Abstract
Background
In frozen embryo transfer (FET), there is limited consensus on the best means of endometrial preparation in terms of the reproductive outcomes in women with polycystic ovary syndrome (PCOS). The present study aimed to compare the pregnancy and neonatal outcomes following artificial cycle FET (AC-FET) with or without gonadotropin-releasing hormone agonist (GnRH-a) pretreatment among women with PCOS.
Methods
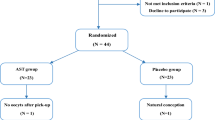
A total of 4503 FET cycles that satisfied the inclusion criteria were enrolled in this retrospective cohort study between 2015 and 2020. The GnRH-a group received GnRH-a pretreatment while the AC-FET group did not. Propensity score matching (PSM) method and multivariate logistic regression analysis were performed to adjust for potential confounding factors.
Results
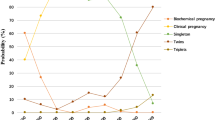
After PSM, women in the GnRH-a group suffered a significantly lower miscarriage rate (11.2% vs. 17.1%, P = 0.033) and a higher live birth rate (LBR) compared with those in the AC-FET group (63.1% vs. 56.8%, P = 0.043). No differences were observed in the rates of biochemical pregnancy, clinical pregnancy and ectopic pregnancy between the two groups. A higher mean gestational age at birth was observed in the GnRH-a group than in the AC-FET group (39.80 ± 2.01 vs. 38.17 ± 2.13, P = 0.009). The incidence of neonatal preterm birth (PTB) in the GnRH-a group was lower than that in the AC-FET group (7.4% vs. 14.9%, P = 0.009). Singleton newborns conceived after GnRH-a group were more likely to be small for gestational age (SGA) than those born after AC-FET group (16.4% vs. 6.8%, P = 0.009). However, no significant differences were found between the two groups in terms of mean birthweight, apgar score, the rates of macrosomia, large for gestational age and low birth weight.
Conclusion(s)
In women with PCOS who underwent AC-FET, GnRH-a pretreatment was significantly associated with a higher live birth rate and a reduced risk of neonatal PTB. However, there was a concomitant increase in the risk of develo** SGA babies.
Similar content being viewed by others
Background
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders in women of reproductive age, accounting for about 70% of anovulatory infertility [1]. Among the strategies for the treatment of infertile PCOS women, frozen embryo transfer (FET) may achieve a higher live birth rate and reduce the risk of ovarian hyperstimulation syndrome (OHSS) compared with fresh embryo transfer [2]. Hence, the application of FET has been recommended as a relatively effective and safer treatment method for this group of infertility patients [3].
Multiple endometrial preparation (EP) cycle protocols have been designed to provide an optimal endometrial environment for embryo implantation in a FET program [4]. However, there is limited consensus on the best means of EP in terms of the reproductive outcomes in women with PCOS [5]. Since PCOS is associated with ovulation dysfunction and irregular menstrual cycles, the most appropriate and frequently used cycle protocol is the artificial cycle FET (AC-FET) [6]. In AC-FET, the endometrium is artificially prepared through consecutive administration of exogenous estrogen and progesterone with or without gonadotropin-releasing hormone agonist (GnRH-a) pretreatment to simulate the natural endocrine environment of the endometrium [7].
GnRH-a is a gonadotropin-releasing hormone (GnRH) analogue with high affinity for pituitary GnRH receptors. After administration, GnRH-a binds to pituitary GnRH receptors and transiently inhibits the hypothalamic–pituitary–gonadal axis, inducing a hypo-estrogenic state [8]. Lower estrogen levels after down-regulation could prevent spontaneous ovulation and prolong the opening period of the “implantation window” to a certain extent [9, 10]. This might be beneficial to the pregnancy outcomes for women undergoing FET.
In assisted reproductive technology (ART), GnRH-a pretreatment combined with AC-FET was found to improve the live birth rate in patients with endometriosis and adenomyosis [11, 30]. However, further studies are needed to validate these findings and clarify the underlying mechanisms.
Strengths and limitations
To the best of our knowledge, this is the largest population study in this field to assess pregnancy-related outcomes following AC-FET with or without GnRH-a pretreatment in women with PCOS. Unlike previous studies that mainly focused on live birth, our study also provided more complete information on the effectiveness and safety of GnRH-a pretreatment by analyzing the neonatal outcomes in PCOS women. Additionally, our study included extensive control for potential confounding differences between GnRH-a and the routine AC-FET groups via PSM and multivariate logistic regression models, thus creating two similar cohorts.
We acknowledge that in this study, we did not obtain information about pregnancy complications, such as gestational hypertension, preeclampsia, and gestational diabetes, which are potential risk factors for adverse neonatal outcomes [39, 40]. This may have a confounding effect on the results. Another limitation is that the neonatal data were obtained through telephone follow-up,which might have led to some information bias.
Conclusions
Our study demonstrated that in women with PCOS who underwent AC-FET, GnRH-a pretreatment was significantly associated with an increase in LBR and a reduced risk of neonatal PTB. However, the incidence of newborns being SGA was also significantly increased at the same time. Therefore, before applying the GnRH-a pretreatment regimen in PCOS women, it seems necessary to take some measures to reduce the risk of neonatal SGA events. Further studies are needed to verify our findings and clarify the underlying mechanisms.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- FET:
-
Frozen embryo transfer
- GnRH-a:
-
Gonadotropin-releasing hormone agonist
- PCOS:
-
Polycystic ovary syndrome
- AC-FET:
-
Artificial cycle FET
- PSM:
-
Propensity score matching
- LBR:
-
Live birth rate
- GA:
-
Gestational age
- LBW:
-
Low birthweight
- PTB:
-
Preterm birth
- SGA:
-
Small for gestational age
- LGA:
-
Large for gestational age
- OHSS:
-
Ovarian hyperstimulation syndrome
- EP:
-
Endometrial preparation
- ART:
-
Assisted reproductive technology
- PGT:
-
Preimplantation genetic testing
- PGS:
-
Preimplantation genetic screening
- E2:
-
Estradiol
- FSH:
-
Follicle-stimulating hormone
- LH:
-
Luteinizing hormone
- hCG:
-
Human chorionic gonadotropin
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- BMI:
-
Body mass index
- HPO:
-
Hypothalamus-pituitary-ovary
- P:
-
Progesterone
- AFC:
-
Antral follicle count
- AMH:
-
Anti-Mullerian hormone
- SD:
-
Standard deviation
- IQR:
-
Inter-quartile range
References
Brassard M, AinMelk Y, Baillargeon JP. Basic infertility including polycystic ovary syndrome. Med Clin North Am. 2008;92(5):1163–92 (xi).
Chen Z, Shi Y, Sun Y, Zhang B, Liang X, Cao Y, et al. Fresh versus Frozen Embryos for Infertility in the Polycystic Ovary Syndrome. N Engl J Med. 2016;375(6):523–33.
Roque M, Haahr T, Geber S, Esteves SC, Humaidan P. Fresh versus elective frozen embryo transfer in IVF/ICSI cycles: a systematic review and meta-analysis of reproductive outcomes. Hum Reprod Update. 2019;25(1):2–14.
Mackens S, Santos-Ribeiro S, van de Vijver A, Racca A, Van Landuyt L, Tournaye H, et al. Frozen embryo transfer: a review on the optimal endometrial preparation and timing. Hum Reprod. 2017;32(11):2234–42.
Kollmann M, Martins WP, Lima ML, Craciunas L, Nastri CO, Richardson A, et al. Strategies for improving outcome of assisted reproduction in women with polycystic ovary syndrome: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2016;48(6):709–18.
Man Y, Bian Y, Zhao S, Zhao R, Xu X, Wei D, et al. The effect of different endometrial preparations on women with polycystic ovary syndrome undergoing initial frozen embryo transfer: A historical cohort analysis. Acta Obstet Gynecol Scand. 2021;100(6):1116–23.
Ghobara T, Gelbaya TA, Ayeleke RO. Cycle regimens for frozen-thawed embryo transfer. Cochrane Database Syst Rev. 2017;7(7):Cd003414.
Xu J, Li SZ, Yin MN, Liang PL, Li P, Sun L. Endometrial Preparation for Frozen-Thawed Embryo Transfer With or Without Pretreatment With GnRH Agonist: A Randomized Controlled Trial at Two Centers. Front Endocrinol (Lausanne). 2021;12:722253.
Ma WG, Song H, Das SK, Paria BC, Dey SK. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci U S A. 2003;100(5):2963–8.
Greco E, Litwicka K, Arrivi C, Varricchio MT, Caragia A, Greco A, et al. The endometrial preparation for frozen-thawed euploid blastocyst transfer: a prospective randomized trial comparing clinical results from natural modified cycle and exogenous hormone stimulation with GnRH agonist. J Assist Reprod Genet. 2016;33(7):873–84.
Niu Z, Chen Q, Sun Y, Feng Y. Long-term pituitary downregulation before frozen embryo transfer could improve pregnancy outcomes in women with adenomyosis. Gynecol Endocrinol. 2013;29(12):1026–30.
Qi Q, Luo J, Wang Y, **e Q. Effects of artificial cycles with and without gonadotropin-releasing hormone agonist pretreatment on frozen embryo transfer outcomes. J Int Med Res. 2020;48(6):300060520918474.
Bosdou JK, Anagnostis P, Kolibianakis EM. Is pretreatment with GnRH agonist necessary for endometrial preparation for frozen embryo transfer cycles in women with polycystic ovary syndrome? BJOG. 2021;128(4):675.
Tsai HW, Wang PH, Lin LT, Chen SN, Tsui KH. Using gonadotropin-releasing hormone agonist before frozen embryo transfer may improve ongoing pregnancy rates in hyperandrogenic polycystic ovary syndrome women. Gynecol Endocrinol. 2017;33(9):686–9.
**e D, Chen F, **e SZ, Chen ZL, Tuo P, Zhou R, et al. Artificial Cycle with or without a Depot Gonadotropin-releasing Hormone Agonist for Frozen-thawed Embryo Transfer: An Assessment of Infertility Type that Is Most Suitable. Current medical science. 2018;38(4):626–31.
Luo L, Chen M, Wen Y, Zhang L, Zhou C, Wang Q. Pregnancy outcome and cost-effectiveness comparisons of artificial cycle-prepared frozen embryo transfer with or without GnRH agonist pretreatment for polycystic ovary syndrome: a randomised controlled trial. BJOG. 2021;128(4):667–74.
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25.
Capital Institute of Pediatrics. Growth standard curves of birth weight, length and head circumference of Chinese newborns of different gestation. Chin J Pediatr. 2020;58(9):738–46.
Garrido MM, Kelley AS, Paris J, Roza K, Meier DE, Morrison RS, et al. Methods for constructing and assessing propensity scores. Health Serv Res. 2014;49(5):1701–20.
Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46(3):399–424.
Zhang J, Liu H, Wang Y, Mao X, Chen Q, Fan Y, et al. Letrozole use during frozen embryo transfer cycles in women with polycystic ovary syndrome. Fertil Steril. 2019;112(2):371–7.
Rosas C, Oróstica L, Poblete C, Carvajal R, Gabler F, Romero C, et al. Hyperandrogenism Decreases GRP78 Protein Level and Glucose Uptake in Human Endometrial Stromal Cells. Reprod Sci. 2016;23(6):761–70.
Schulte MM, Tsai JH, Moley KH. Obesity and PCOS: the effect of metabolic derangements on endometrial receptivity at the time of implantation. Reprod Sci. 2015;22(1):6–14.
Lebbe M, Woodruff TK. Involvement of androgens in ovarian health and disease. Mol Human Reprod. 2013;19(12):828–37.
Qiao J, Feng HL. Extra- and intra-ovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence. Hum Reprod Update. 2011;17(1):17–33.
Cocksedge KA, Saravelos SH, Wang Q, Tuckerman E, Laird SM, Li TC. Does free androgen index predict subsequent pregnancy outcome in women with recurrent miscarriage? Hum Reprod. 2008;23(4):797–802.
Merseburger AS, Hupe MC. An Update on Triptorelin: Current Thinking on Androgen Deprivation Therapy for Prostate Cancer. Adv Ther. 2016;33(7):1072–93.
Chappel SC, Howles C. Reevaluation of the roles of luteinizing hormone and follicle-stimulating hormone in the ovulatory process. Hum Reprod. 1991;6(9):1206–12.
Tesarik J, Hazout A, Mendoza C. Luteinizing hormone affects uterine receptivity independently of ovarian function. Reprod Biomed Online. 2003;7(1):59–64.
Wang B, Zhang J, Zhu Q, Yang X, Wang Y. Effects of different cycle regimens for frozen embryo transfer on perinatal outcomes of singletons. Hum Reprod. 2020;35(7):1612–22.
Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379(9832):2151–61.
Zong L, Liu P, Zhou L, Wei D, Ding L, Qin Y. Increased risk of maternal and neonatal complications in hormone replacement therapy cycles in frozen embryo transfer. Reprod Biol Endocrinol. 2020;18(1):36.
Hu KL, Zhang D, Li R. Endometrium preparation and perinatal outcomes in women undergoing single-blastocyst transfer in frozen cycles. Fertil Steril. 2021;115(6):1487–94.
Lin J, Guo H, Wang B, Chen Q, Zhu Q. Neonatal outcomes in women with polycystic ovary syndrome after frozen-thawed embryo transfer. Fertil Steril. 2021;115(2):447–54.
Bu Z, Zhang J, Hu L, Sun Y. Preterm Birth in Assisted Reproductive Technology: An Analysis of More Than 20,000 Singleton Newborns. Front Endocrinol (Lausanne). 2020;11:558819.
Christ JP, Gunning MN, Meun C, Eijkemans MJC, van Rijn BB, Bonsel GJ, et al. Pre-Conception Characteristics Predict Obstetrical and Neonatal Outcomes in Women With Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2019;104(3):809–18.
Hu S, Xu B, Long R, ** L. The effect of polycystic ovary syndrome without hyperandrogenism on pregnancy-related outcomes: a retrospective cohort study. BJOG. 2021;128(6):1003–10.
Li Y, Ruan X, Wang H, Li X, Cai G, Du J, et al. Comparing the risk of adverse pregnancy outcomes of Chinese patients with polycystic ovary syndrome with and without antiandrogenic pretreatment. Fertil Steril. 2018;109(4):720–7.
Billionnet C, Mitanchez D, Weill A, Nizard J, Alla F, Hartemann A, et al. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia. 2017;60(4):636–44.
Dassah ET, Kusi-Mensah E, Morhe ESK, Odoi AT. Maternal and perinatal outcomes among women with hypertensive disorders in pregnancy in Kumasi, Ghana. PLoS One. 2019;14(10):e0223478.
Acknowledgements
We would like to acknowledge all the participants of this project and the medical staff for their contribution to this work.
Funding
This research was supported by funds from Chongqing medical scientific research project (nos:2022QNXM042), Natural Science Foundation of Chongqing (nos:cstc2021jcyj-msxmX0900) and National Natural Science Foundation of China (NO. 82104923).
Author information
Authors and Affiliations
Contributions
YW and WH performed the data collection, statistical analyses and drafted the manuscript. YQ and MC contributed to data collection. QW, XM, XT, QF and YD were responsible for data acquisition and collection. TL and EA participated in the interpretation of the data and critical revision. ZZ, LG and XL designed the study and directed implementation. All authors were involved in revising the article and have approved this final version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Ethics Committee of Chongqing Medical University. Since this is a retrospective investigation, patients were not asked to participate in this study.
Consent for publication
Not applicable.
Competing interests
None of the authors declared a conflict of interest with this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, Y., Hu, WH., Wan, Q. et al. Effect of artificial cycle with or without GnRH-a pretreatment on pregnancy and neonatal outcomes in women with PCOS after frozen embryo transfer: a propensity score matching study. Reprod Biol Endocrinol 20, 56 (2022). https://doi.org/10.1186/s12958-022-00929-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12958-022-00929-y