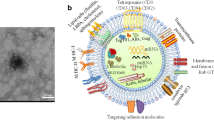
Abstract
Oral and maxillofacial diseases are one of the most prevalent diseases in the world, which not only seriously affect the health of patients’ oral and maxillofacial tissues, but also bring serious economic and psychological burdens to patients. Therefore, oral and maxillofacial diseases require effective treatment. Traditional treatments have limited effects. In recent years, nature exosomes have attracted increasing attention due to their ability to diagnose and treat diseases. However, the application of nature exosomes is limited due to low yield, high impurities, lack of targeting, and high cost. Engineered exosomes can be endowed with better comprehensive therapeutic properties by modifying exosomes of parent cells or directly modifying exosomes, and biomaterial loading exosomes. Compared with natural exosomes, these engineered exosomes can achieve more effective diagnosis and treatment of oral and maxillary system diseases, and provide reference and guidance for clinical application. This paper reviews the engineering modification methods of exosomes and the application of engineered exosomes in oral and maxillofacial diseases and looks forward to future research directions.
Graphical Abstract

Similar content being viewed by others
Introduction
The oral and maxillofacial system is a complex physiological system with various anatomical structures such as teeth, periodontal tissues, jaws, mucous membranes, joints and other organs or tissues. Based on the above anatomical structures, the stomatognathic system has a variety of important functions including respiration, mastication, and speech. Oral and maxillofacial diseases are one of the most prevalent diseases in the world, which not only affect the physiological functions of the oral cavity but may even endanger the patients’ general health status, greatly reducing the quality of life of the affected individuals, and bringing a serious economic burden to the family and the society [1]. Typical oral and maxillofacial diseases, such as pulpitis, periodontitis, and oral cancer, often result in irreversible structural defects and associated dysfunction [2]. Therefore, effective methods are needed to treat oral and maxillofacial diseases. Traditional treatments have limited effects. In recent years, scholars have found that exosomes have a positive therapeutic role among the above mentioned diseases [3].
Exosomes were first proposed in the 1980s by Harding and Pan et al. [4, 5]. Exosomes are one of the extracellular vesicles (EVs) secreted by mother cells in the extracellular environment and are widely distributed in body fluids, which have a bilayered phospholipid membrane structure [6], exosomes contain large amounts of biologically active proteins, lipids, nucleic acids, etc. [7]. The functional status of exosomes-derived cells can be assessed by analyzing their exosomes contents, therefore exosomes can be used as one of the tools for disease diagnosis. Exosomes can also be involved in physiological processes by regulating the function of target cells through intercellular cargo molecular transfer, so they can also be used as a therapeutic tool for a variety of diseases, including tissue regeneration, immunomodulation, and cancer therapy [8, 43]. It has been demonstrated that engineered exosomes can be used to diagnose periodontitis. Studies have shown that salivary exosomes levels of CD9 and CD81 are significantly reduced in periodontitis patients compared to healthy controls [44]. Compared to healthy/gingivitis subjects, the total concentration of EV in gingival crevicular fluid (GCF) increased in periodontitis patients, and the CD63 exosome markers in GCF increased in periodontitis patients [45]. Exosome-based PD-L1 mRNA detection in saliva has the potential to differentiate between periodontitis and health, and its level correlates with the severity/stage of periodontitis [46]. Detection of miR-223-3p expression in salivary exosomes can be an important non-invasive method for diagnosing and assessing the severity of periodontitis [47]. Three significantly increased miRNAs (has-miR-140-5, hsa-miR-146a-5p and hsa-miR-628-5p) were detected in the salivary exosomes of patients with periodontitis compared to healthy controls, which had a good discriminatory power for the diagnosis of periodontitis [48]. Inflammatory periodontal ligament stem cells (PDLSCs) promote M1 macrophage polarization through miR-143-3p-mediated PI3K/AKT/NF-κB signaling regulation in their secreted exosomes. MiR-34c-5p inhibits osteogenic differentiation of PDLSCs via the SATB2/ERK pathway, providing a potential new target for periodontitis diagnose [49, 50].
Engineered exosomes have also been widely used to treat periodontitis. It has been shown that exosomes secreted by stem cells after P2X7 receptor(P2X7R) gene modification can exert a positive effect on their surrounding cells, and that P2X7R gene modification is able to reverse inflammation-mediated damage to periodontal ligament stem cells (PDLSCs) [51]. Exosomes from the inflammatory microenvironment enhanced osteogenic and odontogenic differentiation of PDLSCs in part by switching off LMBR1-targeted miR-758-5p via BMP signaling [52]. Figure 5 depicts exosomes overexpressing C-X-C motif chemokine receptor 4 (CXCR4) and loaded with miR-126 (CXCR4-miR126-Exo) reduced off-target delivery to macrophages and modulated the shift of macrophages towards an anti-inflammatory phenotype. In vivo local injection of CXCR4-miR126-Exo at the site of periodontitis in rats effectively reduced bone resorption and osteoclast formation and inhibited the progression of periodontitis [53]. It has been shown that exosomes released from human adipose-derived stem cells(hADSCs) delivered calcitonin gene-related peptide (CGRP) to human periodontal ligament stem cells (hPDLSCs), thus promoting the osteogenic differentiation potential of hPDLSCs. At the same time, the exosomes were also loaded into poly(lactic acid)-glycolic acid (PLGA) nanocomposite scaffolds grafted with hydroxyapatite (g-HA), and the constructed PLGA/pDA-EV system slowly released exosomes, which were implanted into the alveolar bone defect area of rats and significantly induced the repair of the bone defects [54]. The 3D culture system improved the functionality of MSC-Exo for the treatment of periodontitis, and 3D-Exo exhibited greater enrichment of miR-1246, which inhibits the expression of Nfat5 of Th17, and in turn treats periodontitis more effectively [55]. Shen et al. loaded the exosomes derived from dental pulp stem cells (DPSC-Exo) into chitosan hydrogel. The DPSC-Exo/CS system formed by Shen et al. promoted the conversion of macrophages from a pro-inflammatory phenotype to an anti-inflammatory phenotype in the periodontium of mice with periodontitis by a mechanism that may be related to miR-1246 in DPSC-Exo [56]. Collagen sponges loaded with MSC exosomes enhanced periodontal regeneration in a rat model of periodontal defects [57]. Chew et al. investigated the therapeutic effect of collagen sponges loaded with MSC exosomes in an immunocompetent rat model of periodontal defects, and found that MSC exosomes may enhance periodontal regeneration by increasing periodontal ligament cell carcasses and proliferation [49].
Schematic illustration of CXCR4-miR126-Exo production, targeting, and regulation of periodontitis in vivo [53]
Pulpitis
The dental pulp is rich in nerves and blood vessels, which respond to bacterial attack and injury by providing neuronal sensitivity and transmitting mechanical stimuli for repair and regeneration [58]. One of the most common endodontic diseases is pulp infection or pulp necrosis. Loss of pulp tissue can lead to lack of blood supply to the tissue of tooth, loss of tooth vitality, loss of hardness of the dental tissues, and lead to the disintegration and fragility of the teeth. The traditional treatment for endodontics is root canal therapy (RCT) [59].
A number of studies have been conducted to confirm that engineered exosomes are more effective than conventional therapies in the treatment of pulpitis. Human dental pulp stem cells (hDPSCs) released EVs in a mild inflammatory microenvironment were able to promote pulp regeneration through functional healing rather than scar healing [60]. Exosomes (Hypo-Exo) secreted from hypoxia-pretreated human deciduous papillary stem cells (SHED) promote angiogenesis by transferring let-7f-5p and miR-210-3p, as could be developed as a pro-angiogenic therapeutic strategy during pulp regeneration [61]. Exosomes derived from DPSCs and encapsulated exosomes overexpression of miR-125a-3p in exosomes promoted macrophage phenotypic shift to M2 type and enhanced BMP2 release from macrophages, which in turn promoted DPSCs-guided pulp regeneration [62]. Wang et al. developed a hydroxypropyl chitin (HPCH)/chitin whisker (CW) thermosensitive hydrogel with enhanced mechanical properties and bioactive. They embedded exosomes isolated from human dental pulp stem cells (hDPSCs) directly into HPCH/CW pre-gel to form exosome-loaded hydrogels (HPCH/CW/Exo), and experiments demonstrated that the delivery of exosomes significantly enhanced the hydrogel’s ability to promote pulp regeneration and angiogenesis [63]. Schwann cells (SCs) play a key role in the support, maintenance and regeneration of nerve fibers in the dental pulp. LPS may alter the intercellular signaling of hDPSCs, and exosomes secreted from hDPSCs after pretreatment with LPS promote the proliferation, migration and odontogenic differentiation of SCs, which may mediate the odontogenic differentiation of SCs [64].
Temporomandibular disorders
Temporomandibular disorders (TMD) are a group of disorders involving the orofacial region, divided into those affecting the masticatory muscles and those affecting the temporomandibular joint (TMJ). Typical features include temporomandibular joint pain, limited jaw joint movement and temporomandibular joint sounds [65]. Engineered exosomes have also shown some efficacy in the treatment of TMD. Liu et al. found that inflammation-stimulated adipose-derived mesenchymal stem cell (ADSC)-derived exosomes (IAE) promoted cell proliferation and migration. Moreover, IAE significantly promoted M2 macrophage differentiation, and the high miR-27b-3p expression level in IAE may regulate macrophages by targeting macrophage colony-stimulating factor-1 (CSF-1). The TMJ condylar osteochondral defect model also showed that IAE significantly promoted TMJ regeneration [66]. Lee et al. successfully loaded molecules such as miR-140 into exosomes acting as an RNA delivery system by freeze-thawing and demonstrated the biologically active role of exosomes loaded with miR-140 in inducing the differentiation of BMSCs into chondrocytes and the facilitation of cartilage healing of the articular discs of the TMJ [67].
Peri-implantitis
Peri-implantitis is a pathological condition that occurs in the tissues surrounding dental implants and is characterized by inflammation of the peri-implant mucosa and progressive loss of peri-implant bone tissue [68, 69]. Increased exosome concentration and downregulation of miRNA-21-3p and miRNA-150-5p expression may be associated with the development of peri-implantitis [70].
Engineered exosomes have shown promising effects on improving the peri-implant inflammatory environment and enhancing peri-implant osseointegration. Many clinical studies have focused on bone resorption of immediate implants, but few basic studies have been conducted on the mechanism between immediate implants and bone resorption. Wang et al. found that overexpression of ALKBH5 in MC3T3-E1-derived exosomes significantly rescued peri-implant bone loss, and that ALKBH5 acted on the circ_0008542 via demethylation, making circ_0008542 unsuitable for binding to the miR-185-5p/RANK axis. After reducing its molecular sponge effect, osteoclast differentiation and bone resorption around implants were also reduced. The potential value of this study lies in providing a way to enhance immediate implant resistance through the use of exosomes that release ALKBH5 [71]. The multistage titanium morphology of the micro/nanotube texture promotes the secretion of hBMSCs-derived exosomes through a cell proliferative effect and improves peri-implant osseointegration [72]. Xu et al. developed micro-arc oxidized titanium implants and loaded them with engineered exosomes (S-Exo) in order to promote osseointegration at the implant interface. They first transferred Smurf1-shRNA into BMSC using a viral vector to prepare S-Exo, which was subsequently immobilized on the surface of the micro-arc titanium oxide implant. The immobilized S-Exo could be released slowly and uniformly. The S-Exo subsequently phagocytosed by BMSC and macrophages, and this S-Exo coating exhibited the dual effects of promoting osseointegration and facilitating macrophage M2 polarization (Fig. 6) [73]. Li et al. constructed a fusion peptide (PEP) to act as a drug delivery system (DDS), exosomes derived from bone marrow mesenchymal stem cells (BMSC-Exo) have been shown to trigger osteogenic differentiation and mineralization of MSC. Both in vitro and in vivo experiments demonstrated that PEP retained the ability to bind both titanium and exosomes, and that the DDS gained the ability to target exosomes to the surface of titanium implants after enhancing post-implantation osseointegration, this Exo-PEP system could provide accurate and effective treatment for therapeutic implants [74].
Schematic diagram of the construction of Ti-MAO@PEI-S EXO and its biological mechanism. A Preparation of Ti-MAO@PEI-S-EXO. B Mechanism of Ti-MAO@PEI-S-EXO to promote osteogenesis [73]
Sjogren’s syndrom
Sjogren's Syndrom (SS) is a systemic chronic autoimmune disease of unknown etiology characterized by immune-mediated damage to the salivary and lacrimal glands, which results in dry mouth and dry eyes in patients. When evaluating patients with primary dry syndrom, non-invasive and more accurate diagnostic techniques are needed for a long time, and engineered exosomes can play this role. One study used liquid chromatography-mass spectrometry (LC–MS) to perform proteomic analyses of EVs isolated from saliva and tears of SS patients, and found that dozens of proteins were significantly up-regulated in the salivary EVs of SS patients compared to controls. Only 2 proteins were upregulated in the tears of SS patients due to the low volume of tears collected [75]. MiR-1290 and let-7b-5p have increased ratios that could serve as a novel and non-invasive diagnostic marker for SS [76]. Cortes-Troncoso et al. found that T-cells release exosomes containing a specific miRNA (miR-142-3p), miR-142-3p can target key proteins (SERCA2B, RyR2, AC9) that are critical components of the Ca2+ and cAMP pathways in salivary gland secretion [77]. The mechanism diagram is shown in Fig. 7. Therefore, an increased proportion of miR-142-3p may also serve as a novel and non-invasive diagnostic marker for SS.
T cell exosome–derived miR-142-3p as a pathogenic driver of immunopathology in SS. MiR-142-3p is overexpressed in salivary gland lesions of SS patients [77]
Oral lichen planus
Oral lichen planus (OLP) is a chronic inflammatory T-cell-mediated oral mucosal disease of unknown etiology. The main manifestations of OLP are white streaks, white papules, white plaques, erythema, erosion, or vesicles primarily involving the buccal mucosa, tongue, and gingiva [78]. Yang et al. found that OLP T-Exo induced increased expression of macrophage inflammatory protein (MIP)-1α/β, IL-10, and IL-17A thereby affecting cytokine secretion by T cells, and that MIP-1α/β may drive CD8+ T cell trafficking after binding to CCR1/5 in OLP, contributing to the development of OLP [79]. Thus OLP T-Exo may be used to diagnose OLP. MiR-4484 was significantly upregulated in salivary exosomes from patients with oral lichen planus [80]. Circulating plasma exosomes can also be used as potential diagnostic biomarkers for OLP. Peng et al. isolated exosome miRNA from the plasma of both OLP patients and healthy individuals, and by comparing them by miRNA array analysis they found that circulating exosome miR-34 a-5p was significantly upregulated in patients with OLP, and that exosome miR-34a-5p was positively correlation [81]. It is common for exosomes to play a diagnostic role in OLP, and there is still a lack of studies related to exosome therapy for OLP.
Hard and soft tissue trauma defects of the maxillofacial region
Maxillofacial injuries are very common in Oral and maxillofacial diseases, and maxillofacial injuries usually trigger heavy bleeding because of the abundance of facial vascularization and the special location of the maxillofacial region, where the quality of healing and the speed of scarring healing are often of concern to the patient [82]. Engineered exosomes can promote the healing of hard and soft tissue trauma defects in the oral and maxillofacial region by modulating inflammation, improving angiogenesis, and promoting the proliferation and migration of tissue cells.
Engineered exosomes to regulate inflammation
In the early stages of wound healing, congestion, plasma exudate, leukocyte infiltration and localized redness are early signs of inflammation. In general, a mild inflammatory response is helpful because it helps to eliminate inflammatory factors, fight infection, and remove cellular debris, all of which help to regenerate injured tissue. In contrast, prolonged inflammation severely interferes with the wound healing process [83]. Su et al. infected the human melanoma cells (SK-MEL-5) with lentiviruses to get a stable cell line expressing human PD-L1. High concentrations of PD-L1 were obtained in secreted exosomes from genetically engineered cells overexpressing PD-L1 or stimulating MSC with IFN-γ. It was found that exosome PD-L1 binds specifically to PD-1 on the surface of T-cells and inhibits T-cell activation, thereby modulating the inflammatory response [84]. Exosomes from MSC stimulated with inflammatory factors such as tumor necrosis factor (TNF-α) and interferon (IFN-γ) have been found to reduce the release of pro-inflammatory cytokines and have the ability to improve the inflammatory environment [85]. It has been found that MSC-derived exosomes after LPS pretreatment have a better ability to regulate macrophage homeostasis as they upregulate the expression of anti-inflammatory cytokines and promote M2 macrophage activation through the let 7 b/TLR 4 pathway [86].
Engineered exosomes to improve angiogenesis
Angiogenesis is an intrinsic repair pathway for wound healing and tissue regeneration. Louis J Born et al. designed to observe the therapeutic potential of exosomes from mesenchymal stem cells (MSC) self-transfected to overexpress the long non-coding RNA HOX transcript antisense RNA (HOTAIR). HOTAIR has been found to be essential in mediating the angiogenic effects of endothelial cells, and MSCs were selected as exosome-generating cells for this study due to their widely reported intrinsic angiogenic properties. The experimental results showed that MSCs overexpressed HOTAIR (HOTAIR-MSC) produced exosomes with overexpression of HOTAIR. The HOTAIR-MSC-Exo promoted angiogenesis and wound healing in diabetic mice [87]. It was shown that superparamagnetic iron oxide NP-treated exosomes are precisely targeted and that they accumulate in damaged areas and significantly increase angiogenesis [88]. Synthetic exosomes with specific protein composition and RNA loading significantly promote angiogenesis [89]. Wang et al. loaded VH298 into epidermal stem cell (ESC)-derived exosomes and prepared photocrosslinked hydrogel gelatin methacryloyl (GelMA) containing VH-EVs (Gel-VH-EVs). Figure 8 shows the results revealed that VH298 improved angiogenesis by stabilizing HIF-1α and that Gel-VH-EVs had a significant therapeutic effect on skin defect repair [90].
Schematic illustration of experimental procedure of the study and Underlying mechanisms of VH-EVs released from GelMA hydrogel for enhancing angiogenesis via stabilizing HIF-1α in HUVECs [90]
Engineered exosomes to promote soft tissue wound healing
Fibroblasts play an important role in the healing of soft tissue wounds by synthesizing large amounts of collagen, whose orderly and adequate deposition is a key step in the proliferation and remodeling stages [91]. Wang et al. found that the survival and proliferation of adipose stem cells (ADSC) were significantly enhanced after hypoxia induction compared to anorexia. They also found that hypoxic adipose stem cell exosomes (HypADSC-Exo) can regulate the expression of various growth factors to promote fibroblast proliferation and migration, as well as angiogenesis. The HypADSC-Exo can accelerate diabetic wound healing by activating the PI3K/AKT pathway [29]. It has also been found that engineered stem cell exosomes carrying H19 can also increase fibroblast proliferation and inhibit apoptosis by affecting the H19/miR-152-3p/PTEN axis, which in turn regulates the PI3K/AKT signaling pathway, and ultimately promotes diabetic wound healing [92].
Engineered exosomes promote bone tissue regeneration
Engineered exosomes have also shown superior performance in promoting hard tissue regeneration. MiR-26a as an osteogenesis-related miRNA, Lai et al. extracted exosomes from the culture supernatant of miR-26a-modified BMSC by ultracentrifugation, and their experimental results revealed that miR-26a could be encapsulated into exosomes by DP7-C (a novel immunomodulatory peptide), and that exosomes loaded with miR-26a could promote osteogenesis and inhibit bone loss in experimental periodontitis and serve as a basis for new therapeutic strategies for periodontitis [93]. Figure 9 depicts the sustained release of bioactive bone morphogenetic protein-2 (BMP2) is important for bone regeneration, Yang et al. enriched the Bmp2 plasmid into BMSC-Exo by co-transfection, and the engineered exosome was loaded into GelMA hydrogel, and in an in vivo skull defect model, the ExoBMP2 + NoBody-loaded GelMA showed a powerful ability to promote bone regeneration [94]. Dual functional regulation of angiogenesis and osteogenesis is essential for desired bone regeneration. Yao et al. developed a cell-free tissue engineering system that construct gene-activated engineered exosomes by using ATDC5 chondrogenic progenitor cell line-derived exosomes to encapsulate the VEGF gene. The specific exosome anchor peptide CP05 was used as a flexible connector to effectively combine engineered exosome nanoparticles with 3D-printed porous bone scaffolds, and engineered exosome-mediated bone scaffolds effectively induced mostly vascularized bone regeneration [95]. Liu et al. found that BMSCs-derived exosomes formed from Sr-CS-Exo after strontium-substituted calcium silicate (Sr-CS) stimulation had superior ability of promoting angiogenesis and osteogenesis [96].
Schematical showing the preparation of ExoBMP2 + NoBody-loaded GelMA and its effect on bone regeneration [94]
Oral cancer
Squamous cell carcinoma of the head and neck is the seventh most common malignancy worldwide with an annual incidence of > 600,000, about half of which are located in the oral cavity [97]. Squamous cell carcinoma arising from the oral mucosal epithelium is a fatal disease due to invasion of tumors, orofacial disruption, cervical lymph node metastasis and ultimately hematogenous dissemination. Oral cancer (OC) can be prevented and cured in its early stages. However, a significant number of OC cases are not diagnosed until the progressive stage, which is one of the major reasons for the poorer treatment responsiveness and prognosis [98]. Patients with oral cancer are at high risk of secondary cancers, and there are no available biomarkers to detect them until the patient develops a visible lesion and is diagnosed by biopsy. Exosomes secreted by cancer cells are involved in growth of tumors, invasion and metastasis, the use of exosomes is a very promising biomarker for the diagnosis of oral cancer. OC show a different expression of exosome markers: lower expression of CD81 and CD9 and higher expression of CD63 [99]. Salivary exosomes of oral and oropharyngeal squamous cell carcinomas showed elevated expression of miR-486-5p, miR-1307-5p, miR-10b, miR-130a, miR-210, and miR-365, and decreased expression of miR-10b-5p, compared to healthy controls [100,101,102,103,104]. While miR-512-3p and miR-412-3p were upregulated in salivary exosome from oral squamous cell carcinoma (OSCC) patients, miR-302b-3p and miR-517b-3p were expressed only in exosome from OSCC patients [105]. Salivary exosome miR-24-3p maintains OSCC cell proliferation by targeting PER1, miR-24-3p is a potential novel diagnostic biomarker for OSCC [106]. Has_circ_0069313 is an exosome circRNA, has_circ_0069313 induced oral squamous cell immune escape via the miR-325-3p-Foxp3 axis in oral squamous cells and Treg cells, has_circ_0069313 is up-regulated in oral squamous cell carcinoma tissues [107]. Deng et al. used a combined strategy of microarray of exosome circRNAs and qRT-PCR validation, found that three types of circRNAs from OSCC were associated with the risk of preoperative lymph node metastasis (LNM) risk, including hsa_circRNA_047733, hsa_circRNA_024144, and hsa_circRNA_403472. They also demonstrated that hsa_circRNA_047733 may be a novel biomarker for LNM in OSCC [108]. Circulating PD-L1 on the surface of exosomes isolated from plasma emerged as useful indicators of disease and immune activity in patients with head and neck squamous cell carcinoma (HNSCC) [109]. OSCC LN1-1 cells showed greater capacity for lymphatic genesis and lymph node metastasis than their parental OEC-M1 cells, in addition to the ability to enhance migration and tube formation of lymphatic endothelial cells (LECs). Uptake of laminin γ2-rich exosomes by LECs enhanced the formation of lymphatic vessels in vitro, and thus laminin-332-carrying exosomes are a viable biomarker for OSCC [110]. One study examined serum exosomes (SE) in OSCC patients with lymph node metastases (LNM), and they concluded that PF4V1, CXCL7, F13A1, and ApoA1 in SE may be associated with OSCC metastasis, which could be helpful in the diagnosis of OSCC [111].
A large body of evidence suggests that engineered exosomes containing therapeutic agents can attenuate the oncogenic activity of human cancer cells, and therefore there is an urgent need to develop specific OSCC-targeted Exosomes (oct-Exosome). Hypoxia is a common feature of solid tumors and is associated with aggressiveness and poor patient prognosis. Li et al. found that exosomes derived from hypoxic oral squamous cell carcinoma cells increased migration and invasion of oral squamous cells by a HIF-1α and HIF-2α-dependent manner. And miRNA sequencing was performed on anorexia and hypoxic OSCC-derived exosomes, in which miR-21 was one of the most significantly upregulated miRNAs under hypoxic conditions. The results prompts further studies on the therapeutic value of exosome inhibition for cancer treatment [112]. Studies have shown that miRNA-34a has inhibitory effects on the proliferation, migration and invasion of OSCC. However, the lack of a safe and effective delivery system limits the clinical application of miRNA-34a in oral cancer therapy. Deng et al. loaded cholesterol-modified miRNA-34a into exosomes of HEK293T cells by co-incubation, it was finally taken up by oral squamous carcinoma cell. Exosomes loaded with miRNA-34a inhibited oral squamous carcinoma cell proliferation, migration and invasion by down-regulating SATB6 expression. It provides a new approach for the treatment of oral cancer [113]. Yutaro Kase et al. constructed exosomes of normal fibroblasts transfected with Epstein-Barr virus-inducible 3 (EBI3) cDNA by electroporation with siRNA for lymphocyte cytoplasmic protein 1 (LCP1) as engineered exosomes. The experiments showed that engineered exosomes stably and efficiently transferred siLCP1 into OSCC cells, the LCP1 was down-regulated in OSCC cells using engineering Exosomes, which resulted in in vitro and in vivo producing significant tumor suppressor effects [114]. Exosomes loaded with miR-155 inhibitors can reverse chemoresistance in oral cancer, thus providing an alternative therapeutic strategy for the treatment of refractory oral cancer patients [115]. Zhang et al. developed a pH/photosensitive drug system based on milk exosome for the treatment of oral small cell carcinoma. It is called exosome-doxorubicin-anthracene peroxide derivatives (Exo@Dox-EPT1, NPs).The main scheme of their study is depicted in Fig. 10. The milk exosome binds to doxorubicin, in addition, is loaded with endoperoxide and chlorine e6 (Ce6), which releases single-linear oxygen to kill cancer cells. The new milk exosome-based drug delivery system has been shown to be effective in the treatment of OSCC [116]. One study recently developed a therapeutic drug candidate exoASO-STAT6 carrying engineered exosome, which provides antisense oligonucleotides (ASOs) targeting signal transducer and activator of transcription 6 (STAT6). ASOs selectively silence STAT6 expression in TAM (tumor-associated macrophages), exoASO-STAT6 monotherapy produced an effective anti-tumor response, prompting clinical researchers to start investigating new cancer therapies as effective monotherapy candidates for other types of cancer, including OSCC [117].
A Schematic illustration of the synthesis process for Exo@Dox–EPT1 (NPs). B Therapeutic mechanism of NPs under acidic tumor microenvironment. C PTT mechanism of NP under 808 nm near-IR light irradiation [116]
Conclusions and prospects
Considerable progress has been made in the field of exosomes over the past decade and we have gained a deeper understanding of their biological origin, molecular content and biological function. The role of exosomes in diagnosing and treating diseases of the oral and maxillofacial system has received considerable research attention, due to the potential role of exosomes as biomarkers and therapeutic agents. The use of exosomes as biomarkers and therapeutic agents in clinical applications has several advantages. The use of exosomes as diagnosis has the advantages of minimal invasiveness and wide availability in various body fluids. The diversity of exosome content provides a variety of diagnostic indicators that can improve the sensitivity and specificity of diagnosis. In the treatment of oral and maxillofacial diseases, exosomes are mediators of intercellular communication, transporting their contents to recipient cells and influencing oral disease progression by modulating host-associated immune responses, angiogenesis, drug resistance or invasive metastasis. Exosomes accommodate a variety of biomolecule types, which simultaneously exert different therapeutic mechanisms.
More importantly, due to the unique structure and physicochemical characteristics of exosomes, lead to the exosomes can be modified, and a large number of studies have shown that engineered exosomes can exhibit better diagnostic and therapeutic effects than nature exosomes. This paper we briefly discuss the engineering methods of exosomes, including modifying maternal cells, directly modifying exosomes, or biomaterial loading exosomes, focusing on the application of engineered exosomes in periodontitis, pulpitis, oral cancer, etc. Overall, engineered exosomes can exhibit better properties, such as stronger targeting, more active ingredients, and higher transport efficiency, which are not available in natural exosomes. But there is still a lot of room for improvement in the methods of exosome isolation and purification. Despite the current challenges, the idea of using engineered exosomes as a diagnostic and therapeutic tool for oral and maxillofacial diseases is promising, and the use of engineered exosomes is expected to be used more and more widely in oral and maxillofacial diseases in the near future.
References
Peres MA, Macpherson LMD, Weyant RJ, Daly B, Venturelli R, Mathur MR, et al. Oral diseases: a global public health challenge. Lancet. 2019;394:249–60.
Im SH, Kim CY, Jung Y, Jang Y, Kim SH. Biodegradable vascular stents with high tensile and compressive strength: a novel strategy for applying monofilaments via solid-state drawing and shaped-annealing processes. Biomater Sci. 2017;5:422–31.
Veneruso V, Rossi F, Villella A, Bena A, Forloni G, Veglianese P. Stem cell paracrine effect and delivery strategies for spinal cord injury regeneration. J Control Release. 2019;300:141–53.
Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97:329–39.
Pan B-T, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967–78.
Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, et al. Reassessment of exosome composition. Cell. 2019;177:428-445.e18.
Record M, Carayon K, Poirot M, Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell–cell communication and various pathophysiologies. Biochim et Biophys Acta (BBA) - Mol Cell Biol Lipids. 2014;1841:108–20.
Liao W, Du Y, Zhang C, Pan F, Yao Y, Zhang T, et al. Exosomes: the next generation of endogenous nanomaterials for advanced drug delivery and therapy. Acta Biomater. 2019;86:1–14.
**ao C, Song F, Zheng YL, Lv J, Wang QF, Xu N. Exosomes in head and neck squamous cell carcinoma. Front Oncol. 2019;9:894.
Dang S-Y, Leng Y, Wang Z-X, **ao X, Zhang X, Wen T, et al. Exosomal transfer of obesity adipose tissue for decreased miR-141-3p mediate insulin resistance of hepatocytes. Int J Biol Sci. 2019;15:351–68.
Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–83.
Kou M, Huang L, Yang J, Chiang Z, Chen S, Liu J, et al. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: a next generation therapeutic tool? Cell Death Dis. 2022;13:580.
Waldenström A, Ronquist G. Role of exosomes in myocardial remodeling. Circ Res. 2014;114:315–24.
Hannafon BN, Gin AL, Xu Y-F, Bruns M, Calloway CL, Ding W-Q. Metastasis-associated protein 1 (MTA1) is transferred by exosomes and contributes to the regulation of hypoxia and estrogen signaling in breast cancer cells. Cell Commun Signal. 2019;17:13.
Distler JHW, Huber LC, Gay S, Distler O, Pisetsky DS. Microparticles as mediators of cellular cross-talk in inflammatory disease. Autoimmunity. 2006;39:683–90.
Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H, et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol. 2018;20:332–43.
Heneberg P. Paracrine tumor signaling induces transdifferentiation of surrounding fibroblasts. Crit Rev Oncol Hematol. 2016;97:303–11.
Bang C, Thum T. Exosomes: new players in cell–cell communication. Int J Biochem Cell Biol. 2012;44:2060–4.
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977.
Van Den Boorn JG, Schlee M, Coch C, Hartmann G. SiRNA delivery with exosome nanoparticles. Nat Biotechnol. 2011;29:325–6.
Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: a review of its classification, isolation techniques, storage. Diagn Target Ther Appl IJN. 2020;15:6917–34.
Schiffelers R, Kooijmans S, Van Vader D, Van Solinge WW. Exosome mimetics: a novel class of drug delivery systems. IJN. 2012;7:1525–41.
Shao H, Im H, Castro CM, Breakefield X, Weissleder R, Lee H. New technologies for analysis of extracellular vesicles. Chem Rev. 2018;118:1917–50.
Tao S-C, Guo S-C, Li M, Ke Q-F, Guo Y-P, Zhang C-Q. Chitosan wound dressings incorporating exosomes derived from MicroRNA-126-overexpressing synovium mesenchymal stem cells provide sustained release of exosomes and heal full-thickness skin defects in a diabetic rat model. Stem Cells Transl Med. 2016;6(3):736–47.
Li H, Feng Y, Zheng X, Jia M, Mei Z, Wang Y, et al. M2-type exosomes nanoparticles for rheumatoid arthritis therapy via macrophage re-polarization. J Control Release. 2022;341:16–30.
Liu W, Yu M, **e D, Wang L, Ye C, Zhu Q, et al. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 2020;11:259.
Yu M, Liu W, Li J, Lu J, Lu H, Jia W, et al. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res Ther. 2020;11:350.
Shi H, Xu X, Zhang B, Xu J, Pan Z, Gong A, et al. 3,3′-Diindolylmethane stimulates exosomal Wnt11 autocrine signaling in human umbilical cord mesenchymal stem cells to enhance wound healing. Theranostics. 2017;7:1674–88.
Wang J, Wu H, Peng Y, Zhao Y, Qin Y, Zhang Y, et al. Hypoxia adipose stem cell-derived exosomes promote high-quality healing of diabetic wound involves activation of PI3K/Akt pathways. J Nanobiotechnol. 2021;19:202.
Sun D, Zhuang X, **ang X, Liu Y, Zhang S, Liu C, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18:1606–14.
Johnsen KB, Gudbergsson JM, Skov MN, Christiansen G, Gurevich L, Moos T, et al. Evaluation of electroporation-induced adverse effects on adipose-derived stem cell exosomes. Cytotechnology. 2016;68:2125–38.
Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015;207:18–30.
Yue H, Yuan L, Zhang W, Zhang S, Wei W, Ma G. Macrophage responses to the physical burden of cell-sized particles. J Mater Chem B. 2018;6:393–400.
Yang Z, Shi J, **e J, Wang Y, Sun J, Liu T, et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat Biomed Eng. 2019;4:69–83.
Wan T, Zhong J, Pan Q, Zhou T, ** Y, Liu X. Exosome-mediated delivery of Cas9 ribonucleoprotein complexes for tissue-specific gene therapy of liver diseases. Sci Adv. 2022;8:eabp9435.
**ao S, **ao C, Miao Y, Wang J, Chen R, Fan Z, et al. Human acellular amniotic membrane incorporating exosomes from adipose-derived mesenchymal stem cells promotes diabetic wound healing. Stem Cell Res Ther. 2021;12:255.
Tao S-C, Guo S-C, Li M, Ke Q-F, Guo Y-P, Zhang C-Q. Chitosan wound dressings incorporating exosomes derived from MicroRNA-126-overexpressing synovium mesenchymal stem cells provide sustained release of exosomes and heal full-thickness skin defects in a diabetic rat model. Stem Cells Transl Med. 2017;6:736–47.
Shiekh PA, Singh A, Kumar A. Exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing OxOBand alleviate diabetic and infectious wound healing. Biomaterials. 2020;249:120020.
**g X, Wang S, Tang H, Li D, Zhou F, **n L, et al. Dynamically bioresponsive DNA hydrogel incorporated with dual-functional stem cells from apical papilla-derived exosomes promotes diabetic bone regeneration. ACS Appl Mater Interfaces. 2022;14:16082–99.
Cao H, Chen M, Cui X, Liu Y, Liu Y, Deng S, et al. Cell-free osteoarthritis treatment with sustained-release of chondrocyte-targeting exosomes from umbilical cord-derived mesenchymal stem cells to rejuvenate aging chondrocytes. ACS Nano. 2023;17:13358–76.
Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. 2017;3:17038.
Haritha A, Jayakumar A. Syndromes as they relate to periodontal disease. Periodontol. 2000;2011(56):65–86.
Ghallab NA. Diagnostic potential and future directions of biomarkers in gingival crevicular fluid and saliva of periodontal diseases_ review of the current evidence. Arch Oral Biol. 2018;87:115–24.
Tobón-Arroyave SI, Celis-Mejía N, Córdoba-Hidalgo MP, Isaza-Guzmán DM. Decreased salivary concentration of CD 9 and CD 81 exosome-related tetraspanins may be associated with the periodontal clinical status. J Clin Periodontol. 2019;46:470–80.
Chaparro Padilla A, Weber Aracena L, Realini Fuentes O, Albers Busquetts D, Hernández Ríos M, Ramírez Lobos V, et al. Molecular signatures of extracellular vesicles in oral fluids of periodontitis patients. Oral Dis. 2020;26:1318–25.
Yu J, Lin Y, **ong X, Li K, Yao Z, Dong H, et al. Detection of exosomal PD-L1 RNA in saliva of patients with periodontitis. Front Genet. 2019;10:202.
**a Y, Zhou K, Sun M, Shu R, Qian J, **e Y. The miR-223-3p regulates pyroptosis through NLRP3-Caspase 1-GSDMD signal axis in periodontitis. Inflammation. 2021;44:2531–42.
Han P, Bartold PM, Salomon C, Ivanovski S. Salivary small extracellular vesicles associated miRNAs in periodontal status—a pilot study. IJMS. 2020;21:2809.
Wang Y, Zhang X, Wang J, Zhang Y, Ye Q, Wang Y, et al. Inflammatory periodontal ligament stem cells drive M1 macrophage polarization via exosomal miR-143-3p-mediated regulation of PI3K/AKT/NF-κB signaling. Stem Cells. 2023;41:184–99.
Lin C, Yang Y, Wang Y, **g H, Bai X, Hong Z, et al. Periodontal ligament fibroblasts-derived exosomes induced by PGE2 inhibit human periodontal ligament stem cells osteogenic differentiation via activating miR-34c-5p/SATB2/ERK. Exp Cell Res. 2022;419:113318.
Xu X-Y, Tian B-M, **a Y, **a Y-L, Li X, Zhou H, et al. Exosomes derived from P2X7 receptor gene-modified cells rescue inflammation-compromised periodontal ligament stem cells from dysfunction. Stem Cells Transl Med. 2020;9:1414–30.
Yan C, Li N, **ao T, Ye X, Fu L, Ye Y, et al. Extracellular vesicles from the inflammatory microenvironment regulate the osteogenic and odontogenic differentiation of periodontal ligament stem cells by miR-758-5p/LMBR1/BMP2/4 axis. J Transl Med. 2022;20:208.
Luo H, Chen D, Li R, Li R, Teng Y, Cao Y, et al. Genetically engineered CXCR4-modified exosomes for delivery of miR-126 mimics to macrophages alleviate periodontitis. J Nanobiotechnol. 2023;21:116.
Yang Y, Zhang B, Yang Y, Peng B, Ye R. PLGA containing human adipose-derived stem cell-derived extracellular vesicles accelerates the repair of alveolar bone defects via transfer of CGRP. Oxid Med Cell Longev. 2022;2022:1–14.
Zhang Y, Chen J, Fu H, Kuang S, He F, Zhang M, et al. Exosomes derived from 3D-cultured MSCs improve therapeutic effects in periodontitis and experimental colitis and restore the Th17 cell/Treg balance in inflamed periodontium. Int J Oral Sci. 2021;13:43.
Shen Z, Kuang S, Zhang Y, Yang M, Qin W, Shi X, et al. Chitosan hydrogel incorporated with dental pulp stem cell-derived exosomes alleviates periodontitis in mice via a macrophage-dependent mechanism. Bioact Mater. 2020;5:1113–26.
Chew JRJ, Chuah SJ, Teo KYW, Zhang S, Lai RC, Fu JH, et al. Mesenchymal stem cell exosomes enhance periodontal ligament cell functions and promote periodontal regeneration. Acta Biomater. 2019;89:252–64.
Sui B, Chen C, Kou X, Li B, Xuan K, Shi S, et al. Pulp stem cell-mediated functional pulp regeneration. J Dent Res. 2019;98:27–35.
Ahmed GM, Abouauf EA, AbuBakr N, Dörfer CE, El-Sayed KF. Tissue engineering approaches for enamel, dentin, and pulp regeneration: an update. Stem Cells Int. 2020;2020:1–15.
Chen WJ. The role of small extracellular vesicles derived from lipopolysaccharide-preconditioned human dental pulp stem cells in dental pulp regeneration. J Endod. 2021;47(6):961–9.
Liu P, Qin L, Liu C, Mi J, Zhang Q, Wang S, et al. Exosomes derived from hypoxia-conditioned stem cells of human deciduous exfoliated teeth enhance angiogenesis via the transfer of let-7f-5p and miR-210-3p. Front Cell Dev Biol. 2022;10:879877.
Zheng J, Kong Y, Hu X, Li Z, Li Y, Zhong Y, et al. MicroRNA-enriched small extracellular vesicles possess odonto-immunomodulatory properties for modulating the immune response of macrophages and promoting odontogenesis. Stem Cell Res Ther. 2020;11:517.
Wang S, **ng X, Peng W, Huang C, Du Y, Yang H, et al. Fabrication of an exosome-loaded thermosensitive chitin-based hydrogel for dental pulp regeneration. J Mater Chem B. 2023;11:1580–90.
Li J, Ju Y, Liu S, Fu Y, Zhao S. Exosomes derived from lipopolysaccharide-preconditioned human dental pulp stem cells regulate Schwann cell migration and differentiation. Connect Tissue Res. 2021;62:277–86.
Siéssere S, Vitti M, Semprini M, Regalo SCH, Iyomasa MM, Dias FJ, et al. Macroscopic and microscopic aspects of the temporomandibular joint related to its clinical implication. Micron. 2008;39:852–8.
Liu Y, Zhang Z, Wang B, Dong Y, Zhao C, Zhao Y, et al. Inflammation-stimulated MSC-derived small extracellular vesicle miR-27b-3p regulates macrophages by targeting CSF-1 to promote temporomandibular joint condylar Regeneration. Small. 2022;18:2107354.
Won Lee G, Thangavelu M, Joung Choi M, Yeong Shin E, Sol Kim H, Seon Baek J, et al. Exosome mediated transfer of miRNA-140 promotes enhanced chondrogenic differentiation of bone marrow stem cells for enhanced cartilage repair and regeneration. J Cell Biochem. 2020;121:3642–52.
Lang NP, Berglundh T, on Behalf of Working Group 4 of the Seventh European Workshop on Periodontology. Periimplant diseases: where are we now? – Consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol. 2011;38:178–81.
Lindhe J, Meyle J, on behalf of Group D of the European Workshop on Periodontology. Peri-implant diseases: consensus report of the sixth European workshop on periodontology. J Clinic Periodontol. 2008;35:282–5.
Chaparro A, Atria P, Realini O, Monteiro LJ, Betancur D, Acuña-Gallardo S, et al. Diagnostic potential of peri-implant crevicular fluid microRNA-21-3p and microRNA-150-5p and extracellular vesicles in peri-implant diseases. J Periodontol. 2021;92(6):11–21.
Wang W, Qiao S-C, Wu X-B, Sun B, Yang J-G, Li X, et al. Circ_0008542 in osteoblast exosomes promotes osteoclast-induced bone resorption through m6A methylation. Cell Death Dis. 2021;12:628.
Zhang Z, Xu R, Yang Y, Liang C, Yu X, Liu Y, et al. Micro/nano-textured hierarchical titanium topography promotes exosome biogenesis and secretion to improve osseointegration. J Nanobiotechnol. 2021;19:78.
Xu H, Chai Q, Xu X, Li Z, Bao W, Man Z, et al. Exosome-functionalized Ti6Al4V scaffolds promoting osseointegration by modulating endogenous osteogenesis and osteoimmunity. ACS Appl Mater Interfaces. 2022;14:46161–75.
Li X, Liu Z, Xu S, Ma X, Zhao Z, Hu H, et al. A drug delivery system constructed by a fusion peptide capturing exosomes targets to titanium implants accurately resulting the enhancement of osseointegration peri-implant. Biomater Res. 2022;26:89.
Aqrawi LA, Galtung HK, Vestad B, Øvstebø R, Thiede B, Rusthen S, et al. Identification of potential saliva and tear biomarkers in primary Sjögren’s syndrome, utilising the extraction of extracellular vesicles and proteomics analysis. Arthritis Res Ther. 2017;19:14.
Yamashiro K, Hamada T, Mori K, Nishi K, Nakamura M, Beppu M, et al. Exosome-derived microRNAs from mouthrinse have the potential to be novel biomarkers for Sjögren syndrome. JPM. 2022;12:1483.
Cortes-Troncoso J, Jang S-I, Perez P, Hidalgo J, Ikeuchi T, Greenwell-Wild T, et al. T cell exosome–derived miR-142-3p impairs glandular cell function in Sjögren’s syndrome. JCI Insight. 2020;5:e133497.
Roopashree MR, Gondhalekar RV, Shashikanth MC, George J, Thippeswamy SH, Shukla A. Pathogenesis of oral lichen planus - a review: pathogenesis of oral lichen planus. J Oral Pathol Med. 2010;39:729–34.
Yang J, Zhang J, Lu R, Tan Y, Du G, Zhou G. T cell–derived exosomes induced macrophage inflammatory protein-1α/β drive the trafficking of CD8 + T cells in oral lichen planus. J Cell Mol Med. 2020;24:14086–98.
Byun J, Hong S, Choi J, Jung J, Lee H. Diagnostic profiling of salivary exosomal micro RNA s in oral lichen planus patients. Oral Dis. 2015;21:987–93.
Peng Q, Zhang J, Zhou G. Differentially circulating exosomal microRNAs expression profiling in oral lichen planus. Am J Transl Res. 2018;10(9):2848–58.
Vazquez M-P, Kadlub N, Soupre V, Galliani E, Neiva-Vaz C, Pavlov I, et al. Plaies et traumatismes de la face de l’enfant. Annales de Chirurgie Plastique Esthétique. 2016;61:543–59.
Wang Z-C, Zhao W-Y, Cao Y, Liu Y-Q, Sun Q, Shi P, et al. The roles of inflammation in keloid and hypertrophic scars. Front Immunol. 2020;11:603187.
Su D, Tsai H, Xu Z, Yan F, Wu Y, **ao Y, et al. Exosomal PD-L1 functions as an immunosuppressant to promote wound healing. J Extracell Vesicle. 2020;9:1709262.
Harting MT, Srivastava AK, Zhaorigetu S, Bair H, Prabhakara KS, Toledano Furman NE, et al. Inflammation-stimulated mesenchymal stromal cell-derived extracellular vesicles attenuate inflammation. Stem Cells. 2018;36:79–90.
Ti D, Hao H, Tong C, Liu J, Dong L, Zheng J, et al. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med. 2015;13:308.
Born LJ, Chang K, Shoureshi P, Lay F, Bengali S, Hsu ATW, et al. HOTAIR-loaded mesenchymal stem/stromal cell extracellular vesicles enhance angiogenesis and wound healing. Adv Healthcare Mater. 2022;11:2002070.
Li X, Wang Y, Shi L, Li B, Li J, Wei Z, et al. Magnetic targeting enhances the cutaneous wound healing effects of human mesenchymal stem cell-derived iron oxide exosomes. J Nanobiotechnol. 2020;18:113.
Kim S, Kim Y, Hyun Y-S, Choi H, Kim S-Y, Kim T-G. Exosomes from human cord blood plasma accelerate cutaneous wound healing by promoting fibroblast function, angiogenesis, and M2 macrophage differentiation. Biomater Sci. 2021;9:3028–39.
Wang Y, Cao Z, Wei Q, Ma K, Hu W, Huang Q, et al. VH298-loaded extracellular vesicles released from gelatin methacryloyl hydrogel facilitate diabetic wound healing by HIF-1α-mediated enhancement of angiogenesis. Acta Biomater. 2022;147:342–55.
Singer AJ. Cutaneous wound healing. New Engl J Med. 1999;341(10):738–46.
Li B, Luan S, Chen J, Zhou Y, Wang T, Li Z, et al. The MSC-derived Exosomal lncRNA H19 promotes wound healing in diabetic foot ulcers by upregulating PTEN via MicroRNA-152-3p. Mol Ther—Nucleic Acids. 2020;19:814–26.
Lai S, Deng L, Liu C, Li X, Fan L, Zhu Y, et al. Bone marrow mesenchymal stem cell-derived exosomes loaded with miR-26a through the novel immunomodulatory peptide DP7-C can promote osteogenesis. Biotechnol Lett. 2023;45:905–19.
Yang Z, Li X, Gan X, Wei M, Wang C, Yang G, et al. Hydrogel armed with Bmp2 mRNA-enriched exosomes enhances bone regeneration. J Nanobiotechnol. 2023;21:119.
Zha Y, Li Y, Lin T, Chen J, Zhang S, Wang J. Progenitor cell-derived exosomes endowed with VEGF plasmids enhance osteogenic induction and vascular remodeling in large segmental bone defects. Theranostics. 2021;11:397–409.
Liu L, Yu F, Li L, Zhou L, Zhou T, Xu Y, et al. Bone marrow stromal cells stimulated by strontium-substituted calcium silicate ceramics: release of exosomal miR-146a regulates osteogenesis and angiogenesis. Acta Biomater. 2021;119:444–57.
Kademani D. Oral cancer. Mayo Clin Proc. 2007;82:878–87.
Thomson PJ. Perspectives on oral squamous cell carcinoma prevention—proliferation, position, progression and prediction. J Oral Pathol Med. 2018;47:803–7.
Zlotogorski-Hurvitz A, Dayan D, Chaushu G, Salo T, Vered M. Morphological and molecular features of oral fluid-derived exosomes: oral cancer patients versus healthy individuals. J Cancer Res Clin Oncol. 2016;142:101–10.
Bigagli E, Locatello LG, Maggiore G, Valdarnini F, Bambi F, Gallo O, et al. Extracellular vesicles miR-210 as a potential biomarker for diagnosis and survival prediction of oral squamous cell carcinoma patients. J Oral Pathol Med. 2022;51(4):350–7.
He T, Guo X, Li X, Liao C, Wang X, He K. Plasma-derived exosomal microRNA-130a serves as a noninvasive biomarker for diagnosis and prognosis of oral squamous cell carcinoma. J Oncol. 2021;2021:1–9.
Faur CI, Roman RC, Jurj A, Raduly L, Almășan O, Rotaru H, et al. Salivary exosomal MicroRNA-486-5p and MicroRNA-10b-5p in oral and oropharyngeal squamous cell carcinoma. Medicina. 2022;58:1478.
Patel A, Patel S, Patel P, Mandlik D, Patel K, Tanavde V. Salivary exosomal miRNA-1307-5p predicts disease aggressiveness and poor prognosis in oral squamous cell carcinoma patients. IJMS. 2022;23:10639.
Coon J, Kingsley K, Howard KM. miR-365 (microRNA): potential biomarker in oral squamous cell carcinoma exosomes and extracellular vesicles. Int J Mol Sci. 2020;21(15):5317.
Gai C, Camussi F, Broccoletti R, Gambino A, Cabras M, Molinaro L, et al. Salivary extracellular vesicle-associated miRNAs as potential biomarkers in oral squamous cell carcinoma. BMC Cancer. 2018;18:439.
He L, ** F, Fan Z, Zhang C, Deng M, Cheng B, et al. Salivary exosomal miR-24-3p serves as a potential detective biomarker for oral squamous cell carcinoma screening. Biomed Pharmacother. 2020;121:109553.
Chen Y, Li Z, Liang J, Liu J, Hao J, Wan Q, et al. CircRNA has_circ_0069313 induced OSCC immunity escape by miR-325-3p-Foxp3 axes in both OSCC cells and Treg cells. Aging. 2022;14:4376–89.
Deng Q, Chen Y, Lin L, Lin J, Wang H, Qiu Y, et al. Exosomal hsa_circRNA_047733 integrated with clinical features for preoperative prediction of lymph node metastasis risk in oral squamous cell carcinoma. J Oral Pathol Med. 2023;52:37–46.
Yerneni SS, Hoffmann TK, Gooding WE, Whiteside TL. Clinical significance of PD-L1 Exosomes in plasma of head and neck cancer patients+. Clin Cancer Res. 2018;24(4):896–905.
Wang S, Liou G, Liu S, Chang JS, Hsiao J, Yen Y, et al. Laminin γ2-enriched extracellular vesicles of oral squamous cell carcinoma cells enhance in vitro lymphangiogenesis via integrin α3-dependent uptake by lymphatic endothelial cells. Intl J Cancer. 2019;144:2795–810.
Li C, Zhou Y, Liu J, Su X, Qin H, Huang S, et al. Potential markers from serum-purified exosomes for detecting oral squamous cell carcinoma metastasis. Cancer Epidemiol Biomark Prev. 2019;28:1668–81.
Li L, Li C, Wang S, Wang Z, Jiang J, Wang W, et al. Exosomes derived from hypoxic oral squamous cell carcinoma cells deliver miR-21 to normoxic cells to elicit a prometastatic phenotype. Can Res. 2016;76:1770–80.
Deng W, Meng Y, Wang B, Wang C-X, Hou C-X, Zhu Q-H, et al. In vitro experimental study on the formation of microRNA-34a loaded exosomes and their inhibitory effect in oral squamous cell carcinoma. Cell Cycle. 2022;21:1775–83.
Kase Y, Uzawa K, Wagai S, Yoshimura S, Yamamoto J-I, Toeda Y, et al. Engineered exosomes delivering specific tumor-suppressive RNAi attenuate oral cancer progression. Sci Rep. 2021;11:5897.
Sayyed AA, Gondaliya P, Mali M, Pawar A, Bhat P, Khairnar A, et al. MiR-155 inhibitor-laden exosomes reverse resistance to cisplatin in a 3D tumor spheroid and xenograft model of oral cancer. Mol Pharm. 2021;18:3010–25.
Zhang Q, **ao Q, Yin H, **a C, Pu Y, He Z, et al. Milk-exosome based pH/light sensitive drug system to enhance anticancer activity against oral squamous cell carcinoma. RSC Adv. 2020;10:28314–23.
Kamerkar S, Leng C, Burenkova O, Jang SC, McCoy C, Zhang K, et al. Exosome-mediated genetic reprogramming of tumor-associated macrophages by exoASO-STAT6 leads to potent monotherapy antitumor activity. Sci Adv. 2022;8:eabj7002.
Acknowledgements
This work is supported by Science and Technology Innovation Leader and Key Talent Team Project of Shanxi Province (202204051002034), Key Research and Development Plan of Shanxi Province (202102130501002), Scientific Research Project for Returned Overseas Professionals of Shanxi Province (2022-120), Key national science and technology cooperation project of Shanxi Provincial Department of Science and Technology (202204041101004) , Four “Batches” Innovation Project of Invigorating Medical through Science and Technology of Shanxi Province (2023XM013), Science and technology innovation project of colleges and universities in Shanxi Province (2022L166) , Shanxi Province Basic Research Plan (202203021222256).
Funding
This work is supported by Science and technology innovation project of colleges and universities in Shanxi Province, 2022L166, Shanxi Province Basic Research Plan, 202203021222256, Science and Technology Innovation Leader and Key Talent Team Project of Shanxi Province, 202204051002034, Key Research and Development Plan of Shanxi Province, 202102130501002, Four “Batches” Innovation Project of Invigorating Medical through Science and Technology of Shanxi Province, 2023XM013, Key national science and technology cooperation project of Shanxi Provincial Department of Science and Technology, 202204041101004, Scientific Research Project for Returned Overseas Professionals of Shanxi Province, 2022-120
Author information
Authors and Affiliations
Contributions
JR and XJ contributed equally to this work. JR and XJ designed the structure and content, assembled the literature, and wrote this review. YL, JL, XN, MZ, RZ, HC, JC, BL, and XW provided suggestions and revised this review.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
There are no animal experiments carried out for this article.
Competing interests
There are no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ren, J., **g, X., Liu, Y. et al. Exosome-based engineering strategies for the diagnosis and treatment of oral and maxillofacial diseases. J Nanobiotechnol 21, 501 (2023). https://doi.org/10.1186/s12951-023-02277-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-023-02277-4