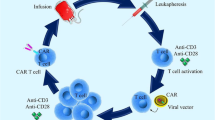
Abstract
Despite several small-molecule drugs that have revolutionized the current treatment strategy for acute myeloid leukemia (AML), hematopoietic stem cell transplantation remains the only curative treatment in most cases to date. Chimeric antigen receptor (CAR)-T cell therapy is one of the most promising next-generation cancer therapies for hematological malignancies and is clinically available for treatment of AML. However, develo** AML-targeted CAR-T therapy is challenging because of the heterogeneity of target antigen expression across leukemic cells and patients, the difficulty in excluding on-/off-target tumor effects, and the immunosuppressive tumor microenvironment. To date, various targets, including CD33, NKG2D, CD123, CLL-1, and CD7, have been actively studied for CAR-T cells. Although no CAR-T cell products are close to practical use, several clinical trials have shown promising results, particularly for CAR-T cells targeting CLL-1 or CD123. Meanwhile, research exploring the ideal target for AML-targeted CAR-T therapy continues. Furthermore, as collecting autologous lymphocytes from patients with AML is difficult, development of off-the-shelf CAR-T products is being actively pursued. This review discusses the challenges in AML-targeted CAR-T cell therapy development from the perspectives of target antigen characteristics and AML-specific on-target/off-tumor toxicity. Moreover, it discusses the clinical development and prospects of AML-targeting CAR-T cells.



Similar content being viewed by others
Data availability statement
This manuscript has data included as electronic supplementary materials.
References
DiNardo CD, Wei AH. How I treat acute myeloid leukemia in the era of new drugs. Blood. 2020;135:85–96.
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–48.
Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380:1726–37.
Schultz LM, Baggott C, Prabhu S, Pacenta HL, Phillips CL, Rossoff J, et al. Disease burden affects outcomes in pediatric and young adult B-cell lymphoblastic leukemia after commercial tisagenlecleucel: a pediatric real-world chimeric antigen receptor consortium report. J Clin Oncol. 2022;40:945–55.
Kantarjian H, Stein A, Gökbuget N, Fielding AK, Schuh AC, Ribera JM, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376:836–47.
Kantarjian HM, DeAngelo DJ, Stelljes M, Liedtke M, Stock W, Gökbuget N, et al. Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer. 2019;125:2474–87.
Atilla E, Benabdellah K. The black hole: CAR T cell therapy in AML. Cancers (Basel). 2023;15:2713.
Fetsch V, Zeiser R. Chimeric antigen receptor T cells for acute myeloid leukemia. Eur J Haematol. 2024;112:28–35.
Ritchie DS, Neeson PJ, Khot A, Peinert S, Tai T, Tainton K, et al. Persistence and efficacy of second generation CAR T cell against the LeY antigen in acute myeloid leukemia. Mol Ther. 2013;21:2122–9.
Wang QS, Wang Y, Lv HY, Han QW, Fan H, Guo B, et al. Treatment of CD33-directed chimeric antigen receptor-modified T cells in one patient with relapsed and refractory acute myeloid leukemia. Mol Ther. 2015;23:184–91.
Tambaro FP, Singh H, Jones E, Rytting M, Mahadeo KM, Thompson P, et al. Autologous CD33-CAR-T cells for treatment of relapsed/refractory acute myelogenous leukemia. Leukemia. 2021;35:3282–6.
Sallman DA, Elmariah H, Sweet K, Mishra A, Cox CA, Chakaith M, et al. Phase 1/1b safety study of Prgn-3006 Ultracar-T in patients with relapsed or refractory CD33-positive acute myeloid leukemia and HIGHER risk myelodysplastic syndromes. Blood. 2022;140(suppl 1):10313–5.
Shah NN, Tasian SK, Kohler ME, Hsieh EM, Baumeister SHC, Summers C, et al. CD33 CAR T-cells (CD33CART) for children and young adults with relapsed/refractory AML: dose-escalation results from a Phase I/II multicenter trial. Blood. 2023;142(suppl 1):771.
Curio S, Jonsson G, Marinović S. A summary of current NKG2D-based CAR clinical trials. Immunother Adv. 2021;1:ltab018.
Baumeister SH, Murad J, Werner L, Daley H, Trebeden-Negre H, Gicobi JK, et al. Phase I trial of autologous CAR T cells targeting NKG2D ligands in patients with AML/MDS and multiple myeloma. Cancer Immunol Res. 2019;7:100–12.
Sallman DA, Kerre T, Havelange V, Poiré X, Lewalle P, Wang ES, et al. CYAD-01, an autologous NKG2D-based CAR T-cell therapy, in relapsed or refractory acute myeloid leukaemia and myelodysplastic syndromes or multiple myeloma (THINK): haematological cohorts of the dose escalation segment of a phase 1 trial. Lancet Haematol. 2023;10:e191–202.
Sallman DA, Brayer J, Sagatys EM, Lonez C, Breman E, Agaugué S, et al. NKG2D-based chimeric antigen receptor therapy induced remission in a relapsed/refractory acute myeloid leukemia patient. Haematologica. 2018;103:e424–6.
Sauter CS, Borthakur G, Mountjoy L, Rotta M, Liu H, Murthy HS, et al. A Phase 1 study of NKX101, a chimeric antigen receptor natural killer (CAR-NK) cell therapy, with fludarabine and cytarabine in patients with acute myeloid leukemia. Blood. 2023;142(suppl 1):2097.
Mardiros A, Dos Santos C, McDonald T, Brown CE, Wang X, Budde LE, et al. T cells expressing CD123-specific chimeric antigen receptors exhibit specific cytolytic effector functions and antitumor effects against human acute myeloid leukemia. Blood. 2013;122:3138–48.
Luo Y, Chang L-J, Hu Y, Dong L, Wei G, Huang H. First-in-man CD123-specific chimeric antigen receptor-modified T cells for the treatment of refractory acute myeloid leukemia. Blood. 2015;126:3778.
Budde L, Song JY, Kim Y, Blanchard S, Wagner J, Stein AS, et al. Remissions of acute myeloid leukemia and blastic plasmacytoid dendritic cell neoplasm following treatment with CD123-specific CAR T cells: a first-in-human clinical trial. Blood. 2017;130(suppl 1):811.
Yao S, Jianlin C, Yarong L, Botao L, Qinghan W, Hongliang F, et al. Donor-derived CD123-targeted CAR T cell serves as a RIC regimen for haploidentical transplantation in a patient with FUS-ERG+ AML. Front Oncol. 2019;9:1358.
Naik S, Madden RM, Lipsitt A, Lockey T, Bran J, Rubnitz JE, et al. Safety and anti-leukemic activity of CD123-CAR T cells in pediatric patients with AML: preliminary results from a Phase 1 trial. Blood. 2022;140(suppl 1):4584–5.
Sallman DA, DeAngelo DJ, Pemmaraju N, Dinner S, Gill S, Olin RL, et al. Ameli-01: a phase I trial of UCART123v1.2, an anti-CD123 allogeneic CAR-T cell product, in adult patients with relapsed or refractory (R/R) CD123+ acute myeloid leukemia (AML). Blood. 2022;140(suppl 1):2371–84.
Peters DT, Savoldo B, Grover NS. Building safety into CAR-T therapy. Hum Vaccin Immunother. 2023;19:2275457.
Loff S, Dietrich J, Meyer JE, Riewaldt J, Spehr J, von Bonin M, et al. Rapidly switchable universal CAR-T cells for treatment of CD123-positive leukemia. Mol Ther Oncolytics. 2020;17:408–20.
Wermke M, Kraus S, Ehninger A, Bargou RC, Goebeler ME, Middeke JM, et al. Proof of concept for a rapidly switchable universal CAR-T platform with UniCAR-T-CD123 in relapsed/refractory AML. Blood. 2021;137:3145–8.
Ehninger G, Kraus S, Sala E, Metzelder SK, Vucinic V, Fiedler W, et al. Phase 1 dose escalation study of the rapidly switchable universal CAR-T therapy Unicar-T-CD123 in relapsed/refractory AML. Blood. 2022;140(suppl 1):2367–8.
Wermke M, Metzelder S, Kraus S, Sala E, Vucinic V, Fiedler W, et al. Updated results from a phase I dose escalation study of the rapidly-switchable universal CAR-T therapy UniCAR-T-CD123 in relapsed/refractory AML. Blood. 2023;142(suppl 1):3465.
Tashiro H, Sauer T, Shum T, Parikh K, Mamonkin M, Omer B, et al. Treatment of acute myeloid leukemia with T cells expressing chimeric antigen receptors directed to C-type lectin-like Molecule 1. Mol Ther. 2017;25:2202–13.
Liu F, Pinz K, Ma Y, Wada M, Chen K, Ma G, et al. First-in-human CLL1-CD33 compound CAR T cells as a two-pronged approach for the treatment of refractory acute myeloid leukemia. HemaSphere. 2018;2:29.
Liu F, Cao Y, Pinz K, Ma Y, Wada M, Chen K, et al. First-in-human CLL1-CD33 compound CAR T cell therapy induces complete remission in patients with refractory acute myeloid leukemia: update on Phase 1 clinical trial. Blood. 2018;132:901.
Zhang H, Wang P, Li Z, He Y, Gan W, Jiang H. Anti-CLL1 chimeric antigen receptor T-cell therapy in children with relapsed/refractory acute myeloid leukemia. Clin Cancer Res. 2021;27:3549–55.
Zhang H, Bu C, Peng Z, Li G, Zhou Z, Ding W, et al. Characteristics of anti-CLL1 based CAR-T therapy for children with relapsed or refractory acute myeloid leukemia: the multi-center efficacy and safety interim analysis. Leukemia. 2022;36:2596–604.
** X, Zhang M, Sun R, Lyu H, **ao X, Zhang X, et al. First-in-human phase I study of CLL-1 CAR-T cells in adults with relapsed/refractory acute myeloid leukemia. J Hematol Oncol. 2022;15:88.
Zhang X, Lv H, **ao X, Bai X, Liu P, Pu Y, et al. A phase I clinical trial of CLL-1 CAR-T cells for the treatment of relapsed/refractory acute myeloid leukemia in adults. Blood. 2023;142:2106.
Ma YJ, Dai HP, Cui QY, Cui W, Zhu WJ, Qu CJ, et al. Successful application of PD-1 knockdown CLL-1 CAR-T therapy in two AML patients with post-transplant relapse and failure of anti-CD38 CAR-T cell treatment. Am J Cancer Res. 2022;12:615–21.
Pei K, Xu H, Wang P, Gan W, Hu Z, Su X, et al. Anti-CLL1-based CAR T-cells with 4-1-BB or CD28/CD27 stimulatory domains in treating childhood refractory/relapsed acute myeloid leukemia. Cancer Med. 2023;12:9655–61.
Zhang X, Yang J, Li J, Qiu L, Liu H, **ong M, et al. Naturally selected CD7-targeted chimeric antigen receptor (CAR)-T cell therapy for refractory/relapsed acute myeloid leukemia: phase I clinical trial. Blood. 2023;142:218.
Chiesa R, Georgiadis C, Syed F, Zhan H, Etuk A, Gkazi SA, et al. Base-edited CAR7 T cells for relapsed T-cell acute lymphoblastic leukemia. N Engl J Med. 2023;389:899–910.
Nakazawa Y, Matsuda K, Kurata T, Sueki A, Tanaka M, Sakashita K, et al. Anti-proliferative effects of T cells expressing a ligand-based chimeric antigen receptor against CD116 on CD34(+) cells of juvenile myelomonocytic leukemia. J Hematol Oncol. 2016;9:27.
Hasegawa A, Saito S, Narimatsu S, Nakano S, Nagai M, Ohnota H, et al. Mutated GM-CSF-based CAR-T cells targeting CD116/CD131 complexes exhibit enhanced anti-tumor effects against acute myeloid leukaemia. Clin Transl Immunol. 2021;10: e1282.
Haubner S, Mansilla-Soto J, Nataraj S, Kogel F, Chang Q, de Stanchina E, et al. Cooperative CAR targeting to selectively eliminate AML and minimize escape. Cancer Cell. 2023;41:1871-1891.e6.
Acknowledgements
The authors also thank ENAGO for the English proofreading.
Funding
This work was supported by the Japan Agency for Medical Research and Development (AMED) (23ck0106821h0001 and 23ae0201006h000).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SS declares no competing interests regarding this work. YN has the following competing interests: leadership position/Advisory role, A-SEEDS Co., Ltd.; Stock ownership or options, A-SEEDS Co., Ltd.; Research founding: BrightPath BioTherapeutics Co., Ltd., AGC Inc., Toshiba Corp., Daiichi Sankyo Co., Ltd., Noile-Immune Biotech Inc., Bourbon Corp. & CSTI Inc.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Saito, S., Nakazawa, Y. CAR-T cell therapy in AML: recent progress and future perspectives. Int J Hematol (2024). https://doi.org/10.1007/s12185-024-03809-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12185-024-03809-w