Abstract
Background
Frizzled (FZD) proteins function as receptors for WNT ligands. Members in FZD family including FZD2, FZD4, FZD7, FZD8 and FZD10 have been demonstrated to mediate cancer cell epithelial-mesenchymal transition (EMT).
Methods
CCLE and TCGA databases were interrogated to reveal the association of FZD5 with EMT. EMT was analyzed by investigating the alterations in CDH1 (E-cadherin), VIM (Vimentin) and ZEB1 expression, cell migration and cell morphology. Transcriptional modulation was determined by ChIP in combination with Real-time PCR. Survival was analyzed by Kaplan–Meier method.
Results
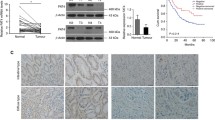
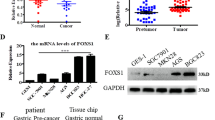
In contrast to other FZDs, FZD5 was identified to prevent EMT in gastric cancer. FZD5 maintains epithelial-like phenotype and is negatively modulated by transcription factors SNAI2 and TEAD1. Epithelial-specific factor ELF3 is a downstream effecter, and protein kinase C (PKC) links FZD5 to ELF3. ELF3 represses ZEB1 expression, further guarding against EMT. Moreover, FZD5 signaling requires its co-receptor LRP5 and WNT7B is a putative ligand for FZD5. FZD5 and ELF3 are associated with longer survival, whereas SNAI2 and TEAD1 are associated with shorter survival.
Conclusions
Taken together, FZD5-ELF3 signaling blocks EMT, and plays a potential tumor-suppressing role in gastric cancer.

Video Abstract
Similar content being viewed by others
Background
Frizzled (FZD) proteins are G protein-coupled receptors (GPCRs). The extracellular N-terminus contains a cysteine-rich domain (CRD) through which FZDs bind WNT ligands. The intracellular C-terminus binds the PDZ domain of Dishevelled (Dvl) and interacts with G proteins. Up to now, 10 FZDs have been identified in human. These receptors mediate β-catenin pathway and various β-catenin-independent pathways depending on cellular context. WNT/β-catenin pathway is oncogenic and involved in almost every aspect of tumor development. In addition, β-catenin-independent WNT/PCP and WNT/Ca2+-PKC pathways are also implicated in tumor progression [1,2,3,4]. In recent years, some novel pathways such as WNT/Stat3 and WNT/Yap-Taz have been successively identified [5,6,7,8], indicating that FZD signalings are far more complicated and still incompletely comprehended.
Epithelial-mesenchymal transition (EMT) in tumor cells endows them with enhanced motility thereby increasing their metastatic potentiality. This process is characterized by reduced expression of epithelial-related factors and increased expression of mesenchymal-related factors. Mechanistically, EMT is driven by some transcription factors such as SNAI1/2 and ZEB1/2. Studies have shown that FZD signalings induce tumor cell EMT and metastasis. FZD4, FZD7 and FZD10 promote EMT through β-catenin pathway in prostate, liver and breast cancer [9,10,11]. FZD2 mediates WNT5-induced EMT dependent on Stat3 signaling in liver, lung, breast and colon cancer [5], and FZD8 mediates WNT11-induced EMT by interacting with TGF-β signaling in prostate cancer [12].
In gastric cancer, FZD4 and FZD7 similarly promote EMT and metastasis by activating β-catenin pathway [13, 14]. Genetic deletion of FZD7 was shown to inhibit the growth of gastric adenomas in vivo [15]. FZD2 and FZD8, through β-catenin pathway but not β-catenin-independent pathways, induce gastric cancer cell proliferation, migration, invasion and metastasis [16, 31]. Actually, WNT7A functions as an EMT inhibitor in lung cancer [32]. Moreover, interrogation of CCLE database showed that FZD5 is also associated with epithelial-like phenotype in lung cancer (data not shown).
As shown in our study, FZD5 is transcriptional inhibited by TEAD1-SNAI2 complex. Activation of tumor-suppressing Hippo pathway results in phosphorylation and degradation of Yap and Taz. When Yap and Taz are not phosphorylated, they translocate into the nucleus to bind transcription factor TEAD. Yap/Taz-TEAD signaling induces gastric cancer cell EMT, stemness, drug resistance and metastasis [33,41, 42], and promotes prostate cancer progression by interacting with NFκB [43]. In gastric cancer, ELF3 maintains epithelial-like phenotype, prevents EMT and is associated with longer survival, acting as a putative tumor suppressor. ELF3 also inhibits EMT in ovarian and breast cancer cells [44, 45]. Mechanistically, ELF3 represses activity and expression of EMT transcription factor ZEB1 [46, 47].
Activation of β-catenin pathway requires not only FZDs, but also their co-receptors LRP5/6 which is not involved in non-canonical β-catenin-independent pathway. A recent study reported that FZDs-LRP5/6 interaction can initiate β-catenin pathway in the absence of WNTs [48]. Surprisingly, another study revealed that FZDs-LRP5/6 interaction can block activation of FZD-mediated non-canonical pathway, further preventing tumor metastasis [49]. These findings, in combination with our observations, suggest that FZDs-LRP5/6 interaction may act as a tumor promoter by activating β-catenin pathway or a tumor suppressor by antagonizing β-catenin-independent pathway, depending on tumor or cell context.
Conclusions
In summary, our study for the first time uncovers that FZD5-ELF3 signaling prevents EMT and is associated with favorable prognosis in gastric cancer. Therefore, FZD5 and ELF3 function as putative tumor suppressors in this type of cancer. Antibodies against FZD5 including ipafricept and vantictumab have been shown effective on certain cancer types [50]. However, our study warns out that targeting FZD5 should be cautious and tumor-specific, since treatment with these antibodies may potentially promote metastasis in some other types of cancers including gastric cancer.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
- FZD:
-
Frizzled
- EMT:
-
Epithelial-mesenchymal transition
- PKC:
-
Protein kinase C
- GPCRs:
-
G protein-coupled receptors
- CCLE:
-
Cancer Cell Line Encyclopedia
- TCGA:
-
The Cancer Genome Atlas
- ChIP:
-
Chromatin Immunoprecipitation
- CoIP:
-
Co-immunoprecipitation
- LRP5:
-
LDL receptor-related proteins 5
References
Asad M, Wong MK, Tan TZ, Choolani M, Low J, Mori S, Virshup D, Thiery JP, Huang RY. FZD7 drives in vitro aggressiveness in Stem-A subtype of ovarian cancer via regulation of non-canonical Wnt/PCP pathway. Cell Death Dis. 2014;5:e1346.
Kaucka M, Plevova K, Pavlova S, Janovska P, Mishra A, Verner J, Prochazkova J, Krejci P, Kotaskova J, Ovesna P, et al. The planar cell polarity pathway drives pathogenesis of chronic lymphocytic leukemia by the regulation of B-lymphocyte migration. Cancer Res. 2013;73(5):1491–501.
Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M, Trent JM. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell. 2002;1(3):279–88.
Dissanayake SK, Wade M, Johnson CE, O’Connell MP, Leotlela PD, French AD, Shah KV, Hewitt KJ, Rosenthal DT, Indig FE, et al. The Wnt5A/protein kinase C pathway mediates motility in melanoma cells via the inhibition of metastasis suppressors and initiation of an epithelial to mesenchymal transition. J Biol Chem. 2007;282(23):17259–71.
Gujral TS, Chan M, Peshkin L, Sorger PK, Kirschner MW, MacBeath G. A noncanonical Frizzled2 pathway regulates epithelial-mesenchymal transition and metastasis. Cell. 2014;159(4):844–56.
Yin P, Wang W, Gao J, Bai Y, Wang Z, Na L, Sun Y, Zhao C. Fzd2 Contributes to breast cancer cell mesenchymal-like stemness and drug resistance. Oncol Res. 2020;28(3):273–84.
Park HW, Kim YC, Yu B, Moroishi T, Mo JS, Plouffe SW, Meng Z, Lin KC, Yu FX, Alexander CM, et al. Alternative Wnt signaling activates YAP/TAZ. Cell. 2015;162(4):780–94.
Luo C, Balsa E, Perry EA, Liang J, Tavares CD, Vazquez F, Widlund HR, Puigserver P. H3K27me3-mediated PGC1alpha gene silencing promotes melanoma invasion through WNT5A and YAP. J Clin Invest. 2020;130(2):853–62.
Gupta S, Il** K, Sara H, Mpindi JP, Mirtti T, Vainio P, Rantala J, Alanen K, Nees M, Kallioniemi O. FZD4 as a mediator of ERG oncogene-induced WNT signaling and epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res. 2010;70(17):6735–45.
Gong C, Qu S, Lv XB, Liu B, Tan W, Nie Y, Su F, Liu Q, Yao H, Song E. BRMS1L suppresses breast cancer metastasis by inducing epigenetic silence of FZD10. Nat Commun. 2014;5:5406.
Nambotin SB, Tomimaru Y, Merle P, Wands JR, Kim M. Functional consequences of WNT3/Frizzled7-mediated signaling in non-transformed hepatic cells. Oncogenesis. 2012;1:e31.
Murillo-Garzon V, Gorrono-Etxebarria I, Akerfelt M, Puustinen MC, Sistonen L, Nees M, Carton J, Waxman J, Kypta RM. Frizzled-8 integrates Wnt-11 and transforming growth factor-beta signaling in prostate cancer. Nat Commun. 2018;9(1):1747.
Li G, Su Q, Liu H, Wang D, Zhang W, Lu Z, Chen Y, Huang X, Li W, Zhang C, et al. Frizzled7 promotes epithelial-to-mesenchymal transition and stemness via activating canonical Wnt/beta-catenin pathway in gastric cancer. Int J Biol Sci. 2018;14(3):280–93.
Li ZT, Zhang X, Wang DW, Xu J, Kou KJ, Wang ZW, Yong G, Liang DS, Sun XY. Overexpressed lncRNA GATA6-AS1 Inhibits LNM and EMT via FZD4 through the Wnt/beta-catenin signaling pathway in GC. Mol Ther Nucleic Acids. 2020;19:827–40.
Flanagan DJ, Barker N, Costanzo NSD, Mason EA, Gurney A, Meniel VS, Koushyar S, Austin CR, Ernst M, Pearson HB, et al. Frizzled-7 is required for Wnt signaling in gastric tumors with and without Apc mutations. Cancer Res. 2019;79(5):970–81.
Tomizawa M, Shinozaki F, Motoyoshi Y, Sugiyama T, Yamamoto S, Ishige N. Gastric cancer cell proliferation is suppressed by frizzled-2 short hairpin RNA. Int J Oncol. 2015;46(3):1018–24.
Chen W, Liu Z, Mai W, **ao Y, You X, Qin L. FZD8 Indicates a poor prognosis and promotes gastric cancer invasion and metastasis via B-catenin signaling pathway. Ann Clin Lab Sci. 2020;50(1):13–23.
Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21(5):449–56.
Szasz AM, Lanczky A, Nagy A, Forster S, Hark K, Green JE, Boussioutas A, Busuttil R, Szabo A, Gyorffy B. Cross-validation of survival associated biomarkers in gastric cancer using transcriptomic data of 1065 patients. Oncotarget. 2016;7(31):49322–33.
Schwab A, Siddiqui A, Vazakidou ME, Napoli F, Bottcher M, Menchicchi B, Raza U, Saatci O, Krebs AM, Ferrazzi F, et al. Polyol pathway links glucose metabolism to the aggressiveness of cancer cells. Cancer Res. 2018;78(7):1604–18.
Tang Y, Feinberg T, Keller ET, Li XY, Weiss SJ. Snail/Slug binding interactions with YAP/TAZ control skeletal stem cell self-renewal and differentiation. Nat Cell Biol. 2016;18(9):917–29.
Kurppa KJ, Liu Y, To C, Zhang T, Fan M, Vajdi A, Knelson EH, **e Y, Lim K, Cejas P, et al. Treatment-induced tumor dormancy through YAP-mediated transcriptional reprogramming of the apoptotic pathway. Cancer Cell. 2020;37(1):104–22.
Wright SC, Canizal MCA, Benkel T, Simon K, Le Gouill C, Matricon P, Namkung Y, Lukasheva V, Konig GM, Laporte SA et al: FZD5 is a Galphaq-coupled receptor that exhibits the functional hallmarks of prototypical GPCRs. Sci Signal. 2018; 11(559).
Erdogan E, Klee EW, Thompson EA, Fields AP. Meta-analysis of oncogenic protein kinase Ciota signaling in lung adenocarcinoma. Clin Cancer Res. 2009;15(5):1527–33.
Yamaguchi H, Kojima T, Ito T, Kimura Y, Imamura M, Son S, Koizumi J, Murata M, Nagayama M, Nobuoka T, et al. Transcriptional control of tight junction proteins via a protein kinase C signal pathway in human telomerase reverse transcriptase-transfected human pancreatic duct epithelial cells. Am J Pathol. 2010;177(2):698–712.
Ali SA, Justilien V, Jamieson L, Murray NR, Fields AP. Protein kinase Ciota drives a NOTCH3-dependent stem-like phenotype in mutant KRAS lung adenocarcinoma. Cancer Cell. 2016;29(3):367–78.
Carmon KS, Loose DS. Secreted frizzled-related protein 4 regulates two Wnt7a signaling pathways and inhibits proliferation in endometrial cancer cells. Mol Cancer Res. 2008;6(6):1017–28.
Yoshioka S, King ML, Ran S, Okuda H, MacLean JA 2nd, McAsey ME, Sugino N, Brard L, Watabe K, Hayashi K. WNT7A regulates tumor growth and progression in ovarian cancer through the WNT/beta-catenin pathway. Mol Cancer Res. 2012;10(3):469–82.
Steinhart Z, Pavlovic Z, Chandrashekhar M, Hart T, Wang X, Zhang X, Robitaille M, Brown KR, Jaksani S, Overmeer R, et al. Genome-wide CRISPR screens reveal a Wnt-FZD5 signaling circuit as a druggable vulnerability of RNF43-mutant pancreatic tumors. Nat Med. 2017;23(1):60–8.
Linke F, Zaunig S, Nietert MM, von Bonin F, Lutz S, Dullin C, Janovska P, Beissbarth T, Alves F, Klapper W, et al. WNT5A: a motility-promoting factor in Hodgkin lymphoma. Oncogene. 2017;36(1):13–23.
Voloshanenko O, Gmach P, Winter J, Kranz D, Boutros M. Map** of Wnt-Frizzled interactions by multiplex CRISPR targeting of receptor gene families. FASEB J. 2017;31(11):4832–44.
Tennis MA, Van Scoyk MM, Freeman SV, Vandervest KM, Nemenoff RA, Winn RA. Sprouty-4 inhibits transformed cell growth, migration and invasion, and epithelial-mesenchymal transition, and is regulated by Wnt7A through PPARgamma in non-small cell lung cancer. Mol Cancer Res. 2010;8(6):833–43.
Tiffon C, Giraud J, Molina-Castro SE, Peru S, Seeneevassen L, Sifre E, Staedel C, Bessede E, Dubus P, Megraud F et al: TAZ controls Helicobacter pylori-induced epithelial-mesenchymal transition and cancer stem cell-like invasive and tumorigenic properties. Cells. 2020; 9(6).
Gao Y, Li J, ** H, Cui J, Zhang K, Zhang J, Zhang Y, Xu W, Liang W, Zhuang Z, et al. Stearoyl-CoA-desaturase-1 regulates gastric cancer stem-like properties and promotes tumour metastasis via Hippo/YAP pathway. Br J Cancer. 2020;122(12):1837–47.
Tang DE, Dai Y, Lin LW, Xu Y, Liu DZ, Hong XP, Jiang HW, Xu SH. STUB1 suppresseses tumorigenesis and chemoresistance through antagonizing YAP1 signaling. Cancer Sci. 2019;110(10):3145–56.
Choi W, Kim J, Park J, Lee DH, Hwang D, Kim JH, Ashktorab H, Smoot D, Kim SY, Choi C, et al. YAP/TAZ initiates gastric tumorigenesis via upregulation of MYC. Cancer Res. 2018;78(12):3306–20.
Giraud J, Molina-Castro S, Seeneevassen L, Sifre E, Izotte J, Tiffon C, Staedel C, Boeuf H, Fernandez S, Barthelemy P, et al. Verteporfin targeting YAP1/TAZ-TEAD transcriptional activity inhibits the tumorigenic properties of gastric cancer stem cells. Int J Cancer. 2020;146(8):2255–67.
Ou H, Chen Z, **ang L, Fang Y, Xu Y, Liu Q, Hu Z, Li X, Huang Y, Yang D. Frizzled 2-induced epithelial-mesenchymal transition correlates with vasculogenic mimicry, stemness, and Hippo signaling in hepatocellular carcinoma. Cancer Sci. 2019;110(4):1169–82.
Tu B, Yao J, Ferri-Borgogno S, Zhao J, Chen S, Wang Q, Yan L, Zhou X, Zhu C, Bang S et al: YAP1 oncogene is a context-specific driver for pancreatic ductal adenocarcinoma. JCI Insight. 2019; 4(21).
Tang Y, Weiss SJ. Snail/Slug-YAP/TAZ complexes cooperatively regulate mesenchymal stem cell function and bone formation. Cell Cycle. 2017;16(5):399–405.
Prescott JD, Poczobutt JM, Tentler JJ, Walker DM, Gutierrez-Hartmann A. Map** of ESE-1 subdomains required to initiate mammary epithelial cell transformation via a cytoplasmic mechanism. Mol Cancer. 2011;10:103.
Prescott JD, Koto KS, Singh M, Gutierrez-Hartmann A. The ETS transcription factor ESE-1 transforms MCF-12A human mammary epithelial cells via a novel cytoplasmic mechanism. Mol Cell Biol. 2004;24(12):5548–64.
Longoni N, Sarti M, Albino D, Civenni G, Malek A, Ortelli E, Pinton S, Mello-Grand M, Ostano P, D’Ambrosio G, et al. ETS transcription factor ESE1/ELF3 orchestrates a positive feedback loop that constitutively activates NF-kappaB and drives prostate cancer progression. Cancer Res. 2013;73(14):4533–47.
Yeung TL, Leung CS, Wong KK, Gutierrez-Hartmann A, Kwong J, Gershenson DM, Mok SC. ELF3 is a negative regulator of epithelial-mesenchymal transition in ovarian cancer cells. Oncotarget. 2017;8(10):16951–63.
Sengez B, Aygun I, Shehwana H, Toyran N, Tercan Avci S, Konu O, Stemmler MP, Alotaibi H: The transcription factor Elf3 is essential for a successful mesenchymal to epithelial transition. Cells. 2019; 8(8).
Sinh ND, Endo K, Miyazawa K, Saitoh M. Ets1 and ESE1 reciprocally regulate expression of ZEB1/ZEB2, dependent on ERK1/2 activity, in breast cancer cells. Cancer Sci. 2017;108(5):952–60.
Liu D, Skomorovska Y, Song J, Bowler E, Harris R, Ravasz M, Bai S, Ayati M, Tamai K, Koyuturk M, et al. ELF3 is an antagonist of oncogenic-signalling-induced expression of EMT-TF ZEB1. Cancer Biol Ther. 2019;20(1):90–100.
Hua Y, Yang Y, Li Q, He X, Zhu W, Wang J, Gan X. Oligomerization of Frizzled and LRP5/6 protein initiates intracellular signaling for the canonical WNT/beta-catenin pathway. J Biol Chem. 2018;293(51):19710–24.
Ren DN, Chen J, Li Z, Yan H, Yin Y, Wo D, Zhang J, Ao L, Chen B, Ito TK, et al. LRP5/6 directly bind to Frizzled and prevent Frizzled-regulated tumour metastasis. Nat Commun. 2015;6:6906.
Fischer MM, Cancilla B, Yeung VP, Cattaruzza F, Chartier C, Murriel CL, Cain J, Tam R, Cheng CY, Evans JW, et al. WNT antagonists exhibit unique combinatorial antitumor activity with taxanes by potentiating mitotic cell death. Sci Adv. 2017;3(6):e1700090.
Acknowledgements
Not applicable
Funding
This work is supported by two grants from Department of Science and Technology of Liaoning Province (2019-MS-363, 2017225028) and a project funded by China Postdoctoral Science Foundation (2020M681014).
Author information
Authors and Affiliations
Contributions
DD, LN and KZ performed the experiments, analyzed the data and wrote the manuscript. ZW, YS, QZ and JG performed the experiments. WW and CZ designed the project, interpreted the data, and drafted the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Consent for publication
Not applicable.
Ethics approval and consent to participate
Gastric cancer tissues were obtained from Sheng**g Hospital China Medical University with the informed consent of the patients. Institutional Research Ethics Committee of China Medical University approved the use of these tissues for research purposes.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dong, D., Na, L., Zhou, K. et al. FZD5 prevents epithelial-mesenchymal transition in gastric cancer. Cell Commun Signal 19, 21 (2021). https://doi.org/10.1186/s12964-021-00708-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12964-021-00708-z