Abstract
Purpose
Thymoma is the most common primary tumor in the anterior mediastinum. The prognostic factors of patients with thymoma still need to be clarified. In this study, we aimed to investigate the prognostic factors of patients with thymoma who received radical resection and establish the nomogram to predict the prognosis of these patients.
Materials and methods
Patients who underwent radical resection for thymoma with complete follow-up data between 2005 and 2021 were enrolled. Their clinicopathological characteristics and treatment methods were retrospectively analyzed. Progression-free survival (PFS) and overall survival (OS) were estimated using the Kaplan–Meier method and compared by the log-rank test. Univariate and multivariate Cox proportional hazards regression analyses were performed to identify the independent prognostic factors. According to the results of the univariate analysis in the Cox regression model, the predictive nomograms were created.
Results
A total of 137 patients with thymoma were enrolled. With a median follow-up of 52 months, the 5-year and 10-year PFS rates were 79.5% and 68.1%, respectively. The 5-year and 10-year OS rates were 88.4% and 73.1%, respectively. Smoking status (P = 0.022) and tumor size (P = 0.039) were identified as independent prognostic factors for PFS. Multivariate analysis showed that a high level of neutrophils (P = 0.040) was independently associated with OS. The nomogram showed that the World Health Organization (WHO) histological classification contributed more to the risk of recurrence than other factors. Neutrophil count was the most important predictor of OS in patients with thymoma.
Conclusion
Smoking status and tumor size are risk factors for PFS in patients with thymoma. A high level of neutrophils is an independent prognostic factor for OS. The nomograms developed in this study accurately predict PFS and OS rates at 5 and 10 years in patients with thymoma based on individual characteristics.
Similar content being viewed by others
Introduction
Thymoma is the most common primary tumor in the anterior mediastinum, although the incidence is generally low [1,2,3]. Moreover, thymoma is associated with a variety of autoimmune diseases, such as hyperthyroidism, pure red cell aplastic anemia, myasthenia gravis (MG), and endocrine disorders [4]. Treatment options for thymoma include surgery, radiotherapy, and systemic therapy. Surgery is the main treatment method for thymoma [5, 6]. Prognostic factors of postoperative thymoma patients include age, tumor size, World Health Organization (WHO) histological classification, paraneoplastic syndrome, great vessel invasion, Masaoka-Koga stage, TNM stage, and completeness of resection [7,8,9,10,11,12,13]. However, all these identified factors are not sufficient to explain the prognosis of patients with thymoma with large heterogeneous features. Therefore, the clinical features and prognostic factors of patients with thymoma still need to be clarified. In this study, we analyzed several indicators after surgical resection of thymoma to determine important prognostic factors. At the same time, the predictive nomogram models were established based on the risk factors of thymoma patients, and the performance of nomogram was measured. We believe that these results will improve our understanding of thymoma and facilitate the development of individualized treatment and optimal follow-up strategies.
Materials and methods
Study design and patient population
Patients who underwent radical resection for thymoma at our hospital with complete follow-up and histopathological data from 2005 to 2021 were enrolled and analyzed retrospectively. Patients were excluded according to the following criteria: (i) received neoadjuvant chemotherapy or radiotherapy; (ii) had recently received continuous steroid therapy, active infection, or other bone marrow disease before surgery; and (iii) had a history of other types of cancer within 1 year. The following data were collected from the electronic medical record system: age, gender, presence of autoimmune disease, surgical approach, extent of resection, adjuvant therapy, tumor size, tumor histology according to WHO current classification, and Masaoka-Koga stage. Pre-treatment neutrophil and lymphocyte counts were obtained from the routine blood test within 1 week before surgery. The research protocol was approved by Ningxia Medical University General Hospital (KYLL-2022–0013). Patient consent was waived due to the retrospective study design.
Treatment methods
The surgery was performed by experienced surgeons, using median sternotomy, muscle-preserving thoracotomy, or video-assisted thoracoscopic surgery (VATS), depending on the tumor size, location, and extension of the thymoma. The resection status was classified as R0 (no residual tumor), R1 (microscopic residual tumor), and R2 (macroscopic residual tumor) according to the pathology of the specimen and the surgical findings. Postoperative treatment options were selected based on resection status, histology, and Masaoka-Koga stage. Patients with type B2 histology and above, non-R0 resection, and stage II and above were considered for radiotherapy. Patients with thymic carcinoma (Tc), R2 resection, and stage IV were recommended for chemotherapy. The most commonly used chemotherapy regimens were CAP (cisplatin, doxorubicin, cyclophosphamide) and TP (paclitaxel and cisplatin). Adjuvant radiotherapy was given 4 to 6 weeks after surgery. The dose range of adjuvant radiotherapy was 45 to 60 Gy. The target mainly covered the postoperative tumor bed.
Follow-up
Follow-up started from the date of completion of the surgery with or without adjuvant therapy and lasted until the patient’s last follow-up or death. Patients were followed up every 3 months in the first year, every 6 months in the following 2 years, and annually thereafter. Follow-up visits included examination of symptoms, chest CT, and blood tests. Patients with MG also underwent neurological monitoring, including clinical evaluation and laboratory tests. Recurrence was defined as histologically confirmed disease recurrence or recurrence that appeared on radiological imaging and response to treatment. Progression-free survival (PFS) is defined as the time from the date of the surgery to the recurrence or the last follow-up. Overall survival (OS) is defined as the time from the date of the surgery to death from any cause or the last follow-up.
Statistical analysis
The clinical and demographic characteristics of the enrolled patients were descriptively analyzed, and the median and range of continuous variables, as well as the absolute value and relative frequency of categorical variables, were analyzed. The optimal cutoff value of neutrophils or neutrophil to lymphocyte ratio (NLR) to predict survival was determined using receiver operating characteristic (ROC) curve analysis based on the maximum Youden index. Survival curves were plotted using the Kaplan–Meier method, and log-rank tests were performed to determine statistical significance. Univariate and multivariate Cox proportional hazard regression models were used to analyze the risk factors of PFS and OS. P < 0.05 was statistically significant. All statistical analyses were performed using SPSS (version 27.0 SPSS Inc., Chicago, IL, USA).
Based on the clinicopathological variables obtained from univariate Cox regression, we created the nomogram, which was completed using R 4.1 and the rms installation package. The concordance index (C-index) value and calibration curve were used to evaluate the performance of the nomogram. Bootstraps with 1000 resamples were used for these activities [14]. A two-sided P < 0.05 was the threshold of significance.
Results
Patients’ characteristics
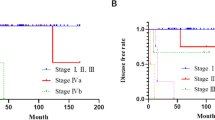
A total of 137 patients were enrolled in this retrospective study (Fig. 1). The clinicopathological characteristics of the enrolled patients are shown in Table 1. The median follow-up time was 52 months (2–208 months). At the time of the last follow-up, local recurrence occurred in 17 patients and distant metastasis in 8 patients. The 5-year and 10-year PFS rates were 79.5% and 68.1%, respectively. In addition, 14 patients died during the follow-up period. One patient died of hemoptysis, two patients died of heart failure, and one patient died for unknown reasons with the last follow-up CT scan negative for recurrence. Other patients died of recurrence or metastasis of thymoma. The 5-year and 10-year OS rates are 88.4% and 73.1%, respectively.
Univariate and multivariate analysis of PFS and OS
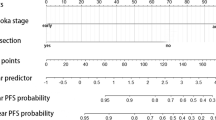
Univariate and multivariate Cox proportional hazard regression models were used to analyze the risk factors of PFS and OS in patients with thymoma. Univariate analysis showed that smoking status, surgical approach, tumor size, resection status, WHO histology classification, Masaoka-Koga stage, adjuvant radiotherapy, and adjuvant chemotherapy were significantly associated with PFS. These factors were included in the multivariate analysis. The results showed that smoking status (P = 0.022) and tumor size (P = 0.039) were independent risk factors for PFS (Table 2 and Fig. 2). Subsequent univariate analysis showed that the risk factors for OS were WHO classification, neutrophils, and NLR. After multivariate analysis, only neutrophils (P = 0.040) remained an independent prognostic factor of OS (Table 3 and Fig. 3).
The nomogram prediction model of PFS and OS
According to the results of the univariate analysis of the Cox regression model, 8 risk factors were selected to establish a nomogram to predict 5-year and 10-year PFS of thymoma patients, and 3 risk factors were selected to establish a nomogram to predict 5-year and 10-year OS. Nomogram showed that WHO classification contributed more to the risk of recurrence than other factors (Fig. 4). Neutrophil count was the most important predictor of OS in patients with thymoma (Fig. 5). Five-year PFS, OS and 10-year PFS, and OS could be estimated according to the total scores of each factor. Ninety-seven patients were enrolled in the training cohort, and other patients were enrolled in the validation cohort. The C-index for predicting PFS was 0.899 (95% CI, 0.842 ~ 0.956). The C-index for predicting OS was 0.804 (95% CI, 0.700 ~ 0.908). The calibration curves of 5-year and 10-year survival probabilities showed good agreement between the predictions of the nomogram and the actual observations (Figs. 4 and 5).
Discussion
Thymic epithelial tumors are relatively rare, with an incidence of 3.2 per 1 million people [1,2,3]. Approximately 80% of thymic epithelial tumors are thymomas [15]. Surgery is the main treatment for thymoma [5, 6]. In this study, we explored patients with thymoma who underwent surgery at our hospital over a 16-year period and analyzed prognostic factors for PFS and OS of these patients. We found that smoking status and tumor size were the major factors affecting PFS. In the subsequent analysis, we revealed that a high level of pretreatment neutrophils was an independent prognostic factor for OS.
The association between smoking and thymoma has been evaluated in several previous studies. In a European multicenter case–control study, Eriksson et al. [16] found that smoking and high spirits intake were risk factors for the development of thymoma. Yanagiya et al. [17] demonstrated that extrathymic malignancies increased the risk of death in patients with thymoma. History of smoking was a risk factor for postoperative extrathymic malignancies. Furthermore, in a recent study, smoking was identified as a critical factor negatively affecting PFS in patients with thymoma [13]. Our results are consistent with previous studies in which we found that smoking was one of the main factors affecting PFS in patients with thymoma. To avoid missed recurrences, postoperative thymoma patients with a history of smoking should be closely followed up. The maximum diameter of most thymomas can be easily measured and obtained from preoperative CT and magnetic resonance imaging (MRI). Therefore, the prognostic value of tumor size in patients with thymoma has been extensively evaluated in previous studies. Several studies have confirmed that thymic tumor size is an independent predictor of recurrence [18,19,20,21]. In the present study, PFS was significantly affected with increasing tumor size, but not OS, suggesting that tumor size is a good predictor of recurrence in patients with thymoma.
Neutrophils are first responders to infection and inflammation and also have a role due to their cancer-promoting properties [22, 31] and can be evaluated in further studies. Therefore, in the future, large, prospective, and randomized trials are needed to fully validate the results of this study.
In conclusion, we retrospectively analyzed the prognostic factors of thymoma patients undergoing surgery. The results showed that smoking status and tumor size were the main factors affecting PFS and that baseline high levels of neutrophils were independent prognostic factors for OS. The nomograms developed in this study accurately predict PFS and OS rates at 5 and 10 years in patients with thymoma based on individual characteristics. Our findings may be helpful to determine treatment and follow-up strategies for thymoma patients after radical resection.
Availability of data and materials
All remaining data are available within the article or available from the corresponding authors upon request.
References
de Jong WK, Blaauwgeers JL, Schaapveld M, Timens W, Klinkenberg TJ, Groen HJ. Thymic epithelial tumours: a population-based study of the incidence, diagnostic procedures and therapy. Eur J Cancer. 2008;44(1):123–30.
Wu J, Wang Z, **g C, Hu Y, Yang B, Hu Y. The incidence and prognosis of thymic squamous cell carcinoma: a Surveillance, Epidemiology, and End Results Program population-based study. Medicine (Baltimore). 2021;100(15): e25331.
Engels EA. Epidemiology of thymoma and associated malignancies. J Thorac Oncol. 2010;5(10 Suppl 4):S260–265.
Zhao J, Bhatnagar V, Ding L, Atay SM, David EA, McFadden PM, Stamnes S, Lechtholz-Zey E, Wightman SC, Detterbeck FC, et al. A systematic review of paraneoplastic syndromes associated with thymoma: treatment modalities, recurrence, and outcomes in resected cases. J Thorac Cardiovasc Surg. 2020;160(1):306-314. e314.
Fu H, Gu ZT, Fang WT, Fu JH, Shen Y, Han YT, Yu ZT, Li Y, Tan LJ, Pang LW, et al. Long-term survival after surgical treatment of thymic carcinoma: a retrospective analysis from the Chinese Alliance for Research of Thymoma Database. Ann Surg Oncol. 2016;23(2):619–25.
Zhao Y, Zhao H, Hu D, Fan L, Shi J, Fang W. Surgical treatment and prognosis of thymic squamous cell carcinoma: a retrospective analysis of 105 cases. Ann Thorac Surg. 2013;96(3):1019–24.
Agrafiotis AC, Siozopoulou V, Hendriks JMH, Pauwels P, Koljenovic S, Van Schil PE. Prognostic factors and genetic markers in thymic epithelial tumors: a narrative review. Thorac Cancer. 2022;13(23):3242–9.
Li J, Liu Y, Zhang X, Zheng X, Qi G. Prognostic factors for overall survival after surgical resection in patients with thymic epithelial tumors: a systematic review and meta-analysis. Medicine (Baltimore). 2022;101(39): e30867.
Knetki-Wroblewska M, Kowalski DM, Olszyna-Serementa M, Krzakowski M, Szolkowska M. Thymic epithelial tumors: do we know all the prognostic factors? Thorac Cancer. 2021;12(3):339–48.
Banna GL, Sheel A, Sheel V, Bille A, Routledge T, Fernando S, Nair A, Lal R. Treatment and prognostic factors of patients with thymic epithelial tumors at first recurrence or progression. Future Oncol. 2017;13(27):2429–39.
Demirci S, Turhan K, Ozsan N, Yalman D, Cakan A, Cok G, Cagirici U, Ozkok S. Prognostic factors for survival in patients with thymic epithelial tumors. Thorac Cardiovasc Surg. 2011;59(3):153–7.
Detterbeck F, Youssef S, Ruffini E, Okumura M. A review of prognostic factors in thymic malignancies. J Thorac Oncol. 2011;6(7 Suppl 3):S1698–1704.
Alkaaki A, Abo Al-Saud A, Di Lena E, Ramirez-GarciaLuna JL, Najmeh S, Spicer J, Ferri L, Mulder D, Sirois C, Cools-Lartigue J. Factors predicting recurrence in thymic epithelial neoplasms. Eur J Cardiothorac Surg. 2022;62(5):ezac274.
Heller G, Mo Q. Estimating the concordance probability in a survival analysis with a discrete number of risk groups. Lifetime Data Anal. 2016;22(2):263–79.
Scorsetti M, Leo F, Trama A, D’Angelillo R, Serpico D, Macerelli M, Zucali P, Gatta G, Garassino MC. Thymoma and thymic carcinomas. Crit Rev Oncol Hematol. 2016;99:332–50.
Eriksson M, Kaerlev L, Johansen P, Afonso N, Ahrens W, Costa-Pereira A, Guenel P, Jockel KH, Gonzalez AL, Merletti F, et al. Tobacco smoking and alcohol consumption as risk factors for thymoma - a European case-control study. Cancer Epidemiol. 2019;61:133–8.
Yanagiya M, Matsumoto J, Kawahara T, Yamaguchi H, Nagayama K, Anraku M, Sato M, Nakajima J. Influence of smoking and histologic subtype on develo** extrathymic malignancy in thymoma patients. Ann Thorac Surg. 2019;107(5):1532–9.
Roden AC, Yi ES, Jenkins SM, Edwards KK, Donovan JL, Cassivi SD, Marks RS, Garces YI, Aubry MC. Modified Masaoka stage and size are independent prognostic predictors in thymoma and modified Masaoka stage is superior to histopathologic classifications. J Thorac Oncol. 2015;10(4):691–700.
Okumura M, Yoshino I, Yano M, Watanabe SI, Tsuboi M, Yoshida K, Date H, Yokoi K, Nakajima J, Toyooka SI, et al. Tumour size determines both recurrence-free survival and disease-specific survival after surgical treatment for thymoma. Eur J Cardiothorac Surg. 2019;56(1):174–81.
Fukui T, Fukumoto K, Okasaka T, Kawaguchi K, Nakamura S, Hakiri S, Ozeki N, Hirakawa A, Tateyama H, Yokoi K. Prognostic impact of tumour size in completely resected thymic epithelial tumours. Eur J Cardiothorac Surg. 2016;50(6):1068–74.
Tseng YC, Hsu HS, Lin YH, Tseng YH, Shu CW, Goan YG, Tseng CJ. Does size affect the prognosis of resectable thymoma beyond the eighth edition TNM? Thorac Cancer. 2022; 13(3):346–352.
Hedrick CC, Malanchi I. Neutrophils in cancer: heterogeneous and multifaceted. Nat Rev Immunol. 2022;22(3):173–87.
**ong S, Dong L, Cheng L. Neutrophils in cancer carcinogenesis and metastasis. J Hematol Oncol. 2021;14(1):173.
Ocana A, Nieto-Jimenez C, Pandiella A, Templeton AJ. Neutrophils in cancer: prognostic role and therapeutic strategies. Mol Cancer. 2017;16(1):137.
Meager A, Wadhwa M, Dilger P, Bird C, Thorpe R, Newsom-Davis J, Willcox N. Anti-cytokine autoantibodies in autoimmunity: preponderance of neutralizing autoantibodies against interferon-alpha, interferon-omega and interleukin-12 in patients with thymoma and/or myasthenia gravis. Clin Exp Immunol. 2003;132(1):128–36.
Okada S, Shimomura M, Tsunezuka H, Ishihara S, Ikebe S, Furuya T, Shimada J, Teramukai S, Inoue M. High neutrophil count as a negative prognostic factor for relapse in patients with thymic epithelial tumor. Ann Surg Oncol. 2020;27(7):2438–47.
Marx A, Chan JKC, Chalabreysse L, Dacic S, Detterbeck F, French CA, Hornick JL, Inagaki H, Jain D, Lazar AJ, et al. The 2021 WHO Classification of Tumors of the Thymus and Mediastinum: what is new in thymic epithelial, germ cell, and mesenchymal tumors? J Thorac Oncol. 2022;17(2):200–13.
Lee GD, Kim HR, Choi SH, Kim YH, Kim DK, Park SI. Prognostic stratification of thymic epithelial tumors based on both Masaoka-Koga stage and WHO classification systems. J Thorac Dis. 2016;8(5):901–10.
Chiappetta M, Lococo F, Pogliani L, Sperduti I, Tabacco D, Bria E, D’Argento E, Massaccesi M, Boldrini L, Meacci E, et al. Masaoka-Koga and TNM staging system in thymic epithelial tumors: prognostic comparison and the role of the number of involved structures. Cancers (Basel). 2021;13(21):5254.
Valdivia D, Cheufou D, Fels B, Puhlvers S, Mardanzai K, Zaatar M, Weinreich G, Taube C, Theegarten D, Stuschke M, et al. Potential prognostic value of preoperative leukocyte count, lactate dehydrogenase and C-reactive protein in thymic epithelial tumors. Pathol Oncol Res. 2021;27: 629993.
Chen Y, Zhou S, Yang S, Mofatteh M, Hu Y, Wei H, Lai Y, Zeng Z, Yang Y, Yu J, et al. Develo** and predicting of early mortality after endovascular thrombectomy in patients with acute ischemic stroke. Front Neurosci. 2022;16:1034472.
Funding
None.
Author information
Authors and Affiliations
Contributions
YYW and JXH conceived and designed the study. RM, XHB, RZ, JXH, and YYW analyzed and interpreted the results of the study. YGJ, MYM, and JJW performed the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was reviewed and approved by the Ethics Committee of the General Hospital of Ningxia Medical University (KYLL-2022–0013). Patient consent was waived due to the retrospective study design.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jiang, YG., Ma, MY., Wu, JJ. et al. Prognostic factors in patients with thymoma who underwent surgery. World J Surg Onc 21, 203 (2023). https://doi.org/10.1186/s12957-023-03068-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-023-03068-9