Abstract
Background
The survival rate for patients with relapsed and refractory acute myeloid leukaemia (R/R-AML) remains poor, and treatment is challenging. Chimeric antigen receptor T cells (CAR-T cells) have been widely used for haematologic malignancies. Current CAR-T therapies for acute myeloid leukaemia mostly target myeloid-lineage antigens, such as CD123 and CD33, which may be associated with potential haematopoietic toxicity. As a lineage-specific receptor, CD7 is expressed in acute myeloid leukaemia cells and T cells but is not expressed in myeloid cells. Therefore, the use of CD7 CAR-T cells for R/R-AML needs to be further explored.
Methods
In this report, immunohistochemistry and flow cytometry were used to analyse CD7 expression in clinical samples from R/R-AML patients and healthy donors (HDs). We designed naturally selected CD7 CAR-T cells to analyse various functions and in vitro antileukaemic efficacy based on flow cytometry, and xenograft models were used to validate in vivo tumour dynamics.
Results
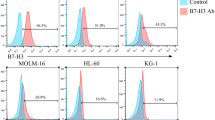
We calculated the percentage of cells with CD7 expression in R/R-AML patients with minimal residual disease (MRD) (5/16, 31.25%) from our institution and assessed CD7 expression in myeloid and lymphoid lineage cells of R/R-AML patients, concluding that CD7 is expressed in T cells but not in myeloid cells. Subsequently, we designed and constructed naturally selected CD7 CAR-T cells (CD7 CAR). We did not perform CD7 antigen knockdown on CD7 CAR-T cells because CD7 molecule expression is naturally eliminated at Day 12 post transduction. We then evaluated the ability to target and kill CD7+ acute myeloid leukaemia cells in vitro and in vivo. Naturally selected CD7 CAR-T cells efficiently killed CD7+ acute myeloid leukaemia cells and CD7+ primary blasts of R/R-AML patients in vitro and significantly inhibited leukaemia cell growth in a xenograft mouse model.
Conclusion
Naturally selected CD7 CAR-T cells represent an effective treatment strategy for relapsed and refractory acute myeloid leukaemia patients in preclinical studies.
Similar content being viewed by others
Background
Acute myeloid leukaemia (AML) is a malignancy characterized by abnormal clonal expansion of myeloid blasts in the bone marrow that impairs normal haematopoiesis, causing infection, bleeding, and anaemia [1]. Despite standard treatment with chemotherapeutic agents, including anthracycline and cytarabine, to achieve complete remission (CR) for patients with acute myeloid leukaemia, many patients still relapse within a short period [2, 3]. Only 35–45% of patients under the age of 60 years experience long-term remission with conventional treatment, and the proportion drops to 10–15% for those older than 60 years [2, 4]. Relapse and leukaemia-related complications are the most common causes of death, and only 10% of patients with a first relapse survive long term [4, 5].
In recent years, cellular immunotherapy of chimeric antigen receptor T cells (CAR-T) for relapsed or refractory acute myeloid leukaemia (R/R-AML) targeting myeloid-lineage antigens, such as CD123, CLL-1, and CD33, has shown promising prospects in many preclinical studies [6,7,8,9,48]. Similarly, we demonstrated this finding in a subset of 16 R/R AML patients (5/16, 31.25%) with minimal residual disease (MRD). Similar to previous studies, both R/R AML patients and HDs had high expression of CD7 in their normal cell populations, such as NK cells and T cells. In contrast, B cells, monocytes, and neutrophils did not express CD7 [15, 49]. The lack of CD7 expression in myeloid cells prevents the killing of myeloid cells.
CD7-positive T-cells cultured with CD7 CAR-T cells lead to diminished proliferation and increased cell death, as demonstrated in our study and previous studies [15]. Despite the diminished proliferation, our previous study demonstrated that the required dose of naturally selected CD7 CAR-T cells for transfusion back to patients could be achieved [34].
In addition, similar to our previous report, CD7 CAR-T cells were dominated by CD7-negative subpopulations on Day 12 post transduction possibly due to antigenic masking/intracellular sequestration by CD7 CAR [34]. This finding suggests that the naturally selected CD7 CAR-T cells remained CD7 negative before they were administered back to the patients.
Finally, CD7 was positively expressed on T cells and NK cells, which are important components of the human immune system [49, 50]. Previous studies have shown that CD7 CAR-T cells cause defects in CD7+ T cells in recipients, but CD7− T-cell subsets appear to replace their function to some extent, thereby alleviating treatment-related T-cell immunodeficiency. In addition, CD7+ T-cell populations and NK cell populations were restored after bridging allogeneic haematopoietic stem cell transplantation [30, 34]. Compared with other myeloid-targeted CAR-T cells, CD7 CAR-T cells can avoid damage to normal myeloid cells and reduce haematopoietic toxicity to a certain extent because CD7 is not expressed on other myeloid cells.
Persistence, a key factor affecting CAR-T-cell efficacy, has been widely investigated in previous studies, and it has been shown that the T-cell memory phenotype subpopulation is an important factor in maintaining CAR-T-cell persistence [41, 42]. In the present study, we demonstrated that both CD4+ and CD8+ T-cell subpopulations exhibited a higher percentage of cells with central memory phenotypes in the naturally selected CD7 CAR-T cells compared with the NTR group, which facilitated a more durable effect of CD7 CAR-T cells. In addition, there was no evidence of accelerated terminal differentiation, even in the CD8+ T-cell subpopulation, and the proportion of TEff with naturally selected CD7 CAR-T cells decreased. The above study demonstrated the persistence of naturally selected CD7 CAR T cells in the in vitro phase of the study. However, further validation of its durability in comparison with other reported CD7 CAR-T cells and in clinical trials of R/R-AML needs to be performed [15, 51,52,53,54].
Naturally selected CD7 CAR-T exhaustion marker assays were performed, and the results suggested a trend or statistically significant increase in both PD-1 and TIM-3 expression. In a previous clinical report of T-ALL/LBL, TIM-3 and PD-1 expression levels were not significantly different in patients who achieved CR compared to those who achieved less than CR [34]. Therefore, the impact of increased exhaustion markers on naturally selected CD7 CAR-T cells for R/R-AML needs to be validated by further clinical trials and long-term observations.
In addition, CD7+ cell lines and primary AML blasts from R/R-AML patients in vitro and CD7+AML xenograft models were used to assess the antileukaemic ability of naturally selected CD7 CAR-T cells, demonstrating powerful cytotoxic effects on CD7+ AML cells.
Although our proposed naturally selected CD7 CAR-Ts exhibit diminished proliferation due to cell death at the in vitro culture stage, the CAR-T dose for patient transfusion can still be achieved. In addition, CD7 CAR-Ts may be less costly and exhibit lower risks associated with gene editing. In addition, in recent years, the construction of CAR-T cells by isolating CD7-negative cell populations or the use of ibrutinib and dasatinib to inhibit fratricide has demonstrated good potential value [52, 54]. Hai-** Dai et al. demonstrated the safety and efficacy of CD7 CAR-T cells using protein expression blockers to block CD7 expression at the CAR-T-cell membrane for the treatment of R/R early T-cell precursor lymphoblastic leukaemia/lymphoma (ETP-ALL/LBL) in patients with TP53 mutations [51]. Yongxian Hu et al. investigated allogeneic CD7 CAR-T cells for R/R CD7-positive haematologic malignancies, including one patient with CD7-positive acute myeloid leukaemia and 11 patients with T-cell leukaemia/lymphoma. This study demonstrated the safety and efficacy of allogeneic CD7 CAR-T cells for CD7-positive haematologic malignancies [53]. Both the abovementioned studies and our proposed naturally selected CD7 CAR-T cells have explored the use of CD7 CAR-T cells in the treatment of haematologic malignancies. However, it seems that CAR-T cells targeting CD7 show different characteristics in clinical trials compared with CAR-T cells targeting CD19. Thus, the actual performance of CD7 CAR-T cells in the treatment of R/R-AML patients should be assessed in further clinical trials and long-term clinical observations [55].
In conclusion, naturally selected CD7 CAR-T cells, as a CAR-T treatment strategy without additional treatments, such as CD7 knockdown, can reduce the cost and additional unknown risks associated with gene knockdown to some extent. Patients can benefit from avoiding the risk of additional gene knockouts and reduced production costs of naturally selected CD7 CAR-T cells as a bridging allogeneic HSCT pretreatment for R/R-AML. This study demonstrated the feasibility of naturally selected CD7 CAR-T cells in the treatment of patients with CD7+ R/R-AML in the preclinical study phase. However, R/R-AML is a complex disease type, and the effectiveness of naturally selected CD7 CAR-T therapy needs to be further tested in clinical trials.
Availability of data and materials
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Abbreviations
- R/R AML:
-
Relapsed and refractory acute myeloid leukemia
- CAR-T:
-
Chimeric antigen receptor T cell
- MRD:
-
Minimal residual disease
- HD:
-
Healthy donor
- HSCT:
-
Hematopoietic stem cell transplantation
- NTR:
-
Non-transduced
- CR:
-
Complete remission
- PBMCs:
-
Peripheral blood mononuclear cells
References
Ishii H, Yano S. New therapeutic strategies for adult acute myeloid leukemia. Cancers. 2022;14(11):2806.
Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–47.
Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European leukemianet. Blood. 2010;115(3):453–74.
Short NJ, Rytting ME, Cortes JE. Acute myeloid leukaemia. Lancet. 2018;392(10147):593–606.
DeWolf S, Tallman MS. How I treat relapsed or refractory AML. Blood. 2020;136(9):1023–32.
Tettamanti S, Marin V, Pizzitola I, Magnani CF, Giordano Attianese GM, Cribioli E, et al. Targeting of acute myeloid leukaemia by cytokine-induced killer cells redirected with a novel CD123-specific chimeric antigen receptor. Br J Haematol. 2013;161(3):389–401.
Pizzitola I, Anjos-Afonso F, Rouault-Pierre K, Lassailly F, Tettamanti S, Spinelli O, et al. Chimeric antigen receptors against CD33/CD123 antigens efficiently target primary acute myeloid leukemia cells in vivo. Leukemia. 2014;28(8):1596–605.
Collinson-Pautz MR, Chang WC, Lu A, Khalil M, Crisostomo JW, Lin PY, et al. Constitutively active MyD88/CD40 costimulation enhances expansion and efficacy of chimeric antigen receptor T cells targeting hematological malignancies. Leukemia. 2019;33(9):2195–207.
Petrov JC, Wada M, Pinz KG, Yan LE, Chen KH, Shuai X, et al. Compound CAR T-cells as a double-pronged approach for treating acute myeloid leukemia. Leukemia. 2018;32(6):1317–26.
Wang J, Chen S, **ao W, Li W, Wang L, Yang S, et al. CAR-T cells targeting CLL-1 as an approach to treat acute myeloid leukemia. J Hematol Oncol. 2018;11(1):7.
Ataca Atilla P, McKenna MK, Tashiro H, Srinivasan M, Mo F, Watanabe N, et al. Modulating TNFalpha activity allows transgenic IL15-expressing CLL-1 CAR T cells to safely eliminate acute myeloid leukemia. J Immunother Cancer. 2020;8(2): e001229.
Taussig DC, Pearce DJ, Simpson C, Rohatiner AZ, Lister TA, Kelly G, et al. Hematopoietic stem cells express multiple myeloid markers: implications for the origin and targeted therapy of acute myeloid leukemia. Blood. 2005;106(13):4086–92.
Kenderian SS, Ruella M, Shestova O, Klichinsky M, Aikawa V, Morrissette JJ, et al. CD33-specific chimeric antigen receptor T cells exhibit potent preclinical activity against human acute myeloid leukemia. Leukemia. 2015;29(8):1637–47.
Wang QS, Wang Y, Lv HY, Han QW, Fan H, Guo B, et al. Treatment of CD33-directed chimeric antigen receptor-modified T cells in one patient with relapsed and refractory acute myeloid leukemia. Mol Ther. 2015;23(1):184–91.
Gomes-Silva D, Atilla E, Atilla PA, Mo F, Tashiro H, Srinivasan M, et al. CD7 CAR T cells for the therapy of acute myeloid leukemia. Mol Ther. 2019;27(1):272–80.
Gomes-Silva D, Srinivasan M, Sharma S, Lee CM, Wagner DL, Davis TH, Rouce RH, Bao G, Brenner MK, Mamonkin M. CD7-edited T cells expressing a CD7-specific CAR for the therapy of T-cell malignancies. Blood. 2017 Jul 20;130(3):285–296.
McMahon CM, Luger SM. Relapsed T Cell ALL: Current Approaches and New Directions. Curr Hematol Malig Rep. 2019 Apr;14(2):83–93.
Zheng J, Wang X, Hu Y, Yang J, Liu J, He Y, et al. A correlation study of immunophenotypic, cytogenetic, and clinical features of 180 AML patients in China. Cytometry B Clin Cytom. 2008;74(1):25–9.
Venditti A, Del Poeta G, Buccisano F, Tamburini A, Cox-Froncillo M, Aronica G, et al. Prognostic relevance of the expression of Tdt and CD7 in 335 cases of acute myeloid leukemia. Leukemia. 1998;12(7):1056–63.
Valet G, Repp R, Link H, Ehninger A, Gramatzki MM. Pretherapeutic identification of high-risk acute myeloid leukemia (AML) patients from immunophenotypic, cytogenetic, and clinical parameters. Cytometry B Clin Cytom. 2003;53(1):4–10.
Haubner S, Perna F, Kohnke T, Schmidt C, Berman S, Augsberger C, et al. Coexpression profile of leukemic stem cell markers for combinatorial targeted therapy in AML. Leukemia. 2019;33(1):64–74.
Saxena A, Sheridan DP, Card RT, McPeek AM, Mewdell CC, Skinnider LF. Biologic and clinical significance of CD7 expression in acute myeloid leukemia. Am J Hematol. 1998;58(4):278–84.
Sathe P, Pang SHM, Delconte R, Elwood N, Huntington ND. Identification of novel human NK cell progenitor subsets. Int J Mol Sci. 2017;18(12):2716.
Hauser A, Schrattbauer K, Najdanovic D, Schlossnickel R, Koch A, Hejtman M, et al. Optimized quantification of lymphocyte subsets by use of CD7 and CD33. Cytometry A. 2013;83(3):316–23.
Hao QL, Zhu J, Price MA, Payne KJ, Barsky LW, Crooks GM. Identification of a novel, human multilymphoid progenitor in cord blood. Blood. 2001;97(12):3683–90.
Rabinowich H, Pricop L, Herberman RB, Whiteside TL. Expression and function of CD7 molecule on human natural killer cells. J Immunol. 1994;152(2):517–26.
Hoebeke I, De Smedt M, Stolz F, Pike-Overzet K, Staal FJ, Plum J, et al. T-, B- and NK-lymphoid, but not myeloid cells arise from human CD34(+)CD38(-)CD7(+) common lymphoid progenitors expressing lymphoid-specific genes. Leukemia. 2007;21(2):311–9.
Bonilla FA, Kokron CM, Swinton P, Geha RS. Targeted gene disruption of murine CD7. Int Immunol. 1997;9(12):1875–83.
Lee DM, Staats HF, Sundy JS, Patel DD, Sempowski GD, Scearce RM, et al. Immunologic characterization of CD7-deficient mice. J Immunol. 1998;160(12):5749–56.
Pan J, Tan Y, Wang G, Deng B, Ling Z, Song W, et al. Donor-derived CD7 chimeric antigen receptor T cells for T-cell acute lymphoblastic leukemia: first-in-human. Phase I Trial J Clin Oncol. 2021;39(30):3340–51.
Cooper ML, Choi J, Staser K, Ritchey JK, Devenport JM, Eckardt K, et al. An “off-the-shelf” fratricide-resistant CAR-T for the treatment of T cell hematologic malignancies. Leukemia. 2018;32(9):1970–83.
Png YT, Vinanica N, Kamiya T, Shimasaki N, Coustan-Smith E, Campana D. Blockade of CD7 expression in T cells for effective chimeric antigen receptor targeting of T-cell malignancies. Blood Adv. 2017;1(25):2348–60.
Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L. “Off-the-shelf” allogeneic CAR T cells: development and challenges. Nat Rev Drug Discov. 2020;19(3):185–99.
Lu P, Liu Y, Yang J, Zhang X, Yang X, Wang H, et al. Naturally selected CD7 CAR-T therapy without genetic manipulations for T-ALL/LBL: first-in-human phase 1 clinical trial. Blood. 2022;140(4):321–34.
Baum W, Steininger H, Bair HJ, Becker W, Hansen-Hagge TE, Kressel M, et al. Therapy with CD7 monoclonal antibody TH-69 is highly effective for xenografted human T-cell ALL. Br J Haematol. 1996;95(2):327–38.
Hudecek M, Sommermeyer D, Kosasih PL, Silva-Benedict A, Liu L, Rader C, et al. The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol Res. 2015;3(2):125–35.
Wang X, Chang WC, Wong CW, Colcher D, Sherman M, Ostberg JR, et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood. 2011;118(5):1255–63.
Ma F, Ho JY, Du H, Xuan F, Wu X, Wang Q, et al. Evidence of long-lasting anti-CD19 activity of engrafted CD19 chimeric antigen receptor-modified T cells in a phase I study targeting pediatrics with acute lymphoblastic leukemia. Hematol Oncol. 2019;37(5):601–8.
Ho JY, Wang L, Liu Y, Ba M, Yang J, Zhang X, et al. Promoter usage regulating the surface density of CAR molecules may modulate the kinetics of CAR-T cells in vivo. Mol Ther Methods Clin Dev. 2021;21:237–46.
Peipp M, Küpers H, Saul D, Schlierf B, Greil J, Zunino SJ, et al. A recombinant CD7-specific single-chain immunotoxin is a potent inducer of apoptosis in acute leukemic T cells. Cancer Res. 2002;62(10):2848–55.
Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici I, Gohil M, Lundh S, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018;24(5):563–71.
Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118(23):6050–6.
Kawalekar OU, O’Connor RS, Fraietta JA, Guo L, McGettigan SE, Posey AD Jr, et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T Cells. Immunity. 2016;44(2):380–90.
Larbi A, Fulop T. From, “truly naïve” to “exhausted senescent” T cells: when markers predict functionality. Cytometry A. 2014;85(1):25–35.
Daver N, Alotaibi AS, Bücklein V, Subklewe M. T-cell-based immunotherapy of acute myeloid leukemia: current concepts and future developments. Leukemia. 2021;35(7):1843–63.
Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56.
Budde L, Song JY, Kim Y, Blanchard S, Wagner J, Stein AS, et al. Remissions of acute myeloid leukemia and blastic plasmacytoid dendritic cell neoplasm following treatment with CD123-specific CAR T cells: a first-in-human clinical trial. Blood. 2017;130(1):811.
Chang H, Salma F, Yi Q-l, Patterson B, Brien B, Minden MD. Prognostic relevance of immunophenoty** in 379 patients with acute myeloid leukemia. Leuk Res. 2004;28(1):43–8.
Cai Y, Dai Y, Wang Y, Yang Q, Guo J, Wei C, et al. Single-cell transcriptomics of blood reveals a natural killer cell subset depletion in tuberculosis. EBioMed. 2020;53: 102686.
Chiossone L, Dumas PY, Vienne M, Vivier E. Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol. 2018;18(11):671–88.
Dai HP, Cui W, Cui QY, Zhu WJ, Meng HM, Zhu MQ, et al. Haploidentical CD7 CAR T-cells induced remission in a patient with TP53 mutated relapsed and refractory early T-cell precursor lymphoblastic leukemia/lymphoma. Biomark Res. 2022;10(1):6.
Freiwan A, Zoine J, Crawford JC, Vaidya A, Schattgen SA, Myers J, Patil SL, Khanlari M, Inaba H, Klco JM, Mullighan CG, Krenciute G, Chockley P, Naik S, Langfitt D, Mamonkin M, Obeng EA, Thomas PG, Gottschalk S, Velasquez MP. Engineering Naturally Occurring CD7 Negative T Cells for the Immunotherapy of Hematological Malignancies. Blood. 2022;blood.2021015020.
Hu Y, Zhou Y, Zhang M, Zhao H, Wei G, Ge W, et al. Genetically modified CD7-targeting allogeneic CAR-T cell therapy with enhanced efficacy for relapsed/refractory CD7-positive hematological malignancies: a phase I clinical study. Cell Res. 2022;32(11):995–1007.
Watanabe N, Mo F, Zheng R, Ma R, Bray VC, van Leeuwen DG, et al. Feasibility and preclinical efficacy of CD7-unedited CD7 CAR T cells for T cell malignancies. Mol Ther. 2022;S1525-0016(22)00557-3.
Zhang X, Zhang G, Li W, Qiu L, Wang D, Yang J, et al. Evolution and proliferation of CD7 CAR-T cells compared to CD19 CAR-T cells therapies for acute leukemia. Blood. 2021;138(1):2820.
Acknowledgements
We thank members of our laboratory for helpful discussion. In the meantime, we are grateful for the help provided by Hebei Senlang Biotechnolngy co.
Funding
This study were supported by the Natural Science Foundation of Hebei Province (H2020206403) and Science and Technology Department Talent Special Project of Hebei Province(205A2402H).
Author information
Authors and Affiliations
Contributions
FW and JL provided funding and designed the experiments and helped revise the manuscript. YuL completed animal testing, clinical sample assays and statistics, and completed the manuscript. SW and XZ assisted with some experiments, manuscript writing and revision. YingL and NK completed the CAR construct and completed in vitro experiments, and helped revise the manuscript. All authors contributed to the article and approved the submitted version. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
By the Declaration of Helsinki, the Second Hospital of Hebei Medical University will have reviewed and approved studies involving human participants. Patients/participants provided written informed consent to participate in this study (approval number: 2022-T009). Animal trials were reviewed and approved by the Fourth Hospital of Hebei Medical University (approval No. LACUC-4th hos hebmu-2021230).
Consent for publication
All subjects have written informed consent.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lu, Y., Liu, Y., Wen, S. et al. Naturally selected CD7 CAR-T therapy without genetic editing demonstrates significant antitumour efficacy against relapsed and refractory acute myeloid leukaemia (R/R-AML). J Transl Med 20, 600 (2022). https://doi.org/10.1186/s12967-022-03797-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-022-03797-7