 receptor alpha), and forkhead box protein P3 (Foxp3), play an obligatory role in immune homeostasis by suppressing excessive immune responses [14]. However, the role of Tregs in the central nervous system is inconclusive [15, 16]. In Alzheimer’s mice, transient depletion of Tregs promotes β-amyloid plaque clearance by inducing leukocytes recruitment through the choroid plexus [17], but accelerates memory loss by limiting the recruitment of microglia toward amyloid plaques [18]. It is also reported that Tregs inhibit astrogliosis and promote neural recovery [19], but impair cerebral microvasculature [20] in a mouse stroke model.
Thus, the present study was designed to investigate the participation of Tregs in a mouse POCD model subjected to tibial fractures internal fixation surgery.
Materials and methods
Animals
Male C57BL/6 mice, 18-month-old and 10-week-old, were purchased from Shanghai Jiesijie Company (Shanghai, China). B6.129(Cg)-Foxp3tm4(YFP/icre)Ayr/J (Foxp3YFP) transgenic mice were used on the C57BL/6 background (Cyagen, China). Mice had free access to food and water. All animals were housed separately under specific pathogen-free conditions with 12-h light/dark cycles in the Laboratory Animal Unit of Zhongshan Hospital, Fudan University (Shanghai, China). The experimental design was approved by the Animal Ethics Committee of Zhongshan Hospital, Fudan University [SYXK (Shanghai) 2021-#0022].
POCD model and Tregs intervention
The POCD model was built by conducting internal fixation of tibial fractures as previously described [13]. Briefly, mice were anesthetized with 1% sodium pentobarbital (8 mg/kg, intraperitoneal injection). A 0.3–0.6 cm vertical incision was made near the tibial tubercle, followed by a needle insertion into the tibial tubercle. After the surgery, butorphanol (2 mg/kg) was administered subcutaneously to relieve the pain.
To block Tregs function, an anti-CD25 antibody (500 µg/mouse; 553864, BD Pharmingen, USA) and its isotopic antibody were administered intraperitoneally [21, 22] (Fig. 1A).
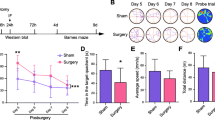
Aged mice have impaired cognitive function after surgery. A Experimental scheme of the research. B Escape latency of young and old mice after surgery. C Number of crossing the target platform of young and old mice after surgery. *P < 0.05 n = 6–9. D Representative tracing in the swimming pool on the test day. Yellow blocks in the pool indicated the target platform in the acquisition training. E, F. Number of peripheral Tregs in young and old mice after surgery. *P < 0.05 n = 5–7
To increase peripheral Tregs, all-trans-retinoic acid (ATRA, 8 mg/kg for young mice, 4 mg/kg for aged mice; R2625, Sigma, Germany) was injected intraperitoneally every 48 h for a week. [23] Four injections of ATRA significantly increased counts of splenic CD4+CD25+Foxp3+ Tregs, but not CD4 or CD8 cells in young mice. However, ATRA had lethal effects on aged mice (Additional file 1: Fig. S1A–C). Therefore, transfusion of Tregs was used to increase peripheral Tregs in the present study. Splenic Tregs from young or aged mice were isolated by a regulatory T cell isolation kit (130-091-041, Miltenyi Biotec, Germany) and verified by flow cytometry (Additional file 1: Fig. S2). A total of 2 × 106 Tregs were intravenously injected through the tail vein 1 day before the surgery [24]. Aged mice were transfused with Tregs from young or aged mice (Fig. 1A).
Morris water maze
The hippocampus-dependent spatial learning and memory capacity were examined by the Morris water maze test [2, 25, 26]. In brief, mice were acclimated to the maze for 3 days. Spatial acquisition training was conducted for 5 consecutive days (D3–D7). In the Morris test (D8), swimming paths, counts of the target platform crossing, and time spent on each quadrant were documented (Jiliang, China) (Fig. 1A).
Flow cytometry
To obtain Tregs from spleens, spleens were carefully collected after mice were anesthetized. The spleens were grounded and filtered through a 70 µm filter for single-cell suspensions. Tregs were incubated with surface antibodies and viability stain (565388, BD Pharmingen, USA) for 30 min at 4 °C. After permeabilization with Foxp3/Transcription Factor Staining Buffer (00-5523-00, Thermo Fisher, USA), the cells were further incubated with intracellular antibodies for 30 min at 4 °C (Table 1). A separate group of cells was challenged with a leukocyte activation cocktail (0.2 µl/106 cells; 550583, BD Pharmingen, USA) containing phorbol 12-myristate-13-acetate (PMA), ionomycin, and brefeldin-A for 4 h, following the protocol of the manufactory. The flow cytometry assays were performed on a BD FAC Symphony (BD Germany).
Enzyme-linked immunosorbent assay (ELISA)
Mice hippocampal tissue (10 mg) or plasma (100 µl) were collected for ELISA. Levels of cytokines in plasma and hippocampal tissues were measured using Bioplex suspension chip reagent Bio-Plex Pro Mouse Cytokine 23-plex (M60009RDPD, Biorad, USA) and normalized with protein concentration in samples.
Gene-expression profiling assay
Gene-expression profiling assays on splenic Tregs, collected from both young and aged mice, were performed by the Shanghai Institute of Immunology. The gene expression files were analyzed with R-3.4.1 software. Differentially expressed genes (DEGs) were defined when an adjusted P value was less than 0.05. DEGs were calculated with the limma package [27]. Database for Annotation, Visualization and Integrated Discovery (v6.8) was used to analyze gene function and potential pathways [28]. Bubble Plots were performed by the ggplot2 package [29]. Ligand–receptor interaction analysis was performed using the iTALK package [30].
Immunofluorescence
To examine the blood–brain barrier permeability, 40 kDa dextran (20–25 mg/kg; D1829 Thermo Fisher, USA) was injected via the tail vein 24 h before the euthanization. After anesthetizing with pentobarbital, the mice were transcardially perfused with 40 ml ice-cold PBS for 20 min. Brain samples were dehydrated in the 30% (w/v) sucrose solution and embedded in an optimum cutting temperature compound (OCT, 4583, Sakura, USA). The frozen brain tissue was prepared in 5-μm thickness. Brain slides were blocked with 5% goat serum and then incubated with primary antibodies, anti-CD31 (24590, Abcam, UK), anti-claudin1 (15098, Abcam, UK), and anti-claudin5 (15106, Abcam, UK), overnight at 4 °C. On the 2nd day, slides were incubated with secondary antibodies for 1 h at 37 °C. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) for 10 min at 37 °C. Images were taken using a fluorescence microscope (Olympus BX51, Japan).
The presence of Tregs in the hippocampus was examined by immunofluorescence in two approaches. Tregs from Foxp3YFP mice, in which the Foxp3 protein was knocked in a yellow fluorescent protein, were injected into aged mice subjected to the surgery. The YFP signal was detected in the choroid plexus, but not the hippocampi of the mice. (Fig. 1A, Additional file 1: Fig. S3). In addition, brain slides were also incubated with primary antibodies, anti-CD4 (557307, BD, USA) and anti-Foxp3 (NB100-39002SS, Novus, USA).
Statistical analysis
Prism 9 (GraphPad, USA) software was used in the present study. Data are presented as means ± SEM. Flow cytometry data were analyzed with FlowJo v10.0.8 (BD, USA). The statistical analysis was done by one-way ANOVA followed by post hoc Bonferroni comparison. The comparison between Tregs with or without PMA/Ionomycin stimulation was performed by paired t test. P < 0.05 was considered statistically significant.
Results
Aged mice subjected to the surgery exhibit cognitive dysfunction
In the acquisition course, surgery significantly increased escape latency in aged mice, but not young mice (Fig. 1B). In the maze test, the surgery did not affect cognitive scores in young mice, but significantly reduced target platform crossings in aged mice compared with their age-matched counterparts (Fig. 1C, D).
In aged mice, but not young ones, the surgery of internal fixation of tibial fractures significantly increased splenic Tregs, and the increase occurred since day 1 (Fig. 1E, F).
In the maze test, aged mice transfused with young Tregs had more crossings on the target platform than those transfused with aged Tregs (Fig. 2A, B). Transfusion with young Tregs, but not aged Tregs, increased protein presence of junction protein claudin1 and claudin5 in the CA3 region of the hippocampus of aged mice (Fig. 2C-F). Transfused Tregs were not detected in the hippocampi of aged mice (Additional file 1: Fig. S3). In addition, transfusion with young Tregs accelerated the swimming speed of aged mice (Additional file 1: Fig. S4).
Tregs transfusion changes mice cognitive function and structure of the blood–brain barrier. A Escape latency of old mice with Tregs transfusion. B Counts of crossing the target platform of old mice with Tregs transfusion. *P < 0.05 n = 6. Quantification of claudin1 (C) and claudin5 (D) in the CA3 region of the hippocampus. Representative immunofluorescence signals of claudin1 (E) and claudin5 (F). DAPI labeled the nuclei (blue), CD31 labeled endothelial cells (red), and claudin1 or claudin5 stained green. Magnification, ×200, *P < 0.05 n = 6
Of note, ARTA administration, a pharmacological approach to increase peripheral Tregs, had lethal effects on aged mice (Additional file 1: Fig. S1).
Aged mice possess impaired Tregs
To explore Tregs functions in aging, splenic Tregs of young and aged mice were collected for RNA-sequencing and flow cytometry.
A total of 2910 DEGs were identified in Tregs of young and aged mice (Fig. 3A). Kyoto Encyclopedia of Genes and Genomes pathway analysis revealed that DEGs were enriched in cytokine–cytokine receptor interaction, Chagas disease, tumor necrosis factor (TNF) signaling pathway, Salmonella infection, and NF-kappa B signaling pathway (Fig. 3B). Ligand–receptor interaction analysis confirmed that TNF was paired with upregulated tumor necrosis factor receptor superfamily member (TNFRSF) 1B and downregulated TNFRSF21, while upregulated TNFSF13B was paired with downregulated TNFRSF13C. In addition, increased expressions of transforming growth factor-β1 (TGF-β1) corresponded with downregulated TGF-β1 receptors 1 and 2 (TFGBR1 and TGFBR2). Interleukin (IL)-1β, IL-12, and IL-17 were paired with IL-1 receptor2 (IL-1R2), IL-12 receptor B1 (IL12B1), and IL-17 receptor (IL17RA), respectively. C–C motif ligand (CCL) 3 was paired with downregulated C–C motif chemokine receptor (CCR) 3 and CCR4, as well as upregulated CCR1 and CCR5. CCL4 was paired with downregulated CCR4 and upregulated CCR1, CCR5, and CCR8 (Fig. 3C).
Flow cytometry revealed that aged mice had higher counts of CD4+CD25+Foxp3+ Tregs and CD4+CD25-Foxp3+ cells than their young counterparts. Under the basal condition, compared with young mice, aged mice had increased protein expressions of 5′-nucleotidase (5′-NT), CCR4, IL-10, ectonucleoside triphosphate diphosphohydrolase 1 (NTPDase 1), programmed cell death protein 1 (PD-1), and TNFRSF18, reduced expressions of interferon regulatory factor-4 (IRF-4) and leucine-rich repeat-containing 32 (LRRC32), and unchanged expressions of cytotoxic T-lymphocyte protein 4 (CTLA-4), IL-2, zinc finger protein Helios, lymphocyte activation gene 3 protein (LAG-3), and TNFRSF4. Combined stimulation of PMA and ionomycin induced comparable expressions of CCR4, CTLA-4, Granzyme B, IRF-4, LRRC32, and TNFRSF18 in Tregs of both young and old mice. The stimulation did not increase 5′-NT, NTPDase 1, IL-10, Perforin-1, TGF-β1, or TNFRSF4 protein expressions in the aged Tregs. The simulation significantly increased IL-2 expression in aged, but not in young, ones (Fig. 4, Additional file 1: Fig. S5).
Changes of candidate proteins in Tregs under basal and stimulated conditions. A Tregs and CD25-Foxp3 cells proportion in CD4+ cells. Changes of candidate proteins B 5-NT, C CCR4, D CTLA-4, E GranzymeB, F IL-2, G IL-10, H IRF-4, I LRRC32, J NTPDase, K PD-1, L Perforin-1, M TGF-β, N TNFRSF4, and O TNFRSF18 in Tregs under basal and stimulated conditions. *P < 0.05 n = 6
Blocking the CD25 molecule improves cognitive function in aged mice subjected to surgery
Taken together with data on Tregs dysfunction in aged mice and their impaired cognitive performance, it is plausible that Tregs play a role in the occurrence of POCD in aged mice. To further study the participation of Tregs in POCD, the anti-CD25 antibody was applied in aged mice [31, 32]. Blockade of CD25 did not affect the body weight or general condition in aged mice (Additional file 1: Fig. S6).
Blocking CD25 reduced the escape latency in aged mice compared with those administered with the isotopic antibody (Fig. 5A). In the Morris test, administration with the anti-CD25 antibody significantly increased crossing counts in the target platform than those with the isotopic antibody (Fig. 5B, C).
Tregs ablation restores POCD in aged mice. A Escape latency in mice. B Number of crossing the target platform. C Representative tracing in the swimming pool on the test day (D8). D Protein expression of TNF-α of mice plasma and hippocampi after the surgery. E Protein expression of CXCL1 of mice plasma and hippocampi after the surgery *P < 0.05 vs. D0, #P < 0.05 vs. Surgery + IgG on the same day. n = 5–11
The surgery transiently increased plasma levels of IL-1β, IL-6, IL-10, TNF-α, granulocyte–macrophage colony-stimulating factor (GM-CSF), interferon-γ (IFN-γ), CCL2 and C–X–C motif chemokine 1 (CXCL1) expression on day 1. Blockade of the CD25 epitope increased plasma TNF-α level when compared with the isotopic antibody, but did not affect plasma levels of other inflammatory factors (Fig. 5D, E. Additional file 1: Fig. S7).
In mouse hippocampus, the surgery significantly and consistently increased IL-3, IL-4, IL-5, IL-6, IL-10, IL-12, IL-17, TNF-α, GM-CSF, IFN-γ, CCL2, CCL3 and CXCL1 levels. Blockade of the CD25 epitope partially reduced IL-3, TNF-α, and CXCL1 levels in the hippocampus of aged mice but did not affect levels of other inflammatory factors (Fig. 5D, E, Additional file 1: Fig. S7).
Fluorescent signals of Tregs, CD4, CD25, or Foxp3, were not detected in the brain in young or aged mice subjected to the surgery (Additional file 1: Fig. S3).
BBB permeability was evaluated by fluorescent signals of low-molecule dextran in hippocampi. The signal of low-molecular dextran was observed in the hippocampus of aged mice subjected to the surgery. Compared with isotopic antibody administration, CD25 blockade significantly reduced the fluorescent signals (Fig. 6A). The junction protein, claudin1 and claudin5, were co-localized with CD31+ endothelial cells in the CA3 region of the hippocampus. The surgery significantly reduced the protein presence of junction proteins in mice administrated with the isotype antibody. Compared to the isotope antibody group, CD25 blockade significantly increased the protein presence of claudin1 and claudin5 (Fig. 6B, C).
Tregs ablation restores the structure of the blood–brain barrier in aged mice. A Fluorescence signals and quantification of 40 kDa dextran in the mouse hippocampus. DAPI labeled the nuclei (blue), CD31 labeled endothelial cells (green), and dextran stained red (Texas red). Immunofluorescence signals and quantification of claudin1 (B) and claudin5 (C) in the CA3 region of the hippocampus. DAPI labeled the nuclei (blue), CD31 labeled endothelial cells (red), claudin1 or claudin5 stained green. Magnification, ×200, *P < 0.05 n = 6
Discussion
The present study reports that aging deteriorates Tregs function and impacts mice cognition when subjected to the surgery of internal fixation of tibial fractures. The surgery-associated cognitive impairment in aged mice contributes to the disruption of the blood–brain barrier and enhanced sterile inflammation in the central nervous system. Blocking the CD25 epitope protects the blood–brain barrier, downregulates inflammation in the hippocampus, and restores cognitive function.
Taken into consideration that aged mice had worse cognition than young mice [2, 25], the present study reported that surgery further deteriorates cognitive function in aged mice, supporting the notion that surgery, as an exogenous stimulus, is a potential risk of cognitive dysfunction in the geriatric population [33, 34]. The involvement of Tregs in the development of cognitive impairment was first reported in 2006 [35]. Tregs function was modified by aging, as shown by the abundant differently expressed genes which have extensive involvement in biological processes and the altered responses to stimuli in flow cytometry examination. Together with increased counts of peripheral Tregs upon the surgery, the findings imply that aged Tregs participate in the development of cognitive dysfunction [36, 37]. Indeed, transfusion with young Tregs partially restores cognition in aged mice. Noted, blocking the CD25 epitope [38], the characteristic marker of Tregs, improved cognitive function in aged mice. Thus, the present study provides substantial evidence that aged Tregs are critical to cognitive dysfunction.
Based on the DEGs of aged Tregs and the associated dysfunction from FACS study, aged Tregs are detrimental, especially challenged under pathological conditions. Tregs-mediated immune suppression includes cytolysis, metabolic disruption, and secretion of anti-inflammatory cytokines [39]. Both NTPDase 1 and 5′-NT are cell-surface proteins, in which NTPDase 1 hydrolyzes extracellular ATP and ADP to AMP [40], and 5′-NT converts AMP to anti-inflammatory adenosine [41]. Although aged Tregs had compensatory higher expressions of NTPDase 1 and 5′-NT under the basal condition, the nonresponsiveness of these two molecules in the stimulated state reinforces the incompetence of aged Tregs [41,42,43].
Tregs participate in cytolysis through Granzyme B and Perforin-1. In the present study, aged Tregs did not increase Perforin-1 expression in response to the stimuli, although Granzyme B expressions were comparable to that of young Tregs, suggesting that the cytotoxic effects of aged Tregs are also blunted [39].
Aged Tregs did not produce stimulated TGF-β1 [44], despite its key regulator LRRC32 was upregulated [45]. Together with the unresponsiveness of IL-10 in aged mice, the present results confirmed the impairment of Tregs in aged mice [46].
Both CTLA-4 and PD-1 are immune checkpoint proteins. Loss of CTLA-4 results in massive lymphocyte proliferation [47]. Increased PD-1 expression exhausts T-cell function [48, 49]. Aged Tregs had increased expression of PD-1 under both basal and stimulated conditions, implying the detrimental role of aged Tregs.
In addition, IL-2 production was higher in stimulated aged Tregs, suggesting that aged Tregs possess pro-inflammatory features. In line, aged Tregs had reduced basal expressions of IRF-4 and increased stimulated CCR4 expression in flow cytometry examination. CCR4 is a critical receptor for chemokines, and IRF-4 is associated with enhanced immunosuppression and differentiation of effector Tregs [50] via regulating IL-17, IL-21 [51], and IL-4 [52] production.
The strategy of CD25 blockade has been used to investigate the role of Treg in renal ischemia–reperfusion injury [53], pancreatic intraepithelial neoplasms [38], and mesothelioma model [54]. In the present study, blocking CD25 increased the plasma TNF-α level in aged mice subjected to the surgery, confirming the immunosuppressive role of Tregs in the circulating immune system. Three TNF receptor superfamily members were identified in the RNA sequencing analyses, including TNFRSF1B, TNFRSF21, and TNFRSF13C. TNFRSF1B, also known as TNFR2, is strictly expressed in neurons, oligodendrocytes, myeloid-derived suppressor cells, Tregs, and monocytes [55,56,57,58,59]. TNFRSF1B mediates Tregs proliferation and function [60, 61]. Thus, the upregulated expression of TNFRSF1B exacerbates inflammatory responses [62,63,64]. TNFRSF21 is involved in regulating T helper cells, while TNFRSF13C regulates B cells. Furthermore, the increased basal expression of TNFRSF18 and nonresponsiveness of TNFRSF4 in aged Tregs in flow cytometry study indicate that the immunosuppressive effects of Tregs on inflammatory responses are compromised, since TNFRSF18 [65] and TNFRSF4 [66] are responsible for the Tregs suppression and differentiation [67].
Indeed, the surgery transiently increased plasma levels of inflammatory factors, supporting that the surgery per se is an inflammatory stimulus for patients. However, hippocampal levels of TNF-α, CXCL1, IL-1, IL-3, IL-4, IL-5, IL-6, IL-10, IL-12, and IL-17 were maintained at higher levels, indicating that the immune status in the CNS is not synchronized. The increased levels of cytokines in the hippocampus confirmed that enhanced inflammation is a crucial player in POCD. More important, the protracted inflammatory responses in the hippocampus partially explain the prolonged cognitive impairment in senile patients subjected to surgeries. The presence of Tregs in the central nervous system has been reported in mice after ischemic stroke [19] and in mice with experimental autoimmune encephalomyelitis [68]. Nevertheless, Tregs were not detected in the hippocampus in the present study. Therefore, the divergent changes of cytokines in the present study are probably attributed to the anatomic structure of the blood–brain barrier.
The blood–brain barrier provides primary protection by limiting the solutes in the circulating blood to the extracellular fluid of the central nervous system. In the present study, the increased signals of low-molecule dextran and reduced presence of tight junction proteins in aged mice following the surgery confirm the blood–brain barrier disruption. Treatment of the CD25 antibody restored the protein presence of junction proteins, leading to reduced TNF-α and CXCL1 levels in the hippocampus. The reduced levels of inflammatory factors in mice hippocampi, especially TNF-α, reinforce the crucial role of TNF-α and its pertinent receptors in aged Tregs in POCD [69, 70]. It further demonstrates that Tregs blockade results in cognition-protective effects through modulating TNF-α levels and BBB structures in the hippocampus in aged mice challenged with surgery. Thus, screening for protective substances produced by the blockade of the CD25 molecule and dissecting the mechanisms underlying the upregulation of endothelial junction proteins would enhance our understanding of the connection between immune status in circulating blood and the central nervous system.
In addition, CD4+CD25-Foxp3+ cells, another suppressive group of lymphocytes [71], were also accumulated in aged mice. The involvement of CD4+CD25-Foxp3+ cells in surgery-associated cognitive dysfunction has not been reported and deserves further investigation.
In the present study, transfusion with young Tregs improved swimming velocity in aged mice. The heterochronic parabiosis of young cells in aged mice has been extensively studied [72, 73]. In a mouse model of human Duchenne muscular dystrophy, muscle injury and inflammation is mitigated by Tregs expansion, but exacerbated by Treg depletion [74]. The graft Tregs-exerted protection is probably attributed to tissue repair by acting on parenchymal cells directly [73, 75,76,77].
In conclusion, surgery-associated cognitive decline in aged mice is attributed to Tregs dysfunction. Blocking the CD25 molecule protects the blood–brain barrier, downregulates inflammation in the hippocampus, and restores cognitive function in aged mice. The results of the present study provide a therapeutic strategy for postoperative cognitive dysfunction in the geriatric population.
Availability of data and materials
The RNA-sequencing data produced in the present study were uploaded to the NCBI–GEO database. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE208231.
References
Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International study of post-operative cognitive dysfunction. Lancet. 1998;351(9106):857–61.
Liang T, Ju H, Zhou Y, Yang Y, Shi Y, Fang H. Inhibition of glycogen synthase kinase 3beta improves cognitive function in aged mice by upregulating claudin presences in cerebral endothelial cells. Acta Biochim Biophys Sin (Shanghai). 2020;52(4):363–70.
Robinson TN, Raeburn CD, Tran ZV, Angles EM, Brenner LA, Moss M. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg. 2009;249(1):173–8.
Yang T, Velagapudi R, Terrando N. Neuroinflammation after surgery: from mechanisms to therapeutic targets. Nat Immunol. 2020;21(11):1319–26.
Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57(2):173–85.
Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood–brain barrier. Nat Med. 2013;19(12):1584–96.
Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and dysfunction of the blood–brain barrier. Cell. 2015;163(5):1064–78.
Gate D, Saligrama N, Leventhal O, Yang AC, Unger MS, Middeldorp J, et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature. 2020;577(7790):399–404.
Dulken BW, Buckley MT, Navarro Negredo P, Saligrama N, Cayrol R, Leeman DS, et al. Single-cell analysis reveals T cell infiltration in old neurogenic niches. Nature. 2019;571(7764):205–10.
Vasunilashorn SM, Ngo LH, Dillon ST, Fong TG, Carlyle BC, Kivisakk P, et al. Plasma and cerebrospinal fluid inflammation and the blood–brain barrier in older surgical patients: the Role of Inflammation after Surgery for Elders (RISE) study. J Neuroinflamm. 2021;18(1):103.
Bettcher BM, Tansey MG, Dorothee G, Heneka MT. Peripheral and central immune system crosstalk in Alzheimer disease—a research prospectus. Nat Rev Neurol. 2021;17(11):689–701.
Brosseron F, Kolbe CC, Santarelli F, Carvalho S, Antonell A, Castro-Gomez S, et al. Multicenter Alzheimer’s and Parkinson’s disease immune biomarker verification study. Alzheimers Dement. 2020;16(2):292–304.
Ju H, Wang Y, Shi Q, Zhou Y, Ma R, Wu P, et al. Inhibition of connexin 43 hemichannels improves postoperative cognitive function in aged mice. Am J Transl Res. 2019;11(4):2280–7.
Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–87.
Wang HY, Ye JR, Cui LY, Chu SF, Chen NH. Regulatory T cells in ischemic stroke. Acta Pharmacol Sin. 2022;43(1):1-9.
Carrasco E, Gomez de Las Heras MM, Gabande-Rodriguez E, Desdin-Mico G, Aranda JF, Mittelbrunn M. The role of T cells in age-related diseases. Nat Rev Immunol. 2022;22(2):97-111.
Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159(4):709–13.
Dansokho C, Ait Ahmed D, Aid S, Toly-Ndour C, Chaigneau T, Calle V, et al. Regulatory T cells delay disease progression in Alzheimer-like pathology. Brain. 2016;139(Pt 4):1237–51.
Ito M, Komai K, Mise-Omata S, Iizuka-Koga M, Noguchi Y, Kondo T, et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature. 2019;565(7738):246–50.
Kleinschnitz C, Kraft P, Dreykluft A, Hagedorn I, Gobel K, Schuhmann MK, et al. Regulatory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood. 2013;121(4):679–91.
Arce Vargas F, Furness AJS, Solomon I, Joshi K, Mekkaoui L, Lesko MH, et al. Fc-optimized anti-CD25 depletes tumor-infiltrating regulatory T cells and synergizes with PD-1 blockade to eradicate established tumors. Immunity. 2017;46(4):577–86.
Setiady YY, Coccia JA, Park PU. In vivo depletion of CD4+FOXP3+ Treg cells by the PC61 anti-CD25 monoclonal antibody is mediated by FcgammaRIII+ phagocytes. Eur J Immunol. 2010;40(3):780–6.
Baruch K, Rosenzweig N, Kertser A, Deczkowska A, Sharif AM, Spinrad A, et al. Breaking immune tolerance by targeting Foxp3(+) regulatory T cells mitigates Alzheimer’s disease pathology. Nat Commun. 2015;6:7967.
Wang L, Zhou Y, Yin J, Gan Y, Wang X, Wen D, et al. Cancer exacerbates ischemic brain injury via Nrp1 (neuropilin 1)-mediated accumulation of regulatory T cells within the tumor. Stroke. 2018;49(11):2733–42.
Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1(2):848–58.
McAlpine CS, Park J, Griciuc A, Kim E, Choi SH, Iwamoto Y, et al. Astrocytic interleukin-3 programs microglia and limits Alzheimer’s disease. Nature. 2021;595(7869):701–6.
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7): e47.
Dennis G Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, et al. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4(5):P3.
Valero-Mora PM. ggplot2: elegant graphics for data analysis. J Stat Softw. 2010. https://doi.org/10.18637/jss.v035.b01.
Wang Y, Wang R, Zhang S, Song S, Jiang C, Han G, et al. iTALK: an R package to characterize and illustrate intercellular communication. bioRxiv. 2019. 2019;507871:1–8.
Stubbe T, Ebner F, Richter D, Engel O, Klehmet J, Royl G, et al. Regulatory T cells accumulate and proliferate in the ischemic hemisphere for up to 30 days after MCAO. J Cereb Blood Flow Metab. 2013;33(1):37–47.
Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299(5609):1033–6.
Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456–66.
Daiello LA, Racine AM, Yun Gou R, Marcantonio ER, **e Z, Kunze LJ, et al. Postoperative delirium and postoperative cognitive dysfunction: overlap and divergence. Anesthesiology. 2019;131(3):477–91.
Gartner D, Hoff H, Gimsa U, Burmester GR, Brunner-Weinzierl MC. CD25 regulatory T cells determine secondary but not primary remission in EAE: impact on long-term disease progression. J Neuroimmunol. 2006;172(1–2):73–84.
Garg SK, Delaney C, Toubai T, Ghosh A, Reddy P, Banerjee R, et al. Aging is associated with increased regulatory T-cell function. Aging Cell. 2014;13(3):441–8.
Lages CS, Suffia I, Velilla PA, Huang B, Warshaw G, Hildeman DA, et al. Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. J Immunol. 2008;181(3):1835–48.
Keenan BP, Saenger Y, Kafrouni MI, Leubner A, Lauer P, Maitra A, et al. A Listeria vaccine and depletion of T-regulatory cells activate immunity against early stage pancreatic intraepithelial neoplasms and prolong survival of mice. Gastroenterology. 2014;146(7):1784-94 e6.
Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8(7):523–32.
Zhao J, Cao Y, Lei Z, Yang Z, Zhang B, Huang B. Selective depletion of CD4+CD25+Foxp3+ regulatory T cells by low-dose cyclophosphamide is explained by reduced intracellular ATP levels. Cancer Res. 2010;70(12):4850–8.
Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204(6):1257–65.
Alam MS, Cavanaugh C, Pereira M, Babu U, Williams K. Susceptibility of aging mice to listeriosis: role of anti-inflammatory responses with enhanced Treg-cell expression of CD39/CD73 and Th-17 cells. Int J Med Microbiol. 2020;310(2): 151397.
He N, Fan W, Henriquez B, Yu RT, Atkins AR, Liddle C, et al. Metabolic control of regulatory T cell (Treg) survival and function by Lkb1. Proc Natl Acad Sci USA. 2017;114(47):12542–7.
Turner JA, Stephen-Victor E, Wang S, Rivas MN, Abdel-Gadir A, Harb H, et al. Regulatory T cell-derived TGF-beta1 controls multiple checkpoints governing allergy and autoimmunity. Immunity. 2020;53(6):1202-14 e6.
Tran DQ, Andersson J, Wang R, Ramsey H, Unutmaz D, Shevach EM. GARP (LRRC32) is essential for the surface expression of latent TGF-beta on platelets and activated FOXP3+ regulatory T cells. Proc Natl Acad Sci USA. 2009;106(32):13445–50.
Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, et al. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195(5):603–16.
Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3(5):541–7.
Kamada T, Togashi Y, Tay C, Ha D, Sasaki A, Nakamura Y, et al. PD-1(+) regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci USA. 2019;116(20):9999–10008.
Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–7.
Cretney E, **n A, Shi W, Minnich M, Masson F, Miasari M, et al. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat Immunol. 2011;12(4):304–11.
Biswas PS, Gupta S, Chang E, Song L, Stirzaker RA, Liao JK, et al. Phosphorylation of IRF4 by ROCK2 regulates IL-17 and IL-21 production and the development of autoimmunity in mice. J Clin Investig. 2010;120(9):3280–95.
Bruchard M, Rebe C, Derangere V, Togbe D, Ryffel B, Boidot R, et al. The receptor NLRP3 is a transcriptional regulator of TH2 differentiation. Nat Immunol. 2015;16(8):859–70.
Koo TY, Lee JG, Yan JJ, Jang JY, Ju KD, Han M, et al. The P2X7 receptor antagonist, oxidized adenosine triphosphate, ameliorates renal ischemia–reperfusion injury by expansion of regulatory T cells. Kidney Int. 2017;92(2):415–31.
Wu L, Yun Z, Tagawa T, Rey-McIntyre K, Anraku M, de Perrot M. Tumor cell repopulation between cycles of chemotherapy is inhibited by regulatory T-cell depletion in a murine mesothelioma model. J Thorac Oncol. 2011;6(9):1578–86.
Chen X, Baumel M, Mannel DN, Howard OM, Oppenheim JJ. Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4+CD25+ T regulatory cells. J Immunol. 2007;179(1):154–61.
Polz J, Remke A, Weber S, Schmidt D, Weber-Steffens D, Pietryga-Krieger A, et al. Myeloid suppressor cells require membrane TNFR2 expression for suppressive activity. Immun Inflamm Dis. 2014;2(2):121–30.
Madsen PM, Desu HL, de Rivero Vaccari JP, Florimon Y, Ellman DG, Keane RW, et al. Oligodendrocytes modulate the immune-inflammatory response in EAE via TNFR2 signaling. Brain Behav Immun. 2020;84:132–46.
Madsen PM, Motti D, Karmally S, Szymkowski DE, Lambertsen KL, Bethea JR, et al. Oligodendroglial TNFR2 mediates membrane TNF-dependent repair in experimental autoimmune encephalomyelitis by promoting oligodendrocyte differentiation and remyelination. J Neurosci. 2016;36(18):5128–43.
Gao H, Danzi MC, Choi CS, Taherian M, Dalby-Hansen C, Ellman DG, et al. Opposing functions of microglial and macrophagic TNFR2 in the pathogenesis of experimental autoimmune encephalomyelitis. Cell Rep. 2017;18(1):198–212.
Joedicke JJ, Myers L, Carmody AB, Messer RJ, Wajant H, Lang KS, et al. Activated CD8+ T cells induce expansion of Vbeta5+ regulatory T cells via TNFR2 signaling. J Immunol. 2014;193(6):2952–60.
Chen X, Oppenheim JJ. The phenotypic and functional consequences of tumour necrosis factor receptor type 2 expression on CD4(+) FoxP3(+) regulatory T cells. Immunology. 2011;133(4):426–33.
Atretkhany KN, Gogoleva VS, Drutskaya MS, Nedospasov SA. Distinct modes of TNF signaling through its two receptors in health and disease. J Leukoc Biol. 2020;107(6):893–905.
Moatti A, Cohen JL. The TNF-alpha/TNFR2 pathway: targeting a brake to release the anti-tumor immune response. Front Cell Dev Biol. 2021;9: 725473.
Vanamee ES, Faustman DL. TNFR2: a novel target for cancer immunotherapy. Trends Mol Med. 2017;23(11):1037–46.
Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3(2):135–42.
Valzasina B, Guiducci C, Dislich H, Killeen N, Weinberg AD, Colombo MP. Triggering of OX40 (CD134) on CD4(+)CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood. 2005;105(7):2845–51.
Mahmud SA, Manlove LS, Schmitz HM, **ng Y, Wang Y, Owen DL, et al. Costimulation via the tumor-necrosis factor receptor superfamily couples TCR signal strength to the thymic differentiation of regulatory T cells. Nat Immunol. 2014;15(5):473–81.
Mykicki N, Herrmann AM, Schwab N, Deenen R, Sparwasser T, Limmer A, et al. Melanocortin-1 receptor activation is neuroprotective in mouse models of neuroinflammatory disease. Sci Transl Med. 2016;8(362):362ra146.
Jung MK, Lee JS, Kwak JE, Shin EC. Tumor necrosis factor and regulatory T Cells. Yonsei Med J. 2019;60(2):126–31.
Choi YS, Jung MK, Lee J, Choi SJ, Choi SH, Lee HW, et al. Tumor necrosis factor-producing T-regulatory cells are associated with severe liver injury in patients with acute hepatitis A. Gastroenterology. 2018;154(4):1047–60.
Nishioka T, Shimizu J, Iida R, Yamazaki S, Sakaguchi S. CD4+CD25+Foxp3+ T cells and CD4+CD25-Foxp3+ T cells in aged mice. J Immunol. 2006;176(11):6586–93.
Sinha M, Jang YC, Oh J, Khong D, Wu EY, Manohar R, et al. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344(6184):649–52.
Panduro M, Benoist C, Mathis D. Tissue Tregs. Annu Rev Immunol. 2016;34:609–33.
Villalta SA, Rosenthal W, Martinez L, Kaur A, Sparwasser T, Tidball JG, et al. Regulatory T cells suppress muscle inflammation and injury in muscular dystrophy. Sci Transl Med. 2014;6(258):258ra142.
Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, et al. A distinct function of regulatory T cells in tissue protection. Cell. 2015;162(5):1078–89.
Kuswanto W, Burzyn D, Panduro M, Wang KK, Jang YC, Wagers AJ, et al. Poor repair of skeletal muscle in aging mice reflects a defect in local, interleukin-33-dependent accumulation of regulatory T cells. Immunity. 2016;44(2):355–67.
Schiaffino S, Pereira MG, Ciciliot S, Rovere-Querini P. Regulatory T cells and skeletal muscle regeneration. FEBS J. 2017;284(4):517–24.
Acknowledgements
We sincerely thank Shanghai Jiao Tong University College of Basic Medical Sciences-Core Facility of Basic Medical Sciences for their scientific input and technological support.
Funding
This work was supported by the National Natural Science Foundation of China [81971863, 82272230 to HF], Natural Science Foundation of Shanghai [22ZR1444700 to HF], and Shanghai Shenkang project for transformation for scientific production [SHDC2022CRD049 to HF].
Author information
Authors and Affiliations
Contributions
YZ, HJ, and YH performed the experiments and analyzed the data; TL, ZC, and YS (Yuan Si) helped with animal experiments. YZ, HJ, XS, YS (Yi Shi), and HF wrote the manuscript; XS and YS (Yi Shi) designed the experiments; XS, YS (Yi Shi), and HF gave final content approval. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Human samples were not used in this study. All animal procedures were performed in accordance with the local welfare legislation and approved by the Animal Ethics Committee of Zhongshan Hospital, Fudan University.
Consent for publication
Not applicable.
Competing interests
The authors declare that no potential competing interests exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Four shots of ATRA significantly increased counts of splenic CD4+CD25+Foxp3+ Tregs but had lethal effects on aged ones. Figure S2. Verification of isolated Tregs by the Regulatory T Cell Isolation Kit. Figure S3. Tregs from Foxp3YFP mice were injected into aged mice via tail vein subjected to the surgery. Figure S4. Swimming velocity documented in the Morris maze test. Figure S5. Changes of LAG-3 (A) and HELIOS (B) proteins in Tregs under basal and stimulated conditions in flow cytometry. Figure S6. Bodyweight of mice with Tregs ablation. Figure S7. Cytokines expressions in plasma and hippocampus of mice with Tregs ablation.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, Y., Ju, H., Hu, Y. et al. Tregs dysfunction aggravates postoperative cognitive impairment in aged mice. J Neuroinflammation 20, 75 (2023). https://doi.org/10.1186/s12974-023-02760-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12974-023-02760-7