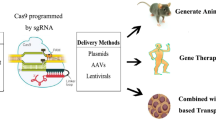
Abstract
Inherited Retinal Diseases (IRDs) are considered one of the leading causes of blindness worldwide. However, the majority of them still lack a safe and effective treatment due to their complexity and genetic heterogeneity. Recently, gene therapy is gaining importance as an efficient strategy to address IRDs which were previously considered incurable. The development of the clustered regularly-interspaced short palindromic repeats (CRISPR)-CRISPR-associated protein 9 (Cas9) system has strongly empowered the field of gene therapy. However, successful gene modifications rely on the efficient delivery of CRISPR-Cas9 components into the complex three-dimensional (3D) architecture of the human retinal tissue. Intriguing findings in the field of nanoparticles (NPs) meet all the criteria required for CRISPR-Cas9 delivery and have made a great contribution toward its therapeutic applications. In addition, exploiting induced pluripotent stem cell (iPSC) technology and in vitro 3D retinal organoids paved the way for prospective clinical trials of the CRISPR-Cas9 system in treating IRDs. This review highlights important advances in NP-based gene therapy, the CRISPR-Cas9 system, and iPSC-derived retinal organoids with a focus on IRDs. Collectively, these studies establish a multidisciplinary approach by integrating nanomedicine and stem cell technologies and demonstrate the utility of retina organoids in develo** effective therapies for IRDs.
Similar content being viewed by others
Introduction
Inherited retinal diseases (IRDs) are a diverse group of rare genetic disorders associated with more than 280 different genes [1]. IRDs manifest varying degrees of clinical severity and variable inheritance patterns [2], leading to blindness in infancy/early childhood [3, 4] or a gradual and progressive vision loss during adulthood [5,6,7,8]. The development of comprehensive and effective treatment proves to be a challenge for scientists, particularly due to the diverse number of genes involved in IRDs. In 2017, Luxturna® (voretigene neparvovec), a gene therapy drug developed by Spark Therapeutics Inc., was approved by the Food and Drug Administration (FDA). Luxturna® uses adeno-associated virus serotype 2 (AAV2) as a delivery vehicle to carry the wild-type Retinal Pigment Epithelium 65 (RPE65) gene into the retinal cells with RPE65 mutation for treating patients with Leber congenital amaurosis (LCA), a rare form of inherited vision loss [9]. AAV-derived vectors have several advantages, including high biosafety, low immunogenicity, stable expression, and high infectivity in several cell types. Although AAV-derived vectors are the safest and most effective viral vectors for gene replacement therapy in the retina, they cannot accommodate genes larger than 4.7 kb, and the generation of neutralizing antibodies against AAV may attenuate the efficacy of AAV-mediated gene therapy [10, 11]. Moreover, the treatment with Luxturna® requires vitrectomy of the retina, followed by the retinal detachment using the air tamponade [12, 13]. This multi-step surgical procedure is a huge burden to patients’ fragile retinas. Another concern is the repeated treatments with Luxturna®, as a single Luxturna® dosage only lasts for five years [14] and patients are required to undergo these invasive procedures routinely. Therefore, innovative topical delivery and highly permeable gene therapy are urgently needed for the therapy of IRDs [15].
The development of the clustered regularly-interspaced short palindromic repeats (CRISPR)-CRISPR-associated protein 9 (Cas9) gene editing technique revolutionized molecular biology and showed great potential for improved gene therapy [16, 17]. The first clinical trial of CRISPR-Cas9 gene editing treatment for IRDs, delivered by the AAV, was launched for the most common cause of inherited childhood blindness, LCA type 10 (LCA10) [18]. Homology-independent targeted integration (HITI) is an advanced CRISPR-Cas9 technique that enables targeted gene insertion in non-dividing cells and presents a new approach to treating genetic disorders [19]. However, one of the greatest challenges in the therapeutic application of CRISPR-Cas9 for retinal diseases is the delivery efficiency of the CRISPR-Cas9 components into the retinal pigment epithelium (RPE) and the neurosensory retinal environment in the posterior pole of human eyeballs [19]. The gene delivery using viral vectors is efficient but associated with several disadvantages, including random insertion, mutagenesis, and biohazard concerns [20]. Recent developments in nanomedicine overcome this difficulty by introducing a nontoxic delivery of CRISPR-Cas9 components that can significantly alleviate safety concerns raised by viral vectors [20]. Today, researchers aim to engineer nanoparticles (NPs) with specialized properties to go beyond viral limitations and create new opportunities towards the application of CRISPR-Cas9 in treating IRDs. However, the translation of such technology to the clinic is hampered by several obstacles. The complexity of the retinal structure poses a significant challenge to the standard measurement of visual performance after treatment and causes unreliable diagnosis [21, 22]. Moreover, in vitro, in vivo, and species variations among disease models limit the ability to fully recapitulate the structure and functions of the retina [23,24,25]. With induced pluripotent stem cells (iPSC) technology's burgeoning field, researchers are now generating three-dimensional (3D) retinal organoids (ROs) from human iPSCs [ During embryonic development, the retina is derived from the prosencephalon, the anterior portion of the brain [21]. The retina’s unique architecture can be classified into two distinct parts: the posterior RPE layer with the most apparent light absorption function and the anterior multilayered neuroretina (Fig. 1). This multi-layered structure of the retina comprises two synaptic layers (the outer and inner plexiform layers) and three specialized neuronal cell layers (Fig. 1). The first neuronal layer comprises rod and cone photoreceptor (PR) cells that convert the absorbed light with different intensities into electrical signals via phototransduction. PR cells then form synapses with the second layers of neuronal cells, horizontal and bipolar cells present in the inner nuclear layer (INL), through the outer plexiform layer (OPL). This eventually leads to the transmission of the signal from PRs to the third class of neuronal layer cells, the retinal ganglion cells (RGCs), through the inner plexiform layer (IPL) (Fig. 1). Finally, the axons of RGCs converge to form the optic nerve, which in turn leads to the transmission of the visual impulses from the eye to the brain [21, 22]. Any impairment in this signaling cascade can result in visual disorder. In addition, the photoreceptor dysfunction or loss can be associated with age, diabetes, and genetics [32,33,34]. The latter causes a specific category of disease described as IRDs, which is the focus of this review. The complex architecture of the retina. The general layout of the retinal layers is shown on the left and cell types on the right. Photoreceptor cells, bipolar cells, and retinal ganglion cells constitute the signal transmission pathway that conveys vision signal to the brain. Horizontal and amacrine cells are interneurons modulating visual signal transmission. Müller glial cells perform the neuronal support functions similar to those of astrocytes in the brain IRDs associated with several clinically and genetically heterogeneous defects belong to a group of progressive retinal degeneration diseases that may lead to vision loss. They exhibit a wide variation in genetic mutations, age of onset, and disease severity [35,36,37], with an estimated incidence of 1 in 2000 to 3000 individuals [38,39,40,41,42]. Due to recent advances toward pathogenesis and characterization of genes responsible for IRDs, with more than 270 genes identified so far, a significant progress has been made in the field of incurable IRDs [43,44,45,46,47,48]. It has led to the development of treatments aimed at restoring vision or delaying the vision loss progression; however thus far, treatment options are still limited. IRD gene variants can be transmitted in an autosomal dominant (e.g. LCA and retinitis pigmentosa (RP)) [49, 50], recessive (e.g. cone-rod dystrophy) [51], and X-linked manner (e.g. X-linked juvenile retinoschisis (XLRS)) [52,53,54,55]. As shown in Table 1, IRDs can be clinically classified into six categories based on the affected retinal regions/cell types and four categories depending on the genetic inheritance mode; other classifications are based on the monogenic and multifactorial nature of IRD and the disease progression [56]. The genetic heterogeneity observed in these diseases manifests in patients with very similar clinical phenotypes but different genetic diagnoses, demanding gene-specific therapies or gene editing treatments to develop treatment strategies [8, 57]. Nevertheless, there is no available cure for IRDs currently, and ophthalmology has been at the forefront of utilizing gene therapy to treat these disorders. Gene therapy application for ophthalmic diseases is a blooming field of research and currently overcoming the barriers for translation to the clinic, which is extensively described in multiple reviews [58,59,60,61,62,63]. AAV vector-based gene therapy has obtained the marketing approval for treating RPE65-associated LCA [18, 64,65,66], Leber hereditary optic neuropathy (LHON) [67, 68], and choroideremia (CHM) [69,70,71]. Treatments for LHON and CHM have entered phase III clinical trials and raised hopes that these approaches might be of practical use to delay or halt disease progression in patients with these IRDs [72,73,74]. Currently, more than 30 gene therapy trials for IRDs are being conducted in the United States, and some have entered the phase III clinical trials. Positive outcomes in these clinical trials were mainly due to the advantages offered by the retina as the target organ and AAV as the carrier [135]. Also, it costs 425,000 US dollars per eye to receive Luxturna® treatment, imposing a heavy economic burden on the society or individuals [1]. Another concern in treating IRDs is the structural peculiarity of the retina, which demands a specific administration route depending on the choice of drug vehicles. With the advent of nanoparticle (NP)-mediated delivery, the unmet needs of efficient genome editing associated with viral vectors are expected to be greatly fulfilled. In therapeutics, the application of NPs as delivery carriers for genes and drugs has been profoundly investigated [136,137,138,139,140,141]. The classification of NPs based on different sizes and structures is shown in Fig. 3. Their nanoscale size enables them to interact with biological systems at the molecular level. In addition, numerous reports have documented that NPs can ensure successful targeted delivery and be transported across biological barriers that can make them an indispensable tool for the drug delivery [142,143,144,145]. The NP-mediated delivery of high molecular weight CRISPR-Cas9 complexes is one of the most significant approaches being developed for genome editing and other evolving applications [146,147,148,149]. Here, we review the promising gene delivery carriers with the potential for IRD treatment based on properties like the nanocarriers and load capacity. To effectively deliver a therapeutic agent to the retina, the particle size and charge are important parameters when develo** nanocarriers [150]. The inner limiting membrane (ILM) with a negative charge located between the vitreous and the retina is a physical and electrostatic barrier [151], which limits the diffusion of NPs [151, 152]. The diffusion of NPs or drugs into the retina varies due to the architecture differences among species, including mice, bovines, and humans [23,24,25]. For example, the pore size of human ILM is about 10 nm, with the variable thickness ranging from 100 nm in the fovea to 4 μm in the thickest area [23]. However, the thickness of the ILM in small animals (such as mice and rats) is less than 100 nm, so the pharmacokinetic distribution of drugs observed in animals often cannot be applicable in clinics [24]. In addition to ILM, the vitreous and retinal cell membranes are all negatively charged. Therefore, after the injection of NPs into the vitreous, the NP’s charge generates an electrostatic interaction which affects the diffusion rate of NPs within the vitreous. Furthermore, the charge of NPs also affects the permeability across the ILM. Therefore, excessive positive and negative charges are not conductive for the drug delivery to the retina [153]. Huang et al. compared lipid NPs with different charges (− 30mv ~ + 50mv) and found that + 35mv lipid NPs can achieve the highest distribution efficiency in the retina [154]. Here, we mainly focus on recent applications of NPs for delivering CRISPR-Cas9 to the retina while an extensive discussion of NP synthesis and design is beyond the scope of this article and has already been reviewed by others [155,156,157,158,159,160,161,162,209]. PEI-modified NPs bind with and condense DNA to form spherical structures, which were fused with the endosome and elicited the “proton sponge effect” to escape endosomal degradation [210]. Several studies have emphasized on the application of this polymer in gene therapy [211,212,213]. In 2015, PEI was utilized for delivering Cas9 and gRNA for the first time, leading to the knockout of the Ptch1 gene in the cerebellum of newborn mice [214]. Liang et al. encapsulated the CRISPR-Cas9 plasmid into an aptamer-functionalized PEG-PEI-Cholesterol nanocarrier to target and reduce vascular endothelial growth factor A (VEGF) gene expression both in vivo and in vitro [168]. However, the moderate toxicity of PEG-PEI-Cholesterol in subsequent clinical trials limited its application for drug delivery [215]. Liao et al. reported that the intravitreal injection of PEI/DNA polyplexes could deliver plasmids into retinal ganglion cells in mice. However, the efficiency of PEI-mediated gene editing was low [216]. β-cyclodextrin (β-CD), an FDA-approved drug [217], is a cyclic oligosaccharide with a diameter of 200 nm and high transfection efficiency for small plasmids [171, 218]. To increase the efficiency of gene transfection by PEI, this polymer was covalently linked with β-CD [219, 220] and showed no reported cytotoxicity in HEK293 cells [221]. This strategy increased the transfection efficiency of the luciferase gene to nearly four folds, compared with PEI alone [171]. Given that β-cyclodextrin-PEI (PEI/β-CD) can condense large plasmids at a high nitrogen-to-phosphorus ratio (N/P ratio; the ratio of positively-chargeable polymer amine (N) groups to negatively-charged nucleic acid phosphate (P) groups), Zhang et al. evaluated the efficiency of PEI/β-CD-mediated delivery of CRISPR-Cas9 system in HeLa cells in which the gene transfection efficiency was about 34%. Meanwhile, this delivery resulted in effient editing at hemoglobin subunit beat loci and rhomboid 5 homolog 1 loci of 19.1% and 7%, respectively [171]. Concerning ocular gene delivery, PEI is one of the most widely investigated polymers. Several studies have explored its potential as an alternative delivery vehicle for the eye [25, 222,223,224,225]. For example, one study showed that the intravitreally injected PEI/DNA could be successfully delivered to mouse RGCs [216]. Conceivably, these findings suggest PEI/β-CD as a potential, efficient, and safe nanocarrier for delivering the CRISPR-Cas9 gene editing system into the retina. However, more investigations and efforts are still needed to elucidate the in vivo utility of PEI/β-CD to deliver CRISPR-Cas9 machinery into the retina. Nanodiamonds (NDs) are a novel class of nanomaterials that have garnered a great deal of attention for their clinical application potential due to their low cost, fluorescent capability, low cytotoxicity, and superior biocompatibility [226,227,228,229,230,231]. It has been reported that most of the NPs destroy the endosomal membrane and release the cargo via the proton sponge effect or chemical reaction [232]. Following the entry of NDs into the cells through endocytosis, the sharp structure of these nanocarriers destroys the endosomal membrane resulting in a quick escape. This unique mechanism of endosomal escape makes NDs more biologically safe and stable [233]. Notably, 2–10 nm NDs provided long-term stability without causing cell death and oxidative stress [231, 234]. In addition, DNA, protein, and drugs can be delivered through different surface modifications of NDs (e.g. carboxylation, hydroxylation, hydrogenation, amination, and halogenation) that improve their intracellular uptake and ability to target specific cells [235,236,237,238]. Despite the progress in ND technology, only one study demonstrated the potential therapeutic application of NDs in treating retinal diseases. In our laboratory, we utilized mCherry protein as a critical linker between 3 nm NDs and DNA in which the amide (–NH) and histidine groups (–His) on mCherry protein bind to the carboxylic groups (–COOH) and phosphate groups (–PO43−) on the ND and DNA molecules, respectively [239]. These chemical reactions formed a stable link between NDs and the components of the CRISPR-Cas9 genome editing system. The final size of this nanocarrier is about 5 nm, which facilitates its penetration and the delivery of CRISPR-Cas9 components to all layers of the retina, including photoreceptor and retinal pigmentation epithelium layers. The resulting NDs effectively promoted the delivery of the CRISPR-Cas9 components to initiate HDR to direct the c.625C > T mutation of RS1 gene in human iPSCs and mouse retina, generating an X-linked retinoschisis-like disease model characterized by severe perturbations of the retinal structure (Fig. 4) [239]. The regulatory approval of new drugs by FDA requires inorganic NPs to be cleared via the kidneys to minimize systemic toxicity and improve drug efficacy [240,241,242]. Since NDs are not biodegradable, using them as the delivery vehicle in therapeutics demands an articulate engineering of NPs to meet FDA standards [231, 243,244,289, 290]. However, the immune issues related to these nanocarriers are a matter of concern, therefore, their clinical applications should be further investigated [291]. To the best of our knowledge, no study explored the potential of DNA nanoclews for drug delivery into the eye and only one study reported the utility of DNA nanoclews for delivering CRISPR-Cas9 as briefly described below [292]. Since this type of NPs is made of ssDNA, a sequence complementary to gRNA can be designed to match the base-pair of the guide portion of Cas9-sgRNA [293]. Sun et al. coated the DNA nanoclews/Cas9 protein/gRNA mixture with PEI to improve its cellular uptake and the endosomal escape. The resulting DNA nanoclews were able to deliver the CRISPR-Cas9 components into the target cells in vitro and in vivo with the editing efficiencies of 36% and 25%, respectively [292]. Nanoscale zeolitic imidazole frameworks (ZIFs), a subclass of metal–organic frameworks (MOFs), are composed of divalent metal cations and imidazolate bridging ligands with pH buffering capacity. These features enable ZIFs to facilitate endosomal escape [294]. ZIFs combine the advantages of the 3D network and porous structure of zeolite with traditional metal–organic clusters [295, 296] that have recently attracted more attention due to their great potential for delivering drugs, genes, and proteins [297,298,299]. Alsaiari et al. reported for the first time that ZIF-8 could encapsulate Cas9 protein and gRNA and subsequently undergo genome editing in Chinese hamster ovary (CHO) cells, with loading and editing efficiencies of 17% and 37%, respectively [294]. To the best of our knowledge, no report on the delivery of CRISPR-Cas9 system to the retina using such nanocarrier has been published. A very recent study utilized a water-in-oil emulsion approach to fabricate a pH-responsive silica–metal–organic framework hybrid NP (SMOF NP) consisting of silica and ZIF. Subretinal injection of SMOF NPs induced efficient genome editing in mouse retinal pigment epithelium. Furthermore, both in vitro loading and delivery efficiencies of CRISPR-Cas9 components by SMOF NPs were high but varied depending on different cell lines [300]. These data introduced a promising nanoplatform that may improve gene therapy in the treatment of IRDs. To summarize, most modern NPs use non-covalent bonding to carry plasmids expressing Cas9 and gRNA or Cas9 protein and gRNA expression plasmids. These NPs are about 100–200 nm in size with a slight positive charge, which may be suitable for intravitreal injection into the eyes of patients with IRDs. We have also described ND carriers as the only carriers smaller than 10 nm. The size may allow the NPs to pass through the human ILM barrier and effectively perform HDR-based CRISPR gene editing in all retinal layers. Also, SMNPs can adapt to various in vivo environments with their unique molecular recognition ability and therefore have the potential to be an alternative approach for the delivery of CRISPR system to the vitreous. Yet, the means of safe and efficient delivery remain to be fully investigated. The properties and advantages/disatvatages of different types of NPs are summarized in Table 2. The heterogeneity of IRDs hampers the development of an effective strategy to tackle a wide range of disorders. One of the major hurdles that hinders the translation of basic retinal research into clinical applications is mainly due to the poor relevance in existing preclinical models. For example, in the mouse model, more than 90% of photoreceptors are rod cells, whereas, in humans, the visual acuity is mostly dependent on cone photoreceptors [301]. Notably, many in vitro and in vivo findings could not be reproduced in humans. Drugs that proved to be safe and effective in animal studies failed to exert the same efficacy in clinical trials. In addition, the information obtained by studying two-dimensional cultures does not recapitulate the heterogeneous complexity and critical features of the microenvironment of cells in vivo [136,137,138]. This gap causes a noticeable lack of fidelity between the aforementioned experimental models and human outcomes. The advent of three-dimensional (3D) multicellular constructs, referred to as the organoid technology, offers a promising complementary model to pursue clinical translation and precision medicine applications. Human pluripotent stem cells (hPSC)-derived retinal organoids hold an excellent value for modeling the human retina features [302,303,304,305,306]. In particular, by using patient-derived cells combined with reprogramming strategies, this technique could represent an efficient pre-clinical approach toward the personalized therapeutic strategies adaptable to a broad number of IRDs and provide the link to disease-specific human drug screening models [307]. The iPSC-derived organoids strategy can provide a means for assessing the efficiency and efficacy of NP-mediated delivery of CRISPR-Cas9 system tailored to each patient's genetic makeup. In this part, we mainly focus on organoid as a technology platform for precision medicine in IRDs, especially with potential translational applications for evaluating new therapeutic drugs. Introducing a new pharmaceutical drug to the market is a complicated and costly process, especially when the in vivo testing result of drug candidates fail to reach the requirements initially fulfilled by the in vitro test. The gap between in vitro validation and clinical application is significant, mainly because the simplicity of the in vitro model cannot mimic the complex nature and heterogeneous characteristics of clinical patients [308]. These critical problems impose significant limitations on the translation of candidate drugs to the clinic and require advanced strategies to improve this shortage. Organoid systems show considerable reliability of recapitulating features and functions of the human system offering a great potential for testing drug efficiency in target organs. Patient-derived organoids (PDOs) can be generated particularly by reprogramming patient-derived cells to induced pluripotent stem cells (iPSCs), followed by the differentiation into the desired cell lineage and organoids [309]. Notably, several reports showed that in vitro PDOs could highly match and reproduce patients’ response to candidate drugs in most cases, highlighting the merit of this system in personalized medicine as a predictor of therapeutic outcome [310,311,312,313]. The organ-like structure technology offers a more efficient screening of candidate drugs prior to in vivo testing, which helps to reduce drug development costs. Organoids have been a powerful tool for functional drug testing, personalized therapy and disease modeling [314,315,316,317,318,319,320]. For iPSC-derived organoids, various organoid models have been generated using human iPSCs, including heart [321, 322], kidney [323], brain [324, 325], intestine [312, 313, 326], liver [327,328,329], lung [330] and retina [302,303,304,305]. Furthermore, organoids have been successfully applied to model human genetic diseases. For example, intestinal organoids derived from cystic fibrosis (CF) patients proved to be a reliable tool for effective drug treatment [314]. Of note, brain [315,316,317] and kidney [318,319,320] organoids generated from patient-derived iPSCs have also been established to model diseases. Xu et al. subjected brain organoids to Zika virus infection and used them as the platform for drug repurposing [331]. Bian et al. demonstrated that the neoplastic cerebral organoids are suitable for targeted drug testing [332]. Saengwimol et al. used retinoblastoma organoids for the evaluation of cellular response to chemotherapy drugs. [333]. However, the consistency and reducibility of this system at a scale consistent with clinically associated cell numbers is still a matter of concern [334]. For retinal organoids, Vergara et al. developed an iPSC-derived retinal organoid-based screening platform that allows the accurate quantification of fluorescent reporters [335]. Despite the progress of some organoid-based researches, the organoid technologies remains immature and not ready for the demands of high-throughput screening in drug screening [336]. It was attributed to the developmental variability and diversity of retinal organoids that may hinder the utility of retinal organoids in the evaluation of therapeutic effects and comparative analysis [337]. Nevertheless, considering that retinal organoids hold promising potential in new drug development, it would be still expected and encouraging to use retinal organoid technologies to augment the existing drug development pipelines. Collectively, these findings highlight the utility of organoids as a part of the drug testing pipeline, creating the opportunities for more effective therapies, especially for patients with rare genetic diseases in a cost-effective manner. To establish a drug testing platform for IRD treatment based on organoid technologies, generating a representative disease model is a fundamental step. As highlighted below, several reports demonstrated the utility of organoids in eye disease modeling. Ohlemacher et al. developed a retinal organoid model using patient-specific iPSC-derived RGCs to study an inherited form of glaucoma [26]. Tucker et al. generated multi-layer optic cup-like structures representing photoreceptor precursor cells for investigating the pathogenesis of RP [27]. A separate study focused on different frameshift mutations in the RPGR gene, one of the most prevalent causes of autosomal recessive RP, and generated patient-specific retinal organoids with defects in morphology and functionality of photoreceptors accompanied with decreased cilia length as a disease model [28]. The constructed vectors for the CRISPR-Cas9 machinery were delivered into the patient-derived iPSCs via electroporation, and the mutation-corrected iPSCs were then differentiated into retinal organoids. Notably, the reversal of morphological and functional defects in retinal organoids with RPGR mutation was observed after the CRISPR-Cas9-mediated gene correction [28]. Using a similar approach, Buskin et al. demonstrated the severe RP defects observed in patient-specific retinal organoids harboring the CRISPR-Cas9-induced PRPF31 mutation [29]. This further proved the effectiveness of this combined strategy toward personalized and targeted gene therapy. Another example for coupling retinal organoids with the genome engineering technique is the application of CRISPR-Cas9 technology to correct RS1 mutation in retinal organoids derived from XLRS-patients [30]. Huang et al. successfully established the XLRS patient-derived retinal organoids that recapitulate the retinal splitting feature of the disease. Meanwhile, they delivered the CRISPR-Cas9 system using electroporation to correct the mutation and showed that CRISPR-Cas9-mediated correction of the disease-associated C625T mutation efficiently rescued the disease phenotype (Fig. 6) [30]. Parfitt et al. used LCA patient-derived iPSCs to generate 3D optic cups with the mutation of a cilia-related gene, CEP290 [31]. Introducing an antisense oligonucleotide to patient iPSC-derived organoids could effectively prevent the aberrant splicing, restore the expression of full-length CEP290 protein, and repaired the cilia defects [31]. Currently, CEP290 treatment is in phase III clinical trial (NCT03913143), paralleling the classic augmentation RPE65 trials initiated in 2007 [338, 339]. Overall, these studies demonstrated that 3D retinal organoids derived from patients with various retinal diseases are able to recapitulate the complex retinal architecture, rendering them an ideal platform for examining the safety and specificity of CRISPR-Cas9 system for the therapeutics applications. Reproduced from our previous study [30] Patient-derived retinal organoids recapitulate disease-specific features. A Bright-field images and B H&E staining of control and XLRS patient-derived retinal organoids exhibit schisis feature at day 150 of differentiation. C Quantification of splitting area in control and XLRS-patient-derived retinal organoids. D Bright-field images of control and XLRS patient-derived retinal organoids at days 90, 100, and 110 of differentiation. E A schematic presentation of the time course for the generation of control and XLRS patient-derived retinal organoids. Disease-specific features can be observed after applying defined differentiation stimuli and time course. IRDs have long been viewed as a class of disorders with no effective treatment. This maxim is now being reversed by tremendous efforts in nanomedicine and gene engineering, which results in promising clinical trials for blinding diseases. As therapeutic strategies for IRDs expand, the importance of molecular diagnosis is gaining momentum. Most IRD gene supplementation therapies are in phase I/II clinical trials, with LCA therapy approved by the FDA to treat patients carrying biallelic RPE65 mutations [59, 62, 340,341,342]. Although these efforts are still evolving, the importance of gene therapy for elevating the life quality of IRD patients has never been more apparent. In this review, we introduced a forward movement of therapy by combining the advances in CRISPR-mediated gene editing, NP-based delivery, and iPSC-derived retinal organoids technologies, to assess the potential safety and efficacy of designed CRISPR-Cas9 components and nanocarriers in a clinically relevant in vitro model. As mentioned earlier, NP-mediated delivery of high molecular weight CRISPR-Cas9 complexes combined with the advanced CRISPR-Cas9 technologies, applicable in non-dividing retinal cells (e.g. HITI), introduces a simple yet efficient approach for precise gene therapy in IRDs. In addition, patient-derived retinal organoids can mimic typical disease features, providing a reliable platform for disease modeling. Collectively, this integrated strategy is expected to facilitate the evaluation of the gene editing in preclinical tests and be a major driver towards advancing IRD’s personalized medicine (Fig. 7). An integrative, multidisciplinary approach for future gene therapy in IRDs. IRD patient’s blood sample can be reprogrammed into induced pluripotent stem cells (iPSCs) followed by the differentiation into retinal organoids. These patient-derived retinal organoids exhibit disease-specific features and can be applied as a reliable platform for assessing disease progression and treatment outcome (e.g. XLRS-patient-derived retinal organoids exhibit severe retinoschisis-like features). Researchers can utilize optimized NPs loaded with plasmid DNA encoding CRISPR-Cas9 machinery to achieve efficient gene delivery and precise gene editing. This results in the rescue of the disease phenotypes associated with the specific IRDs (e.g. the splitting phenotype in XLRS-patient-derived retinal organoids can be rescued as shown above). Integrating patient-derived retinal organoids, CRISPR-Cas9 technologies, and NPs promotes precision gene therapy applications for IRDs During the past few years, our preliminary research on combining CRISPR-Cas9 gene editing, patient-derived retinal organoids, and NPs has been conducted to investigate the potential of this integrated strategy in IRD therapeutics. For example, Yang et al. utilized ND-mediated delivery of CRISPR-Cas9 to introduce the mutated RS1 gene into human iPSCs and mouse retina, leading to the generation of XLRS-like disease model [239], however, the potential of this strategy for gene therapy is yet to be explored. In another study, Chou et al. successfully combined SMNP nanocarrier and CRISPR-Cas9-mediated HITI strategy to knock-in the RS1 gene in the mouse retina [119]. In the future, this strategy can be further applied in patient-derived retinal organoids model to assess its efficiency and efficacy in clinically relevant disease setting. In a separate study, Huang et al. successfully differentiated iPSCs from XLRS patients into retinal organoids presenting disease features. They further coupled this disease model with CRISPR-Cas9 technology and repaired the RS1 gene mutation with 50% efficiency [30]. However, the electroporation method used for the transfection of CRISPR-Cas9 machinery caused a massive cell death [30]. Therefore, future attempts to utilize NPs as an alternative method will be of great interest to resolve this drawback and further boost the therapeutic effects. We hope that this combined strategy will become a treatment modality for other IRDs and elevate the life quality of patients. Although the rationale for this integrated approach is clear, several technology hurdles remain to be addressed. The development of advanced CRISPR-Cas9 systems with high specificity has armed researchers worldwide with a powerful tool to study human diseases. However, utilizing this technique for translational medicine research has inevitable concerns. Since patient-derived iPSCs not only carry the specific mutation intended to be repaired but also harbor the entire human genome, this makes CRISPR-Cas9-mediated gene editing more susceptible to undesired off-target effects. For example, gRNA may recognize sequences similar to the target loci and cause permanent sequence alterations resulting in abnormal gene function. Furthermore, no in vivo study examined how long the nuclease remains active before its degradation and what the possible adverse effect(s) might be. Anti-CRISPR proteins can be used to limit the off-target editing, however, the best time to shut off Cas activity requires optimizations [343]. Another concern is that CRISPR-Cas9-mediated knockout or overexpression of the gene of interest may be compensated by neighboring cells, which would interfere with the expected outcome [344]. As for the organoid technology platform, although the 3D retina organoid has equipped ophthalmology with a unique and relatively accurate representation of the human eye, it still lacks a high level of morphological and functional complexity demonstrated by the mammalian retina in vivo. For example, the 3D retinal organoid with retinal pigment epithelium (RPE) layer provides a more physiologically relevant disease model for photoreceptor-associated diseases. However, both simple and complex organoid models have their pros and cons; thus, the appropriate level of complexity should be designed according to the purpose of the study [345]. A more challenging issue is the disease modeling of late-onset retinal diseases by manipulating culture medium to induce ageing factors. It demands a profound knowledge of factors involved in each specific developmental stage which are yet to be investigated. More in-depth knowledge and assessment of the culture medium composition and distribution are required for modeling complex IRDs. Nevertheless, retinal organoid technologies have only been utilized for monogenic IRDs so far. Lastly, although NP-based delivery has been proposed and proven to be a promising drug delivery vehicle, improving the therapeutic application of NP-based gene therapy remains an important concern. It requires in-depth investigation on the cytotoxicity of the NPs under variable conditions and on the key factors determining the release rate of drugs from NPs to the retina. The successful drug delivery to the neuroretina, and even more specifically to photoreceptors, highly depends on the choice of the NPs most suitable for both the drug and the target tissue. Besides, different gene mutations may affect the complex retina structure and cause anatomical obstacles for nanomedicine drug delivery. Moreover, the choice of delivery route, immunoreactivity, and nucleic acid-based drug stability are critical factors that need to be addressed for a successful clinical application. Nevertheless, in this ever-evolving field, it is crucial to move scientific discoveries into clinics and new therapies for vision restoration in more patients than ever before. Ultimately, the application of such technologies in the clinic and industry should fulfill four criteria: reproducibility, standardization, validation, and quality assurance. Although NP-mediated delivery of the CRISPR-Cas9 system shows a great promise in repairing the IRDs, the time window for treatment is a critical determinant for the therapeutic outcome. For example, in XLRS, the differentiated retinal cells with the splitting phenotype are not responsive to gene therapy, indicating the delayed treatment by the time the disease is already progressed. However, more preclinical data will be required to prove this concept. Taken together, the advances and current progress of basic research hold the promises that laboratory findings can be translated into clinical applications in the near future and bring hope to patients who have blindness and other hereditary diseases. The integration of nanotechnology, CRISPR, and stem cell technologies present a novel platform and is expected to accelerate bridging the basic research and translational medicine, and further promote medical precision therapies.
The complex three-dimensional (3D) architecture of the retina
Inherited retinal diseases and the treatment obstacles
Overview of gene therapy techniques for IRDs
Inorganic nanoparticles
Nanodiamonds (NDs)
Nanoscale zeolitic imidazole frameworks (ZIFs)
The retinal organoids and precision medicine
Organoids as a drug testing platform for translational research
Retinal organoid applications in precision medicine

Conclusions and perspectives
The combination of NPs, CRISPR-Cas9, and retinal organoids as a promising therapeutic platform
Technology hurdles
References
Daiger S, Sullivan L, Bowne S, Rossiter B. RetNet: retinal information network. Na+ Ca2. 2013;5.
Farrar GJ, Carrigan M, Dockery A, Millington-Ward S, Palfi A, Chadderton N, Humphries M, Kiang AS, Kenna PF, Humphries P. Toward an elucidation of the molecular genetics of inherited retinal degenerations. Hum Mol Genet. 2017;26:R2–11.
Koenekoop RK, Sui R, Sallum J, Van Den Born LI, Ajlan R, Khan A, Den Hollander AI, Cremers FP, Mendola JD, Bittner AK. Oral 9-cis retinoid for childhood blindness due to Leber congenital amaurosis caused by RPE65 or LRAT mutations: an open-label phase 1b trial. Lancet. 2014;384:1513–20.
Bennett J, Wellman J, Marshall KA, McCague S, Ashtari M, DiStefano-Pappas J, Elci OU, Chung DC, Sun J, Wright JF. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial. Lancet. 2016;388:661–72.
Finger RP, Fimmers R, Holz FG, Scholl HP. Prevalence and causes of registered blindness in the largest federal state of Germany. Br J Ophthalmol. 2011;95:1061–7.
Liew G, Michaelides M, Bunce C. A comparison of the causes of blindness certifications in England and Wales in working age adults (16–64 years), 1999–2000 with 2009–2010. BMJ Open. 2014;4:e004015.
Khan NW, Falsini B, Kondo M, Robson AG. Inherited retinal degeneration: genetics, disease characterization, and outcome measures. J Ophthalmol. 2017. https://doi.org/10.1155/2017/2109014.
Sullivan LS, Daiger SP. Inherited retinal degeneration: exceptional genetic and clinical heterogeneity. Mol Med Today. 1996;2:380–6.
Gupta PR, Huckfeldt RM. Gene therapy for inherited retinal degenerations: initial successes and future challenges. J Neural Eng. 2017;14: 051002.
Flotte TR. Size does matter: overcoming the adeno-associated virus packaging limit. Respir Res. 2000;1:16–8.
Peng R, Lin G, Li J. Potential pitfalls of CRISPR/Cas9-mediated genome editing. FEBS J. 2016;283:1218–31.
Grieger JC, Samulski RJ. Packaging capacity of adeno-associated virus serotypes: impact of larger genomes on infectivity and postentry steps. J Virol. 2005;79:9933–44.
Selot R S, Hareendran S, Jayandharan G R. Develo** immunologically inert adeno-associated virus (AAV) vectors for gene therapy: possibilities and limitations. Curr Pharm Biotechnol. 2013;14:1072–82.
Weleber RG, Pennesi ME, Wilson DJ, Kaushal S, Erker LR, Jensen L, McBride MT, Flotte TR, Humphries M, Calcedo R. Results at 2 years after gene therapy for RPE65-deficient Leber congenital amaurosis and severe early-childhood–onset retinal dystrophy. Ophthalmology. 2016;123:1606–20.
Thompson DA, Iannaccone A, Ali RR, Arshavsky VY, Audo I, Bainbridge JW, Besirli CG, Birch DG, Branham KE, Cideciyan AV. Advancing clinical trials for inherited retinal diseases: recommendations from the Second Monaciano Symposium. Transl Vis Sci Technol. 2020;9:2–2.
Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8:2281–308.
Schwank G, Koo B-K, Sasselli V, Dekkers JF, Heo I, Demircan T, Sasaki N, Boymans S, Cuppen E, van der Ent CK. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. 2013;13:653–8.
Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358:2231–9.
Moreno AM, Fu X, Zhu J, Katrekar D, Shih YRV, Marlett J, Cabotaje J, Tat J, Naughton J, Lisowski L. In situ gene therapy via AAV-CRISPR-Cas9-mediated targeted gene regulation. Mol Ther. 2018;26:1818–27.
Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:346–58.
Nakamura H, Matsui KA, Takagi S, Fujisawa H. Projection of the retinal ganglion cells to the tectum differentiated from the prosencephalon. Neurosci Res. 1991;11:189–97.
Polyak SL. The retina. Chicago: University of Chicago Press; 1941.
Henrich PB, Monnier CA, Halfter W, Haritoglou C, Strauss RW, Lim RY, Loparic M. Nanoscale topographic and biomechanical studies of the human internal limiting membrane. Invest Ophthalmol Vis Sci. 2012;53:2561–70.
Slijkerman RW, Song F, Astuti GD, Huynen MA, van Wijk E, Stieger K, Collin RW. The pros and cons of vertebrate animal models for functional and therapeutic research on inherited retinal dystrophies. Prog Retin Eye Res. 2015;48:137–59.
Pitkänen L, Pelkonen J, Ruponen M, Rönkkö S, Urtti A. Neural retina limits the nonviral gene transfer to retinal pigment epithelium in an in vitro bovine eye model. AAPS J. 2004;6:72–80.
Ohlemacher SK, Sridhar A, **ao Y, Hochstetler AE, Sarfarazi M, Cummins TR, Meyer JS. Stepwise differentiation of retinal ganglion cells from human pluripotent stem cells enables analysis of glaucomatous neurodegeneration. Stem Cells. 2016;34:1553–62.
Tucker BA, Mullins RF, Streb LM, Anfinson K, Eyestone ME, Kaalberg E, Riker MJ, Drack AV, Braun TA, Stone EM. Patient-specific iPSC-derived photoreceptor precursor cells as a means to investigate retinitis pigmentosa. Elife. 2013;2: e00824.
Deng W-L, Gao M-L, Lei X-L, Lv J-N, Zhao H, He K-W, **a X-X, Li L-Y, Chen Y-C, Li Y-P, et al. Gene correction reverses ciliopathy and photoreceptor loss in iPSC-derived retinal organoids from retinitis pigmentosa patients. Stem Cell Rep. 2018;10:1267–81.
Buskin A, Zhu L, Chichagova V, Basu B, Mozaffari-Jovin S, Dolan D, Droop A, Collin J, Bronstein R, Mehrotra S, et al. Disrupted alternative splicing for genes implicated in splicing and ciliogenesis causes PRPF31 retinitis pigmentosa. Nat Commun. 2018;9:4234.
Huang K-C, Wang M-L, Chen S-J, Kuo J-C, Wang W-J, Nguyen PNN, Wahlin KJ, Lu J-F, Tran AA, Shi M. Morphological and molecular defects in human three-dimensional retinal organoid model of X-linked juvenile retinoschisis. Stem Cell Rep. 2019;13:906–23.
Parfitt DA, Lane A, Ramsden CM, Carr AJ, Munro PM, Jovanovic K, Schwarz N, Kanuga N, Muthiah MN, Hull S, et al. Identification and correction of mechanisms underlying inherited blindness in human iPSC-derived optic cups. Cell Stem Cell. 2016;18:769–81.
Bennett J, Tanabe T, Sun D, Zeng Y, Kjeldbye H, Gouras P, Maguire AM. Photoreceptor cell rescue in retinal degeneration (rd) mice by in vivo gene therapy. Nat Med. 1996;2:649–54.
Jackson GR, Barber AJ. Visual dysfunction associated with diabetic retinopathy. Curr DiabRep. 2010;10:380–4.
Jackson GR, Owsley C, Curcio CA. Photoreceptor degeneration and dysfunction in aging and age-related maculopathy. Ageing Res Rev. 2002;1:381–96.
Huang X-F, Huang F, Wu K-C, Wu J, Chen J, Pang C-P, Lu F, Qu J, ** Z-B. Genotype–phenotype correlation and mutation spectrum in a large cohort of patients with inherited retinal dystrophy revealed by next-generation sequencing. Genet Med. 2015;17:271–8.
Glöckle N, Kohl S, Mohr J, Scheurenbrand T, Sprecher A, Weisschuh N, Bernd A, Rudolph G, Schubach M, Poloschek C. Panel-based next generation sequencing as a reliable and efficient technique to detect mutations in unselected patients with retinal dystrophies. Eur J Hum Genet. 2014;22:99–104.
Bernardis I, Chiesi L, Tenedini E, Artuso L, Percesepe A, Artusi V, Simone ML, Manfredini R, Camparini M, Rinaldi C. Unravelling the complexity of inherited retinal dystrophies molecular testing: added value of targeted next-generation sequencing. Biomed Res Int. 2016;2016:14.
Novak-Lauš K, Kukulj S, Zorić-Geber M, Bastaić O. Primary tapetoretinal dystrophies as the cause of blindness and impaired vision in the republic of Croatia. Acta Clin Croat. 2002;41:23–7.
Haim M. Epidemiology of retinitis pigmentosa in Denmark. Acta Ophthalmol Scand. 2002;80:1.
Grøndahl J. Estimation of prognosis and prevalence of retinitis pigmentosa and Usher syndrome in Norway. Clin Genet. 1987;31:255–64.
Bunker CH, Berson EL, Bromley WC, Hayes RP, Roderick TH. Prevalence of retinitis pigmentosa in Maine. Am J Ophthalmol. 1984;97:357–65.
Broadgate S, Yu J, Downes SM, Halford S. Unravelling the genetics of inherited retinal dystrophies: past, present and future. Prog Retin Eye Res. 2017;59:53–96.
Wright AF, Chakarova CF, Abd El-Aziz MM, Bhattacharya SS. Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. Nat Rev Genet. 2010;11:273–84.
Nash BM, Wright DC, Grigg JR, Bennetts B, Jamieson RV. Retinal dystrophies, genomic applications in diagnosis and prospects for therapy. Transl Pediatr. 2015;4:139–63.
Iannaccone A. The genetics of hereditary retinopathies and optic neuropathies. Compr Ophthalmol Update. 2005;6:39–62.
Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–809.
Daiger S, Rossiter B, Greenberg J, Christoffels A, Hide W. Data services and software for identifying genes and mutations causing retinal degeneration. Invest Ophthalmol Vis Sci. 1998;39:S295.
Bramall AN, Wright AF, Jacobson SG, McInnes RR. The genomic, biochemical, and cellular responses of the retina in inherited photoreceptor degenerations and prospects for the treatment of these disorders. Annu Rev Neurosci. 2010;33:441–72.
Erkilic N, Sanjurjo-Soriano C, Manes G, Dubois G, Hamel CP, Meunier I, Kalatzis V. Generation of a human iPSC line, INMi004-A, with a point mutation in CRX associated with autosomal dominant Leber congenital amaurosis. Stem Cell Res. 2019;38: 101476.
Diakatou M, Manes G, Bocquet B, Meunier I, Kalatzis V. Genome editing as a treatment for the most prevalent causative genes of autosomal dominant retinitis pigmentosa. Int J Mol Sci. 2019;20:2542.
Ahn J, Chiang J, Gorin MB. Novel mutation in SLC4A7 gene causing autosomal recessive progressive rod-cone dystrophy. Ophthalmic Genet. 2020;41:386–9.
Vijayasarathy C, Takada Y, Zeng Y, Bush RA, Sieving PA. Retinoschisin is a peripheral membrane protein with affinity for anionic phospholipids and affected by divalent cations. Invest Ophthalmol Vis Sci. 2007;48:991–1000.
Sauer CG, Gehrig A, Warneke-Wittstock R, Marquardt A, Ewing CC, Gibson A, Lorenz B, Jurklies B, Weber BH. Positional cloning of the gene associated with X-linked juvenile retinoschisis. Nat Genet. 1997;17:164–70.
Hiriyanna KT, Bingham EL, Yashar BM, Ayyagari R, Fishman G, Small KW, Weinberg DV, Weleber RG, Lewis RA, Andreasson S. Novel mutations in XLRS1 causing retinoschisis, including first evidence of putative leader sequence change. Hum Mutat. 1999;14:423–7.
Ali MH, Vajzovic L. X-Linked Juvenile Retinoschisis. In: Toth CA, Ong SS, editors. Handbook of pediatric retinal OCT and the eye-brain connection. Philadelphia: Elsevier; 2020. p. 119–23.
Dalkara D, Sahel J-A. Gene therapy for inherited retinal degenerations. CR Biol. 2014;337:185–92.
Dryja T, Li T. Molecular genetics of retinitis pigmentosa. Hum Mol Genet. 1995;4:1739–43.
Arbabi A, Liu A, Ameri H. Gene therapy for inherited retinal degeneration. J Ocul Pharmacol Ther. 2019;35:79–97.
Lee JH, Wang J-H, Chen J, Li F, Edwards TL, Hewitt AW, Liu G-S. Gene therapy for visual loss: opportunities and concerns. Prog Retin Eye Res. 2019;68:31–53.
Ramlogan-Steel CA, Murali A, Andrzejewski S, Dhungel B, Steel JC, Layton CJ. Gene therapy and the adeno-associated virus in the treatment of genetic and acquired ophthalmic diseases in humans: trials, future directions and safety considerations. Clin Exp Ophthalmol. 2019;47:521–36.
Soofiyani SR, Baradaran B, Lotfipour F, Kazemi T, Mohammadnejad L. Gene therapy, early promises, subsequent problems, and recent breakthroughs. Adv Pharm Bull. 2013;3:249.
Trapani I, Auricchio A. Seeing the light after 25 years of retinal gene therapy. Trends Mol Med. 2018;24:669–81.
Wert KJ, Davis RJ, Sancho-Pelluz J, Nishina PM, Tsang SH. Gene therapy provides long-term visual function in a pre-clinical model of retinitis pigmentosa. Hum Mol Genet. 2013;22:558–67.
Cideciyan AV, Aleman TS, Boye SL, Schwartz SB, Kaushal S, Roman AJ, Pang JJ, Sumaroka A, Windsor EA, Wilson JM. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci. 2008;105:15112–7.
Maguire AM, Simonelli F, Pierce EA, Pugh EN Jr, Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358:2240–8.
Smalley E. First AAV gene therapy poised for landmark approval. Nat Biotech. 2017. https://doi.org/10.1038/nbt1117-998.
Feuer WJ, Schiffman JC, Davis JL, Porciatti V, Gonzalez P, Koilkonda RD, Yuan H, Lalwani A, Lam BL, Guy J. Gene therapy for Leber hereditary optic neuropathy: initial results. Ophthalmology. 2016;123:558–70.
Guy J, Feuer WJ, Davis JL, Porciatti V, Gonzalez PJ, Koilkonda RD, Yuan H, Hauswirth WW, Lam BL. Gene therapy for Leber hereditary optic neuropathy: low-and medium-dose visual results. Ophthalmology. 2017;124:1621–34.
Fischer MD, Ochakovski GA, Beier B, Seitz IP, Vaheb Y, Kortuem C, Reichel FF, Kuehlewein L, Kahle NA, Peters T. Efficacy and safety of retinal gene therapy using adeno-associated virus vector for patients with choroideremia: a randomized clinical trial. JAMA Ophthalmol. 2019;137:1247–54.
Lam BL, Davis JL, Gregori NZ, MacLaren RE, Girach A, Verriotto JD, Rodriguez B, Rosa PR, Zhang X, Feuer WJ. Choroideremia gene therapy phase 2 clinical trial: 24-month results. Am J Ophthalmol. 2019;197:65–73.
MacLaren RE, Groppe M, Barnard AR, Cottriall CL, Tolmachova T, Seymour L, Clark KR, During MJ, Cremers FP, Black GC. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 2014;383:1129–37.
McClements ME, MacLaren RE. Gene therapy for retinal disease. Transl Res. 2013;161:241–54.
Russell S, Bennett J, Wellman J, Chung D, High K, Tillman A. Phase 3 trial update of voretigene neparvovec in biallelic RPE65-mediated inherited retinal disease. Am Acad Ophthalmol AAO. 2017;2017:11–4.
Russell S, Bennett J, Wellman JA, Chung DC, Yu Z-F, Tillman A, Wittes J, Pappas J, Elci O, McCague S. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390:849–60.
Vandenberghe LH, Bell P, Maguire AM, Cearley CN, **ao R, Calcedo R, Wang L, Castle MJ, Maguire AC, Grant R. Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey. Sci Transl Med. 2011;3:88ra54-88ra54.
Koerber JT, Klimczak R, Jang J-H, Dalkara D, Flannery JG, Schaffer DV. Molecular evolution of adeno-associated virus for enhanced glial gene delivery. Mol Ther. 2009;17:2088–95.
Dyka FM, Molday LL, Chiodo VA, Molday RS, Hauswirth WW. Dual ABCA4-AAV vector treatment reduces pathogenic retinal A2E accumulation in a mouse model of autosomal recessive stargardt disease. Hum Gene Ther. 2019;30:1361–70.
Dyka FM, Boye SL, Chiodo VA, Hauswirth WW, Boye SE. Dual adeno-associated virus vectors result in efficient in vitro and in vivo expression of an oversized gene, MYO7A. Hum Gene Ther Methods. 2014;25:166–77.
Zeng Y, Takada Y, Kjellstrom S, Hiriyanna K, Tanikawa A, Wawrousek E, Smaoui N, Caruso R, Bush RA, Sieving PA. RS-1 gene delivery to an adult Rs1h knockout mouse model restores ERG b-wave with reversal of the electronegative waveform of X-linked retinoschisis. Invest Ophthalmol Vis Sci. 2004;45:3279–85.
Park T, Wu Z, Kjellstrom S, Zeng Y, Bush RA, Sieving P, Colosi P. Intravitreal delivery of AAV8 retinoschisin results in cell type-specific gene expression and retinal rescue in the Rs1-KO mouse. Gene Ther. 2009;16:916–26.
Byrne LC, Öztürk BE, Lee T, Fortuny C, Visel M, Dalkara D, Schaffer DV, Flannery JG. Retinoschisin gene therapy in photoreceptors, Müller glia or all retinal cells in the Rs1h−/− mouse. Gene Ther. 2014;21:585–92.
Sengillo JD, Justus S, Tsai YT, Cabral T, Tsang SH. Gene and cell-based therapies for inherited retinal disorders: an update. In: Tan WH, Bird LM, editors. American journal of medical genetics part c: seminars in medical genetics. Toronto: Wiley; 2016. p. 349–66.
Lewin AS, Rossmiller B, Mao H. Gene augmentation for adRP mutations in RHO. Cold Spring Harb Perspect Med. 2014;4: a017400.
Davis JL, Gregori NZ, MacLaren RE, Lam BL. Surgical technique for subretinal gene therapy in humans with inherited retinal degeneration. Retina. 2019;39:S2–8.
Davis JL. The blunt end: surgical challenges of gene therapy for inherited retinal diseases. Am J Ophthalmol. 2018;196:1–3.
Andrieu-Soler C, Bejjani R-A, de Bizemont T, Normand N, BenEzra D, Behar-Cohen F. Ocular gene therapy: a review of nonviral strategies. Mol Vis. 2006;12:1334–47.
Han Z, Conley SM, Naash MI. AAV and compacted DNA nanoparticles for the treatment of retinal disorders: challenges and future prospects. Invest Ophthalmol Vis Sci. 2011;52:3051–9.
Koirala A, Conley SM, Naash MI. A review of therapeutic prospects of non-viral gene therapy in the retinal pigment epithelium. Biomaterials. 2013;34:7158–67.
Cai X, Conley S, Naash M. Nanoparticle applications in ocular gene therapy. Vision Res. 2008;48:319–24.
Han Z, Conley SM, Makkia RS, Cooper MJ, Naash MI. DNA nanoparticle-mediated ABCA4 delivery rescues Stargardt dystrophy in mice. J Clin Investig. 2012;122:3221–6.
Dalkara D, Byrne LC, Klimczak RR, Visel M, Yin L, Merigan WH, Flannery JG, Schaffer DV. In vivo—directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci Transl Med. 2013;5:189ra176-189ra176.
Petrs-Silva H, Dinculescu A, Li Q, Min S-H, Chiodo V, Pang J-J, Zhong L, Zolotukhin S, Srivastava A, Lewin AS. High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol Ther. 2009;17:463–71.
Leroy B, Pennesi M, Ohnsman C. Brave new world: gene therapy for inherited retinal disease. In: Leroy B, Pennesi M, Ohnsman C, editors. American academy of ophthalmology. San Francisco: EyeNet; 2018. p. 1–16.
Lipinski DM, Thake M, MacLaren RE. Clinical applications of retinal gene therapy. Prog Retin Eye Res. 2013;32:22–47.
Pennesi ME, Birch DG, Duncan JL, Bennett J, Girach A. Choroideremia: retinal degeneration with an unmet need. Retina. 2019;39:2059–69.
Liu M, Rehman S, Tang X, Gu K, Fan Q, Chen D, Ma W. Methodologies for improving HDR efficiency. Front Genet. 2019;9:691.
Yang H, Wang H, Shivalila CS, Cheng AW, Shi L, Jaenisch R. One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell. 2013;154:1370–9.
Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153:910–8.
Ran F, Cong L, Yan WX, Scott DA, Gootenberg JS, Kriz AJ, Zetsche B, Shalem O, Wu X, Makarova KS. In vivo genome editing using Staphylococcus aureus Cas9. Nature. 2015;520:186–91.
Le Rhun A, Escalera-Maurer A, Bratovic M, Charpentier E. CRISPR-Cas in Streptococcus pyogenes. RNA Biol. 2019;16:380–9.
Aghaizu ND, Kruczek K, Gonzalez-Cordero A, Ali RR, Pearson RA. Pluripotent stem cells and their utility in treating photoreceptor degenerations. Prog Brain Res. 2017;231:191–223.
Hazim RA, Karumbayaram S, Jiang M, Dimashkie A, Lopes VS, Li D, Burgess BL, Vijayaraj P, Alva-Ornelas JA, Zack JA. Differentiation of RPE cells from integration-free iPS cells and their cell biological characterization. Stem Cell Res Ther. 2017;8:1–17.
Jones MK, Lu B, Girman S, Wang S. Cell-based therapeutic strategies for replacement and preservation in retinal degenerative diseases. Prog Retin Eye Res. 2017;58:1–27.
Reichman S, Terray A, Slembrouck A, Nanteau C, Orieux G, Habeler W, Nandrot EF, Sahel J-A, Monville C, Goureau O. From confluent human iPS cells to self-forming neural retina and retinal pigmented epithelium. Proc Natl Acad Sci. 2014;111:8518–23.
Nami F, Basiri M, Satarian L, Curtiss C, Baharvand H, Verfaillie C. Strategies for in vivo genome editing in nondividing cells. Trends Biotechnol. 2018;36:770–86.
Yamamoto Y, Bliss J, Gerbi SA. Whole organism genome editing: Targeted large DNA insertion via ObLiGaRe nonhomologous end-joining in vivo capture. G3. 2015;5:1843–7.
Ishizu T, Higo S, Masumura Y, Kohama Y, Shiba M, Higo T, Shibamoto M, Nakagawa A, Morimoto S, Takashima S. Targeted genome replacement via homology-directed repair in non-dividing cardiomyocytes. Sci Rep. 2017;7:1–11.
Suzuki K, Tsunekawa Y, Hernandez-Benitez R, Wu J, Zhu J, Kim EJ, Hatanaka F, Yamamoto M, Araoka T, Li Z. In vivo genome editing via CRISPR/Cas9 mediated homology-independent targeted integration. Nature. 2016;540:144–9.
Waldron D. In vivo gene editing in non-dividing cells. Nat Rev Genet. 2017;18:1–1.
Sakuma T, Nakade S, Sakane Y, Suzuki KT, Yamamoto T. MMEJ-assisted gene knock-in using TALENs and CRISPR-Cas9 with the PITCh systems. Nat Protoc. 2016;11:118–33.
Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature. 2016;533:420–4.
Anzalone AV, Randolph PB, Davis JR, Sousa AA, Koblan LW, Levy JM, Chen PJ, Wilson C, Newby GA, Raguram A, Liu DR. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature. 2019;576:149–57.
Dominguez AA, Lim WA, Qi LS. Beyond editing: repurposing CRISPR-Cas9 for precision genome regulation and interrogation. Nat Rev Mol Cell Biol. 2016;17:5–15.
Auer TO, Duroure K, De Cian A, Concordet JP, Del Bene F. Highly efficient CRISPR/Cas9-mediated knock-in in zebrafish by homology-independent DNA repair. Genome Res. 2014;24:142–53.
Suzuki K, Izpisua Belmonte JC. In vivo genome editing via the HITI method as a tool for gene therapy. J Hum Genet. 2018;63:157–64.
He X, Tan C, Wang F, Wang Y, Zhou R, Cui D, You W, Zhao H, Ren J, Feng B. Knock-in of large reporter genes in human cells via CRISPR/Cas9-induced homology-dependent and independent DNA repair. Nucleic Acids Res. 2016;44: e85.
Papapetrou EP, Schambach A. Gene insertion into genomic safe harbors for human gene therapy. Mol Ther. 2016;24:678–84.
Kampmann M. CRISPRi and CRISPRa screens in mammalian cells for precision biology and medicine. ACS Chem Biol. 2018;13:406–16.
Chou SJ, Yang P, Ban Q, Yang YP, Wang ML, Chien CS, Chen SJ, Sun N, Zhu Y, Liu H, et al. Dual supramolecular nanoparticle vectors enable CRISPR/Cas9-mediated knockin of Retinoschisin 1 Gene-A potential nonviral therapeutic solution for X-linked Juvenile Retinoschisis. Adv Sci (Weinh). 2020;7:1903432.
Sikkink SK, Biswas S, Parry NR, Stanga PE, Trump D. X-linked retinoschisis: an update. J Med Genet. 2007;44:225–32.
Tantri A, Vrabec TR, Cu-Unjieng A, Frost A, Annesley WH Jr, Donoso LA. X-linked retinoschisis: a clinical and molecular genetic review. Surv Ophthalmol. 2004;49:214–30.
Bakondi B, Lv W, Lu B, Jones MK, Tsai Y, Kim KJ, Levy R, Akhtar AA, Breunig JJ, Svendsen CN. In vivo CRISPR/Cas9 gene editing corrects retinal dystrophy in the S334ter-3 rat model of autosomal dominant retinitis pigmentosa. Mol Ther. 2016;24:556–63.
Burnight ER, Gupta M, Wiley LA, Anfinson KR, Tran A, Triboulet R, Hoffmann JM, Klaahsen DL, Andorf JL, Jiao C. Using CRISPR-Cas9 to generate gene-corrected autologous iPSCs for the treatment of inherited retinal degeneration. Mol Ther. 2017;25:1999–2013.
Vagni P, Perlini LE, Chenais N, Marchetti T, Parrini M, Contestabile A, Cancedda L, Ghezzi D. Gene editing preserves visual functions in a mouse model of retinal degeneration. Front Neurosci. 2019;13:945.
Yang X, Bayat V, DiDonato N, Zhao Y, Zarnegar B, Siprashvili Z, Lopez-Pajares V, Sun T, Tao S, Li C. Genetic and genomic studies of pathogenic EXOSC2 mutations in the newly described disease SHRF implicate the autophagy pathway in disease pathogenesis. Hum Mol Genet. 2020;29:541–53.
Philippidis A. One small dose, one giant leap for CRISPR gene editing. Hum Gene Ther. 2020;31:402–4.
Suh S, Choi EH, Leinonen H, Foik AT, Newby GA, Yeh WH, Dong Z, Kiser PD, Lyon DC, Liu DR, Palczewski K. Restoration of visual function in adult mice with an inherited retinal disease via adenine base editing. Nat Biomed Eng. 2021;5:169–78.
Liu Y, Li X, He S, Huang S, Li C, Chen Y, Liu Z, Huang X, Wang X. Efficient generation of mouse models with the prime editing system. Cell Discov. 2020;6:27.
Liu P, Liang SQ, Zheng C, Mintzer E, Zhao YG, Ponnienselvan K, Mir A, Sontheimer EJ, Gao G, Flotte TR, et al. Improved prime editors enable pathogenic allele correction and cancer modelling in adult mice. Nat Commun. 2021;12:2121.
Peddle CF, Fry LE, McClements ME, MacLaren RE. CRISPR interference-potential application in retinal disease. Int J Mol Sci. 2020;21:2329.
Keeler AM, Flotte TR. Recombinant adeno-associated virus gene therapy in light of Luxturna (and Zolgensma and Glybera): where are we, and how did we get here? Annu Rev Virol. 2019;6:601–21.
Verdera HC, Kuranda K, Mingozzi F. AAV vector immunogenicity in humans: a long journey to successful gene transfer. Mol Ther. 2020;28:723–46.
Li C, Samulski RJ. Engineering adeno-associated virus vectors for gene therapy. Nat Rev Genet. 2020;21:255–72.
Patel A, Zhao J, Duan D, Lai Y. Design of AAV vectors for delivery of large or multiple transgenes. Methods Mol Biol. 2019;1950:19–33.
Duan D, Yue Y, Engelhardt JF. Expanding AAV packaging capacity with trans-splicing or overlap** vectors: a quantitative comparison. Mol Ther. 2001;4:383–91.
Amreddy N, Babu A, Muralidharan R, Panneerselvam J, Srivastava A, Ahmed R, Mehta M, Munshi A, Ramesh R. Recent advances in nanoparticle-based cancer drug and gene delivery. Adv Cancer Res. 2018;137:115–70.
Kim HS, Sun X, Lee J-H, Kim H-W, Fu X, Leong KW. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv Drug Deliv Rev. 2019;146:209–39.
Kong F-Y, Zhang J-W, Li R-F, Wang Z-X, Wang W-J, Wang W. Unique roles of gold nanoparticles in drug delivery, targeting and imaging applications. Molecules. 2017;22:1445.
Matoba T, Koga JI, Nakano K, Egashira K, Tsutsui H. Nanoparticle-mediated drug delivery system for atherosclerotic cardiovascular disease. J Cardiol. 2017;70:206–11.
Mirza Z, Karim S. Nanoparticles-based drug delivery and gene therapy for breast cancer: recent advancements and future challenges. Semin Cancer Biol. 2019. https://doi.org/10.1016/j.semcancer.2019.10.020.
Zahin N, Anwar R, Tewari D, Kabir MT, Sajid A, Mathew B, Uddin MS, Aleya L, Abdel-Daim MM. Nanoparticles and its biomedical applications in health and diseases: special focus on drug delivery. Environ Sci Pollut Res. 2019. https://doi.org/10.1007/s11356-019-05211-0.
Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941.
Juillerat-Jeanneret L. The targeted delivery of cancer drugs across the blood–brain barrier: chemical modifications of drugs or drug-nanoparticles? Drug Discov Today. 2008;13:1099–106.
Kievit FM, Zhang M. Cancer therapy: cancer nanotheranostics: improving imaging and therapy by targeted delivery across biological barriers (Adv. Mater. 36/2011). Adv Mater. 2011;23:H209–H209.
Steichen SD, Caldorera-Moore M, Peppas NA. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur J Pharm Sci. 2013;48:416–27.
Givens BE, Naguib YW, Geary SM, Devor EJ, Salem AK. Nanoparticle-based delivery of CRISPR/Cas9 genome-editing therapeutics. AAPS J. 2018;20:108.
Nakade S, Yamamoto T, Sakuma T. Cas9, Cpf1 and C2c1/2/3-what’s next? Bioengineered. 2017;8:265–73.
Xu Y, Liu R, Dai Z. Key considerations in designing CRISPR/Cas9-carrying nanoparticles for therapeutic genome editing. Nanoscale. 2020;12:21001–14.
Zhang S, Shen J, Li D, Cheng Y. Strategies in the delivery of Cas9 ribonucleoprotein for CRISPR/Cas9 genome editing. Theranostics. 2021;11:614.
Huang X, Chau Y. Intravitreal nanoparticles for retinal delivery. Drug Discov Today. 2019;24:1510–23.
Jackson TL, Antcliff RJ, Hillenkamp J, Marshall J. Human retinal molecular weight exclusion limit and estimate of species variation. Invest Ophthalmol Vis Sci. 2003;44:2141–6.
Sebag J. Anatomy and pathology of the vitreo-retinal interface. Eye (Lond). 1992;6(Pt 6):541–52.
Tavakoli S, Peynshaert K, Lajunen T, Devoldere J, Del Amo EM, Ruponen M, De Smedt SC, Remaut K, Urtti A. Ocular barriers to retinal delivery of intravitreal liposomes: impact of vitreoretinal interface. J Control Release. 2020;328:952–61.
Huang X, Chau Y. Investigating impacts of surface charge on intraocular distribution of intravitreal lipid nanoparticles. Exp Eye Res. 2019;186: 107711.
Altınoglu S, Wang M, Xu Q. Combinatorial library strategies for synthesis of cationic lipid-like nanoparticles and their potential medical applications. Nanomedicine. 2015;10:643–57.
Freitag F, Wagner E. Optimizing synthetic nucleic acid and protein nanocarriers: the chemical evolution approach. Adv Drug Deliv Rev. 2021;168:30–54.
Fu A, Tang R, Hardie J, Farkas ME, Rotello VM. Promises and pitfalls of intracellular delivery of proteins. Bioconjug Chem. 2014;25:1602–8.
Rahimi H, Salehiabar M, Charmi J, Barsbay M, Ghaffarlou M, Razlighi MR, Davaran S, Khalilov R, Sugiyama M, Nosrati H. Harnessing nanoparticles for the efficient delivery of the CRISPR/Cas9 system. Nano Today. 2020;34: 100895.
Tang H, Zhao X, Jiang X. Synthetic multi-layer nanoparticles for CRISPR-Cas9 genome editing. Adv Drug Deliv Rev. 2020. https://doi.org/10.1016/j.addr.2020.03.001.
Wan T, Niu D, Wu C, Xu F-J, Church G, ** Y. Material solutions for delivery of CRISPR/Cas-based genome editing tools: current status and future outlook. Mater Today. 2019;26:40–66.
Finn JD, Smith AR, Patel MC, Shaw L, Youniss MR, van Heteren J, Dirstine T, Ciullo C, Lescarbeau R, Seitzer J. A single administration of CRISPR/Cas9 lipid nanoparticles achieves robust and persistent in vivo genome editing. Cell Rep. 2018;22:2227–35.
Liu J, Chang J, Jiang Y, Meng X, Sun T, Mao L, Xu Q, Wang M. Fast and efficient CRISPR/Cas9 genome editing in vivo enabled by bioreducible lipid and messenger RNA nanoparticles. Adv Mater. 2019;31:1902575.
Miller JB, Zhang S, Kos P, **ong H, Zhou K, Perelman SS, Zhu H, Siegwart DJ. Non-viral CRISPR/Cas gene editing in vitro and in vivo enabled by synthetic nanoparticle co-delivery of Cas9 mRNA and sgRNA. Angew Chem Int Ed. 2017;56:1059–63.
Zhang L, Wang P, Feng Q, Wang N, Chen Z, Huang Y, Zheng W, Jiang X. Lipid nanoparticle-mediated efficient delivery of CRISPR/Cas9 for tumor therapy. NPG Asia Mater. 2017;9:e441–e441.
Zhang X, Li B, Luo X, Zhao W, Jiang J, Zhang C, Gao M, Chen X, Dong Y. Biodegradable amino-ester nanomaterials for Cas9 mRNA delivery in vitro and in vivo. ACS Appl Mater Interfaces. 2017;9:25481–7.
Ruponen M, Yla-Herttuala S, Urtti A. Interactions of polymeric and liposomal gene delivery systems with extracellular glycosaminoglycans: physicochemical and transfection studies. Biochim Biophys Acta. 1999;1415:331–41.
Anderson DG, Akinc A, Hossain N, Langer R. Structure/property studies of polymeric gene delivery using a library of poly(beta-amino esters). Mol Ther. 2005;11:426–34.
Liang C, Li F, Wang L, Zhang Z-K, Wang C, He B, Li J, Chen Z, Shaikh AB, Liu J. Tumor cell-targeted delivery of CRISPR/Cas9 by aptamer-functionalized lipopolymer for therapeutic genome editing of VEGFA in osteosarcoma. Biomaterials. 2017;147:68–85.
Salameh JW, Zhou L, Ward SM, Santa Chalarca CF, Emrick T, Figueiredo ML. Polymer-mediated gene therapy: recent advances and merging of delivery techniques. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12: e1598.
Wan T, Chen Y, Pan Q, Xu X, Kang Y, Gao X, Huang F, Wu C, ** Y. Genome editing of mutant KRAS through supramolecular polymer-mediated delivery of Cas9 ribonucleoprotein for colorectal cancer therapy. J Control Release. 2020;322:236–47.
Zhang Z, Wan T, Chen Y, Chen Y, Sun H, Cao T, Songyang Z, Tang G, Wu C, ** Y. Cationic polymer-mediated CRISPR/Cas9 plasmid delivery for genome editing. Macromol Rapid Commun. 2019;40:1800068.
Wolfert MA, Dash PR, Nazarova O, Oupicky D, Seymour LW, Smart S, Strohalm J, Ulbrich K. Polyelectrolyte vectors for gene delivery: influence of cationic polymer on biophysical properties of complexes formed with DNA. Bioconjug Chem. 1999;10:993–1004.
D’Souza AA, Shegokar R. Polyethylene glycol (PEG): a versatile polymer for pharmaceutical applications. Expert Opin Drug Deliv. 2016;13:1257–75.
Kim YH, Park JH, Lee M, Kim YH, Park TG, Kim SW. Polyethylenimine with acid-labile linkages as a biodegradable gene carrier. J Control Release. 2005;103:209–19.
Sadekar S, Ghandehari H. Transepithelial transport and toxicity of PAMAM dendrimers: implications for oral drug delivery. Adv Drug Deliv Rev. 2012;64:571–88.
Lv H, Zhang S, Wang B, Cui S, Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J Control Release. 2006;114:100–9.
Chen K, Jiang S, Hong Y, Li Z, Wu Y-L, Wu C. Cationic polymeric nanoformulation: recent advances in material design for CRISPR/Cas9 gene therapy. Prog Nat Sci. 2019;29:617–27.
Zhang H, Bahamondez-Canas TF, Zhang Y, Leal J, Smyth HD. PEGylated chitosan for nonviral aerosol and mucosal delivery of the CRISPR/Cas9 system in vitro. Mol Pharm. 2018;15:4814–26.
Li L, He Z-Y, Wei X-W, Gao G-P, Wei Y-Q. Challenges in CRISPR/CAS9 delivery: potential roles of nonviral vectors. Hum Gene Ther. 2015;26:452–62.
Abedi-Gaballu F, Dehghan G, Ghaffari M, Yekta R, Abbaspour-Ravasjani S, Baradaran B, Dolatabadi JEN, Hamblin MR. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl Mater Today. 2018;12:177–90.
Avila-Salas FN, González RI, Ríos PL, Araya-Durán I, Camarada MB. Effect of the generation of PAMAM dendrimers on the stabilization of gold nanoparticles. J Chem Inform Model. 2020;60:2966–76.
Islam MT, Shi X, Balogh L, Baker JR. HPLC separation of different generations of poly (amidoamine) dendrimers modified with various terminal groups. Anal Chem. 2005;77:2063–70.
Kurbatov AO, Balabaev NK, Mazo MA, Kramarenko EY. Effects of generation number, spacer length and temperature on the structure and intramolecular dynamics of siloxane dendrimer melts: molecular dynamics simulations. Soft Matter. 2020;16:3792–805.
Maiti PK, Çaǧın T, Wang G, Goddard WA. Structure of PAMAM dendrimers: generations 1 through 11. Macromolecules. 2004;37:6236–54.
Pavan GM, Albertazzi L, Danani A. Ability to adapt: different generations of PAMAM dendrimers show different behaviors in binding siRNA. J Phys Chem B. 2010;114:2667–75.
Sebby KB, Walter ED, Usselman RJ, Cloninger MJ, Singel DJ. End-group distributions of multiple generations of spin-labeled PAMAM dendrimers. J Phys Chem B. 2011;115:4613–20.
Vinicius R, Ara D, Santos S, Ferreira EI, Giarolla J. New advances in general biomedical applications of PAMAM dendrimer. Molecules. 2018;23:2849.
Thanh VM, Nguyen TH, Tran TV, Ngoc UT, Ho MN, Nguyen TT, Chau YN, Tran NQ, Nguyen CK, Nguyen DH. Low systemic toxicity nanocarriers fabricated from heparin-mPEG and PAMAM dendrimers for controlled drug release. Mater Sci Eng C. 2018;82:291–8.
Yavuz B, Bozdağ Pehlivan S, Sümer Bolu B, Nomak Sanyal R, Vural İ, Ünlü N. Dexamethasone-PAMAM dendrimer conjugates for retinal delivery: preparation, characterization and in vivo evaluation. J Pharm Pharmacol. 2016;68:1010–20.
Yavuz B, Pehlivan SB, Vural İ, Ünlü N. In vitro/in vivo evaluation of dexamethasone-PAMAM dendrimer complexes for retinal drug delivery. J Pharm Sci. 2015;104:3814–23.
Kretzmann JA, Ho D, Evans CW, Plani-Lam JH, Garcia-Bloj B, Mohamed AE, O’Mara ML, Ford E, Tan DE, Lister R. Synthetically controlling dendrimer flexibility improves delivery of large plasmid DNA. Chem Sci. 2017;8:2923–30.
Liu C, Wan T, Wang H, Zhang S, ** Y, Cheng Y. A boronic acid-rich dendrimer with robust and unprecedented efficiency for cytosolic protein delivery and CRISPR-Cas9 gene editing. Sci Adv. 2019;5:eaaw8922.
Wei S, Shao X, Liu Y, **ong B, Cui P, Liu Z, Li Q. Genome editing of PD-L1 mediated by nucleobase-modified polyamidoamine for cancer immunotherapy. J Mater Chem B. 2022;10:1291–300.
Gref R, Lück M, Quellec P, Marchand M, Dellacherie E, Harnisch S, Blunk T, Müller R. ‘Stealth’corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf B. 2000;18:301–13.
Nunes R, Araújo F, Tavares J, Sarmento B. Surface modification with polyethylene glycol enhances colorectal distribution and retention of nanoparticles. Eur J Pharm Biopharm. 2018. https://doi.org/10.1016/j.ejpb.2018.06.029.
Pelaz B, del Pino P, Maffre P, Hartmann R, Gallego M, Rivera-Fernandez S, de la Fuente JM, Nienhaus GU, Parak WJ. Surface functionalization of nanoparticles with polyethylene glycol: effects on protein adsorption and cellular uptake. ACS Nano. 2015;9:6996–7008.
Ruiz A, Hernandez Y, Cabal C, Gonzalez E, Veintemillas-Verdaguer S, Martinez E, Morales M. Biodistribution and pharmacokinetics of uniform magnetite nanoparticles chemically modified with polyethylene glycol. Nanoscale. 2013;5:11400–8.
Chen CL, Rosi NL. Peptide-based methods for the preparation of nanostructured inorganic materials. Angew Chem Int Ed. 2010;49:1924–42.
Huang H, Li J, Liao L, Li J, Wu L, Dong C, Lai P, Liu D. Poly (l-glutamic acid)-based star-block copolymers as pH-responsive nanocarriers for cationic drugs. Eur Polymer J. 2012;48:696–704.
Li Z, Chen Q, Qi Y, Liu Z, Hao T, Sun X, Qiao M, Ma X, Xu T, Zhao X. Rational design of multifunctional polymeric nanoparticles based on poly (L-histidine) and d-α-Vitamin E Succinate for reversing tumor multidrug resistance. Biomacromol. 2018;19:2595–609.
Liu B, Gao GH, Liu P, Yi HQ, Wei W, Ge ZC, Cai LT. A tunable pH-responsive nanomaterials for cancer delivery. Adv Mater Res. 2013;750–752:1476–9.
Lv S, Tang Z, Li M, Lin J, Song W, Liu H, Huang Y, Zhang Y, Chen X. Co-delivery of doxorubicin and paclitaxel by PEG-polypeptide nanovehicle for the treatment of non-small cell lung cancer. Biomaterials. 2014;35:6118–29.
Shi C, He Y, Feng X, Fu D. ε-Polylysine and next-generation dendrigraft poly-l-lysine: chemistry, activity, and applications in biopharmaceuticals. J Biomater Sci Polym Ed. 2015;26:1343–56.
Yi H, Liu P, Sheng N, Gong P, Ma Y, Cai L. In situ crosslinked smart polypeptide nanoparticles for multistage responsive tumor-targeted drug delivery. Nanoscale. 2016;8:5985–95.
Zhang R, Zheng N, Song Z, Yin L, Cheng J. The effect of side-chain functionality and hydrophobicity on the gene delivery capabilities of cationic helical polypeptides. Biomaterials. 2014;35:3443–54.
Zhou H, Lv S, Zhang D, Deng M, Zhang X, Tang Z, Chen X. A polypeptide based podophyllotoxin conjugate for the treatment of multi drug resistant breast cancer with enhanced efficiency and minimal toxicity. Acta Biomater. 2018;73:388–99.
Yin L, Song Z, Qu Q, Kim KH, Zheng N, Yao C, Chaudhury I, Tang H, Gabrielson NP, Uckun FM, Cheng J. Supramolecular self-assembled nanoparticles mediate oral delivery of therapeutic TNF-α siRNA against systemic inflammation. Angew Chem Int Ed Engl. 2013;52:5757–61.
Wang HX, Song Z, Lao YH, Xu X, Gong J, Cheng D, Chakraborty S, Park JS, Li M, Huang D, et al. Nonviral gene editing via CRISPR/Cas9 delivery by membrane-disruptive and endosomolytic helical polypeptide. Proc Natl Acad Sci USA. 2018;115:4903–8.
Zakeri A, Kouhbanani MAJ, Beheshtkhoo N, Beigi V, Mousavi SM, Hashemi SAR, Karimi Zade A, Amani AM, Savardashtaki A, Mirzaei E, et al. Polyethylenimine-based nanocarriers in co-delivery of drug and gene: a develo** horizon. Nano Rev Exp. 2018;9:1488497.
Pishavar E, Shafiei M, Mehri S, Ramezani M, Abnous K. The effects of polyethylenimine/DNA nanoparticle on transcript levels of apoptosis-related genes. Drug Chem Toxicol. 2017;40:406–9.
Bahadur KR, Uludağ H. PEI and its derivatives for gene therapy. In: Narain R, editor. Polymers and nanomaterials for gene therapy. Amsterdam: Elsevier; 2016. p. 29–54.
Ryu N, Kim MA, Park D, Lee B, Kim YR, Kim KH, Baek JI, Kim WJ, Lee KY, Kim UK. Effective PEI-mediated delivery of CRISPR-Cas9 complex for targeted gene therapy. Nanomed Nanotechnol Biol Med. 2018;14:2095–102.
Wu Y, Wang W, Chen Y, Huang K, Shuai X, Chen Q, Li X, Lian G. The investigation of polymer-siRNA nanoparticle for gene therapy of gastric cancer in vitro. Int J Nanomed. 2010;5:129.
Zuckermann M, Hovestadt V, Knobbe-Thomsen CB, Zapatka M, Northcott PA, Schramm K, Belic J, Jones DT, Tschida B, Moriarity B. Somatic CRISPR/Cas9-mediated tumour suppressor disruption enables versatile brain tumour modelling. Nat Commun. 2015;6:1–9.
Thaker PH, Brady WE, Lankes HA, Odunsi K, Bradley WH, Moore KN, Muller CY, Anwer K, Schilder RJ, Alvarez RD, Fracasso PM. A phase I trial of intraperitoneal GEN-1, an IL-12 plasmid formulated with PEG-PEI-cholesterol lipopolymer, administered with pegylated liposomal doxorubicin in patients with recurrent or persistent epithelial ovarian, fallopian tube or primary peritoneal cancers: an NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol. 2017;147:283–90.
Liao H-W, Yau K-W. In vivo gene delivery in the retina using polyethylenimine. Biotechniques. 2007;42:285–8.
Mendelsohn AR, Larrick JW. Preclinical reversal of atherosclerosis by FDA-approved compound that transforms cholesterol into an anti-inflammatory “prodrug.” Rejuvenation Res. 2016;19:252–5.
Lai WF. Cyclodextrins in non-viral gene delivery. Biomaterials. 2014;35:401–11.
** Y, Liu C, Zhang Z, Liu KL, Chen J, Li J. Chitosan-graft-(PEI-β-cyclodextrin) copolymers and their supramolecular PEGylation for DNA and siRNA delivery. Biomaterials. 2011;32:8328–41.
Li J-M, Wang Y-Y, Zhang W, Su H, Ji L-N, Mao Z-W. Low-weight polyethylenimine cross-linked 2-hydroxypopyl-β-cyclodextrin and folic acid as an efficient and nontoxic siRNA carrier for gene silencing and tumor inhibition by VEGF siRNA. Int J Nanomed. 2013;8:2101.
Forrest ML, Gabrielson N, Pack DW. Cyclodextrin–polyethylenimine conjugates for targeted in vitro gene delivery. Biotechnol Bioeng. 2005;89:416–23.
Borrás T. Recent developments in ocular gene therapy. Exp Eye Res. 2003;76:643–52.
Gomes dos Santos AL, Bochot A, Tsapis N, Artzner F, Bejjani RA, Thillaye-Goldenberg B, De Kozak Y, Fattal E, Behar-Cohen F. Oligonucleotide-polyethylenimine complexes targeting retinal cells: structural analysis and application to anti-TGFbeta-2 therapy. Pharm Res. 2006;23:770–81.
Pitkänen L, Ruponen M, Nieminen J, Urtti A. Vitreous is a barrier in nonviral gene transfer by cationic lipids and polymers. Pharm Res. 2003;20:576–83.
Reinisalo M, Urtti A, Honkakoski P. Freeze-drying of cationic polymer DNA complexes enables their long-term storage and reverse transfection of post-mitotic cells. J Control Release. 2006;110:437–43.
Huang H, Liu M, Jiang R, Chen J, Mao L, Wen Y, Tian J, Zhou N, Zhang X, Wei Y. Facile modification of nanodiamonds with hyperbranched polymers based on supramolecular chemistry and their potential for drug delivery. J Colloid Interface Sci. 2018;513:198–204.
Jung H-S, Cho K-J, Ryu S-J, Takagi Y, Roche PA, Neuman KC. Biocompatible fluorescent nanodiamonds as multifunctional optical probes for latent fingerprint detection. ACS Appl Mater Interfaces. 2020;12:6641–50.
Li J, Zhu Y, Li W, Zhang X, Peng Y, Huang Q. Nanodiamonds as intracellular transporters of chemotherapeutic drug. Biomaterials. 2010;31:8410–8.
van der Laan K, Hasani M, Zheng T, Schirhagl R. Nanodiamonds for in vivo applications. Small. 2018;14:1703838.
Woodhams B, Ansel-Bollepalli L, Surmacki J, Knowles H, Maggini L, De Volder M, Atatüre M, Bohndiek S. Graphitic and oxidised high pressure high temperature (HPHT) nanodiamonds induce differential biological responses in breast cancer cell lines. Nanoscale. 2018;10:12169–79.
Zhu Y, Li J, Li W, Zhang Y, Yang X, Chen N, Sun Y, Zhao Y, Fan C, Huang Q. The biocompatibility of nanodiamonds and their application in drug delivery systems. Theranostics. 2012;2:302.
Chu Z, Zhang S, Zhang B, Zhang C, Fang CY, Rehor I, Cigler P, Chang HC, Lin G, Liu R, Li Q. Unambiguous observation of shape effects on cellular fate of nanoparticles. Sci Rep. 2014;4:4495.
Chu Z, Miu K, Lung P, Zhang S, Zhao S, Chang HC, Lin G, Li Q. Rapid endosomal escape of prickly nanodiamonds: implications for gene delivery. Sci Rep. 2015;5:11661.
Schrand AM, Huang H, Carlson C, Schlager JJ, Omacr Sawa E, Hussain SM, Dai L. Are diamond nanoparticles cytotoxic? J Phys Chem B. 2007;111:2–7.
Fu CC, Lee HY, Chen K, Lim TS, Wu HY, Lin PK, Wei PK, Tsao PH, Chang HC, Fann W. Characterization and application of single fluorescent nanodiamonds as cellular biomarkers. Proc Natl Acad Sci USA. 2007;104:727–32.
Chu HL, Chen HW, Tseng SH, Hsu MH, Ho LP, Chou FH, Li MP, Chang YC, Chen PH, Tsai LY, et al. Development of a growth-hormone-conjugated nanodiamond complex for cancer therapy. ChemMedChem. 2014;9:1023–9.
Mochalin VN, Pentecost A, Li XM, Neitzel I, Nelson M, Wei C, He T, Guo F, Gogotsi Y. Adsorption of drugs on nanodiamond: toward development of a drug delivery platform. Mol Pharm. 2013;10:3728–35.
Jung HS, Neuman KC. Surface modification of fluorescent nanodiamonds for biological applications. Nanomaterials (Basel). 2021;11:153.
Yang TC, Chang CY, Yarmishyn AA, Mao YS, Yang YP, Wang ML, Hsu CC, Yang HY, Hwang DK, Chen SJ, et al. Carboxylated nanodiamond-mediated CRISPR-Cas9 delivery of human retinoschisis mutation into human iPSCs and mouse retina. Acta Biomater. 2020;101:484–94.
Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res. 2016;33:2373–87.
Mohammadpour R, Dobrovolskaia MA, Cheney DL, Greish KF, Ghandehari H. Subchronic and chronic toxicity evaluation of inorganic nanoparticles for delivery applications. Adv Drug Deliv Rev. 2019;144:112–32.
Yang G, Phua SZF, Bindra AK, Zhao Y. Degradability and clearance of inorganic nanoparticles for biomedical applications. Adv Mater. 2019;31:1805730.
Eidi H, David M-O, Crépeaux G, Henry L, Joshi V, Berger M-H, Sennour M, Cadusseau J, Gherardi RK, Curmi PA. Fluorescent nanodiamonds as a relevant tag for the assessment of alum adjuvant particle biodisposition. BMC Med. 2015;13:1–13.
Mohan N, Chen C-S, Hsieh H-H, Wu Y-C, Chang H-C. In vivo imaging and toxicity assessments of fluorescent nanodiamonds in Caenorhabditis elegans. Nano Lett. 2010;10:3692–9.
Moore L, Yang J, Lan TTH, Osawa E, Lee DK, Johnson WD, ** J, Chow EK, Ho D. Biocompatibility assessment of detonation nanodiamond in non-human primates and rats using histological, hematologic, and urine analysis. ACS Nano. 2016;10:7385–400.
Balfourier A, Luciani N, Wang G, Lelong G, Ersen O, Khelfa A, Alloyeau D, Gazeau F, Carn F. Unexpected intracellular biodegradation and recrystallization of gold nanoparticles. Proc Natl Acad Sci USA. 2020;117:103–13.
Bernard K, Thannickal VJ. NADPH oxidase inhibition in fibrotic pathologies. Antioxid Redox Signal. 2020;33:455–79.
Lee K, Conboy M, Park HM, Jiang F, Kim HJ, Dewitt MA, Mackley VA, Chang K, Rao A, Skinner C, et al. Nanoparticle delivery of Cas9 ribonucleoprotein and donor DNA in vivo induces homology-directed DNA repair. Nat Biomed Eng. 2017;1:889–901.
Castaneda MT, Merkoçi A, Pumera M, Alegret S. Electrochemical genosensors for biomedical applications based on gold nanoparticles. Biosens Bioelectron. 2007;22:1961–7.
da Silva AB, Rufato KB, de Oliveira AC, Souza PR, da Silva EP, Muniz EC, Vilsinski BH, Martins AF. Composite materials based on chitosan/gold nanoparticles: from synthesis to biomedical applications. Int J Biol Macromol. 2020. https://doi.org/10.1016/j.ijbiomac.2020.06.113.
Fan J, Cheng Y, Sun M. Functionalized gold nanoparticles: synthesis, properties and biomedical applications. Chem Rec. 2020;20:1474–504.
Tiwari PM, Vig K, Dennis VA, Singh SR. Functionalized gold nanoparticles and their biomedical applications. Nanomaterials. 2011;1:31–63.
Chen F, Alphonse M, Liu Q. Strategies for nonviral nanoparticle-based delivery of CRISPR/Cas9 therapeutics. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12: e1609.
Glass Z, Li Y, Xu Q. Nanoparticles for CRISPR–Cas9 delivery. Nat Biomed Eng. 2017;1:854–5.
Shankar SS, Ahmad A, Pasricha R, Sastry M. Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J Mater Chem. 2003;13:1822–6.
Wang P, Zhang L, Zheng W, Cong L, Guo Z, **e Y, Wang L, Tang R, Feng Q, Hamada Y. Thermo-triggered release of CRISPR-Cas9 system by lipid-encapsulated gold nanoparticles for tumor therapy. Angew Chem Int Ed. 2018;57:1491–6.
Wang P, Zhang L, Zheng W, Cong L, Guo Z, **e Y, Wang L, Tang R, Feng Q, Hamada Y, et al. Thermo-triggered release of CRISPR-Cas9 system by lipid-encapsulated gold nanoparticles for tumor therapy. Angew Chem Int Ed Engl. 2018;57:1491–6.
Wang P, Zhang L, **e Y, Wang N, Tang R, Zheng W, Jiang X. Genome editing for cancer therapy: delivery of Cas9 Protein/sgRNA plasmid via a gold nanocluster/lipid core-shell nanocarrier. Adv Sci (Weinh). 2017;4:1700175.
Karakoçak BB, Raliya R, Davis JT, Chavalmane S, Wang WN, Ravi N, Biswas P. Biocompatibility of gold nanoparticles in retinal pigment epithelial cell line. Toxicol In Vitro. 2016;37:61–9.
Söderstjerna E, Bauer P, Cedervall T, Abdshill H, Johansson F, Johansson UE. Silver and gold nanoparticles exposure to in vitro cultured retina—studies on nanoparticle internalization, apoptosis, oxidative stress, glial- and microglial activity. PLoS ONE. 2014;9: e105359.
Song HB, Wi JS, Jo DH, Kim JH, Lee SW, Lee TG, Kim JH. Intraocular application of gold nanodisks optically tuned for optical coherence tomography: inhibitory effect on retinal neovascularization without unbearable toxicity. Nanomedicine. 2017;13:1901–11.
Kim JH, Kim JH, Kim KW, Kim MH, Yu YS. Intravenously administered gold nanoparticles pass through the blood-retinal barrier depending on the particle size, and induce no retinal toxicity. Nanotechnology. 2009;20: 505101.
Dasari Shareena TP, McShan D, Dasmahapatra AK, Tchounwou PB. A review on graphene-based nanomaterials in biomedical applications and risks in environment and health. Nano Micro Lett. 2018;10:53.
Liu J, Cui L, Losic D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013;9:9243–57.
Lammel T, Boisseaux P, Fernández-Cruz ML, Navas JM. Internalization and cytotoxicity of graphene oxide and carboxyl graphene nanoplatelets in the human hepatocellular carcinoma cell line Hep G2. Part Fibre Toxicol. 2013;10:27.
Linares J, Matesanz MC, Vila M, Feito MJ, Gonçalves G, Vallet-Regí M, Marques PA, Portolés MT. Endocytic mechanisms of graphene oxide nanosheets in osteoblasts, hepatocytes and macrophages. ACS Appl Mater Interfaces. 2014;6:13697–706.
Burnett M, Abuetabh Y, Wronski A, Shen F, Persad S, Leng R, Eisenstat D, Sergi C. Graphene oxide nanoparticles induce apoptosis in wild-type and CRISPR/Cas9-IGF/IGFBP3 knocked-out osteosarcoma cells. J Cancer. 2020;11:5007.
Carboni V, Maaliki C, Alyami M, Alsaiari S, Khashab N. Synthetic vehicles for encapsulation and delivery of CRISPR/Cas9 gene editing machinery. Adv Ther. 2019;2:1800085.
Liu Z, Robinson JT, Sun X, Dai H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J Am Chem Soc. 2008;130:10876–7.
Shen H, Liu M, He H, Zhang L, Huang J, Chong Y, Dai J, Zhang Z. PEGylated graphene oxide-mediated protein delivery for cell function regulation. ACS Appl Mater Interfaces. 2012;4:6317–23.
Yue H, Zhou X, Cheng M, **ng D. Graphene oxide-mediated Cas9/sgRNA delivery for efficient genome editing. Nanoscale. 2018;10:1063–71.
Yan L, Wang Y, Xu X, Zeng C, Hou J, Lin M, Xu J, Sun F, Huang X, Dai L, et al. Can graphene oxide cause damage to eyesight? Chem Res Toxicol. 2012;25:1265–70.
Li B, Zhang X-Y, Yang J-Z, Zhang Y-J, Li W-X, Fan C-H, Huang Q. Influence of polyethylene glycol coating on biodistribution and toxicity of nanoscale graphene oxide in mice after intravenous injection. Int J Nanomed. 2014;9:4697–707.
Vázquez E, Villaverde A. Engineering building blocks for self-assembling protein nanoparticles. Microb Cell Fact. 2010;9:101.
Mastrobattista E, van der Aa MA, Hennink WE, Crommelin DJ. Artificial viruses: a nanotechnological approach to gene delivery. Nat Rev Drug Discov. 2006;5:115–21.
Li L, Song L, Liu X, Yang X, Li X, He T, Wang N, Yang S, Yu C, Yin T, et al. Artificial virus delivers CRISPR-Cas9 system for genome editing of cells in mice. ACS Nano. 2017;11:95–111.
Mejia-Ariza R, Kronig GA, Huskens J. Size-controlled and redox-responsive supramolecular nanoparticles. Beilstein J Org Chem. 2015;11:2388–99.
Rädler JO, Koltover I, Salditt T, Safinya CR. Structure of DNA-cationic liposome complexes: DNA intercalation in multilamellar membranes in distinct interhelical packing regimes. Science. 1997;275:810–4.
Patel S, Ryals RC, Weller KK, Pennesi ME, Sahay G. Lipid nanoparticles for delivery of messenger RNA to the back of the eye. J Control Release. 2019;303:91–100.
Ewert KK, Zidovska A, Ahmad A, Bouxsein NF, Evans HM, McAllister CS, Samuel CE, Safinya CR. Cationic liposome-nucleic acid complexes for gene delivery and silencing: pathways and mechanisms for plasmid DNA and siRNA. Top Curr Chem. 2010;296:191–226.
Ahmad A, Evans HM, Ewert K, George CX, Samuel CE, Safinya CR. New multivalent cationic lipids reveal bell curve for transfection efficiency versus membrane charge density: lipid-DNA complexes for gene delivery. J Gene Med. 2005;7:739–48.
Cheng X, Lee RJ. The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery. Adv Drug Deliv Rev. 2016;99:129–37.
Leung AK, Tam YY, Cullis PR. Lipid nanoparticles for short interfering RNA delivery. Adv Genet. 2014;88:71–110.
Caracciolo G, Pozzi D, Capriotti AL, Cavaliere C, Laganà A. Effect of DOPE and cholesterol on the protein adsorption onto lipid nanoparticles. J Nanoparticle Res. 2013. https://doi.org/10.1007/s11051-013-1498-4.
Adams D, Gonzalez-Duarte A, O’Riordan WD, Yang C-C, Ueda M, Kristen AV, Tournev I, Schmidt HH, Coelho T, Berk JL. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379:11–21.
Akinc A, Maier MA, Manoharan M, Fitzgerald K, Jayaraman M, Barros S, Ansell S, Du X, Hope MJ, Madden TD. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat Nanotechnol. 2019;14:1084–7.
Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–79.
Ali MM, Li F, Zhang Z, Zhang K, Kang DK, Ankrum JA, Le XC, Zhao W. Rolling circle amplification: a versatile tool for chemical biology, materials science and medicine. Chem Soc Rev. 2014;43:3324–41.
Sun W, Jiang T, Lu Y, Reiff M, Mo R, Gu Z. Cocoon-like self-degradable DNA nanoclew for anticancer drug delivery. J Am Chem Soc. 2014;136:14722–5.
Liu C, Zhang L, Liu H, Cheng K. Delivery strategies of the CRISPR-Cas9 gene-editing system for therapeutic applications. J Control Release. 2017;266:17–26.
Tian J, Avalos AM, Mao S-Y, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–96.
Sun W, Ji W, Hall JM, Hu Q, Wang C, Beisel CL, Gu Z. Self-assembled DNA nanoclews for the efficient delivery of CRISPR-Cas9 for genome editing. Angew Chem Int Ed Engl. 2015;54:12029–33.
Gasiunas G, Barrangou R, Horvath P, Siksnys V. Cas9–crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci. 2012;109:E2579–86.
Alsaiari SK, Patil S, Alyami M, Alamoudi KO, Aleisa FA, Merzaban JS, Li M, Khashab NM. Endosomal escape and delivery of CRISPR/Cas9 genome editing machinery enabled by Nanoscale Zeolitic Imidazolate Framework. J Am Chem Soc. 2018;140:143–6.
Zhang J, Tan Y, Song W-J. Zeolitic imidazolate frameworks for use in electrochemical and optical chemical sensing and biosensing: a review. Microchim Acta. 2020;187:234.
Miensah ED, Khan MM, Chen JY, Zhang XM, Wang P, Zhang ZX, Jiao Y, Liu Y, Yang Y. Zeolitic imidazolate frameworks and their derived materials for sequestration of radionuclides in the environment: a review. Crit Rev Environ Sci Technol. 2020;50:1874–934.
Chen TT, Yi JT, Zhao YY, Chu X. Biomineralized metal-organic framework nanoparticles enable intracellular delivery and endo-lysosomal release of native active proteins. J Am Chem Soc. 2018;140:9912–20.
Poddar A, Conesa JJ, Liang K, Dhakal S, Reineck P, Bryant G, Pereiro E, Ricco R, Amenitsch H, Doonan C, et al. Encapsulation, visualization and expression of genes with biomimetically mineralized Zeolitic Imidazolate Framework-8 (ZIF-8). Small. 2019;15: e1902268.
Yang X, Tang Q, Jiang Y, Zhang M, Wang M, Mao L. Nanoscale ATP-Responsive Zeolitic Imidazole Framework-90 as a general platform for cytosolic protein delivery and genome editing. J Am Chem Soc. 2019;141:3782–6.
Wang Y, Shahi PK, **e R, Zhang H, Abdeen AA, Yodsanit N, Ma Z, Saha K, Pattnaik BR, Gong S. A pH-responsive silica-metal-organic framework hybrid nanoparticle for the delivery of hydrophilic drugs, nucleic acids, and CRISPR-Cas9 genome-editing machineries. J Control Release. 2020;324:194–203.
Mustafi D, Engel AH, Palczewski K. Structure of cone photoreceptors. Prog Retin Eye Res. 2009;28:289–302.
Chichagova V, Hilgen G, Ghareeb A, Georgiou M, Carter M, Sernagor E, Lako M, Armstrong L. Human iPSC differentiation to retinal organoids in response to IGF1 and BMP4 activation is line-and method-dependent. Stem Cells. 2020;38:195–201.
Lukovic D, Castro AA, Kaya KD, Munezero D, Gieser L, Davó-Martínez C, Corton M, Cuenca N, Swaroop A, Ramamurthy V. Retinal organoids derived from hiPSCs of an AIPL1-LCA patient maintain cytoarchitecture despite reduced levels of mutant AIPL1. Sci Rep. 2020;10:1–13.
Regent F, Chen HY, Kelley RA, Qu Z, Swaroop A, Li T. A simple and efficient method for generating human retinal organoids. Mol Vis. 2020;26:97.
Sridhar A, Hoshino A, Finkbeiner CR, Chitsazan A, Dai L, Haugan AK, Eschenbacher KM, Jackson DL, Trapnell C, Bermingham-McDonogh O. Single-cell transcriptomic comparison of human fetal retina, hPSC-derived retinal organoids, and long-term retinal cultures. Cell Rep. 2020;30(1644–1659): e1644.
Völkner M, Zschätzsch M, Rostovskaya M, Overall RW, Busskamp V, Anastassiadis K, Karl MO. Retinal organoids from pluripotent stem cells efficiently recapitulate retinogenesis. Stem Cell Rep. 2016;6:525–38.
Wiegand C, Banerjee I. Recent advances in the applications of iPSC technology. Curr Opin Biotechnol. 2019;60:250–8.
Fatehullah A, Tan SH, Barker N. Organoids as an in vitro model of human development and disease. Nat Cell Biol. 2016;18:246–54.
Streeter I, Harrison PW, Faulconbridge A, Consortium H, Flicek P, Parkinson H, Clarke L. The human-induced pluripotent stem cell initiative—data resources for cellular genetics. Nucleic Acids Res. 2017;45:D691–7.
Kim M, Mun H, Sung CO, Cho EJ, Jeon H-J, Chun S-M, Shin TH, Jeong GS, Kim DK, Choi EK. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat Commun. 2019;10:1–15.
Lee SH, Hu W, Matulay JT, Silva MV, Owczarek TB, Kim K, Chua CW, Barlow LJ, Kandoth C, Williams AB. Tumor evolution and drug response in patient-derived organoid models of bladder cancer. Cell. 2018;173(515–528): e517.
Nadkarni RR, Abed S, Cox BJ, Bhatia S, Lau JT, Surette MG, Draper JS. Functional enterospheres derived in vitro from human pluripotent stem cells. Stem Cell Rep. 2017;9:897–912.
Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–9.
Dekkers JF, Wiegerinck CL, De Jonge HR, Bronsveld I, Janssens HM, De Winter-de Groot KM, Brandsma AM, De Jong NW, Bijvelds MJ, Scholte BJ. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med. 2013;19:939–45.
Brawner AT, Xu R, Liu D, Jiang P. Generating CNS organoids from human induced pluripotent stem cells for modeling neurological disorders. Int J Physiol Pathophysiol Pharmacol. 2017;9:101.
Gabriel E, Gopalakrishnan J. Generation of iPSC-derived human brain organoids to model early neurodevelopmental disorders. J Vis Exper. 2017;14:e55372.
Sun G, Chiuppesi F, Chen X, Wang C, Tian E, Nguyen J, Kha M, Trinh D, Zhang H, Marchetto MC. Modeling human cytomegalovirus-induced microcephaly in human iPSC-derived brain organoids. Cell Rep Med. 2020;1:100002.
Forbes TA, Howden SE, Lawlor K, Phipson B, Maksimovic J, Hale L, Wilson S, Quinlan C, Ho G, Holman K. Patient-iPSC-derived kidney organoids show functional validation of a ciliopathic renal phenotype and reveal underlying pathogenetic mechanisms. Am J Hum Genet. 2018;102:816–31.
Freedman BS, Lam AQ, Sundsbak JL, Iatrino R, Su X, Koon SJ, Wu M, Daheron L, Harris PC, Zhou J. Reduced ciliary polycystin-2 in induced pluripotent stem cells from polycystic kidney disease patients with PKD1 mutations. J Am Soc Nephrol. 2013;24:1571–86.
Shimizu T, Mae S-I, Araoka T, Okita K, Hotta A, Yamagata K, Osafune K. A novel ADPKD model using kidney organoids derived from disease-specific human iPSCs. Biochem Biophys Res Commun. 2020;529:1186–94.
Hulot JS. Modeling cardiac arrhythmias with organoids. J Am Coll Cardiol. 2019. https://doi.org/10.1016/j.jacc.2019.01.076.
Lee J, Sutani A, Kaneko R, Takeuchi J, Sasano T, Kohda T, Ihara K, Takahashi K, Yamazoe M, Morio T. In vitro generation of functional murine heart organoids via FGF4 and extracellular matrix. Nat Commun. 2020;11:1–18.
Takasato M, Pei XE, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, de Sousa Lopes SMC. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2015;526:564–8.
Lancaster MA, Renner M, Martin C-A, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–9.
Qian X, Jacob F, Song MM, Nguyen HN, Song H, Gl M. Generation of human brain region-specific organoids using a miniaturized spinning bioreactor. Nat Protoc. 2018;13:565.
Forster R, Chiba K, Schaeffer L, Regalado SG, Lai CS, Gao Q, Kiani S, Farin HF, Clevers H, Cost GJ. Human intestinal tissue with adult stem cell properties derived from pluripotent stem cells. Stem Cell Rep. 2014;2:838–52.
Ang LT, Tan AKY, Autio MI, Goh SH, Choo SH, Lee KL, Tan J, Pan B, Lee JJH, Lum JJ. A roadmap for human liver differentiation from pluripotent stem cells. Cell Rep. 2018;22:2190–205.
Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang R-R, Ueno Y, Zheng Y-W, Koike N. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–4.
Takebe T, Sekine K, Kimura M, Yoshizawa E, Ayano S, Koido M, Funayama S, Nakanishi N, Hisai T, Kobayashi T. Massive and reproducible production of liver buds entirely from human pluripotent stem cells. Cell Rep. 2017;21:2661–70.
Dye BR, Dedhia PH, Miller AJ, Nagy MS, White ES, Shea LD, Spence JR. A bioengineered niche promotes in vivo engraftment and maturation of pluripotent stem cell derived human lung organoids. Elife. 2016;5: e19732.
Xu M, Lee EM, Wen Z, Cheng Y, Huang WK, Qian X, Tcw J, Kouznetsova J, Ogden SC, Hammack C, et al. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat Med. 2016;22:1101–7.
Bian S, Repic M, Guo Z, Kavirayani A, Burkard T, Bagley JA, Krauditsch C, Knoblich JA. Genetically engineered cerebral organoids model brain tumor formation. Nat Methods. 2018;15:631–9.
Saengwimol D, Rojanaporn D, Chaitankar V, Chittavanich P, Aroonroch R, Boontawon T, Thammachote W, **awath N, Hongeng S, Kaewkhaw R. A three-dimensional organoid model recapitulates tumorigenic aspects and drug responses of advanced human retinoblastoma. Sci Rep. 2018;8:15664.
Fernandes TG, Rodrigues CA, Diogo MM, Cabral JM. Stem cell bioprocessing for regenerative medicine. J Chem Technol Biotechnol. 2014;89:34–47.
Vergara MN, Flores-Bellver M, Aparicio-Domingo S, McNally M, Wahlin KJ, Saxena MT, Mumm JS, Canto-Soler MV. Three-dimensional automated reporter quantification (3D-ARQ) technology enables quantitative screening in retinal organoids. Development. 2017;144:3698–705.
Aasen DM, Vergara MN. New drug discovery paradigms for retinal diseases: a focus on retinal organoids. J Ocul Pharmacol Ther. 2020;36:18–24.
Kruczek K, Swaroop A. Pluripotent stem cell-derived retinal organoids for disease modeling and development of therapies. Stem Cells. 2020;38:1206–15.
Pierce EA, Bennett J. The status of RPE65 gene therapy trials: safety and efficacy. Cold Spring Harb Perspect Med. 2015;5: a017285.
Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL, Rossi S, Marshall K, Banfi S, Surace EM. Gene therapy for Leber’s congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther. 2010;18:643–50.
Benati D, Patrizi C, Recchia A. Gene editing prospects for treating inherited retinal diseases. J Med Genet. 2020;57:437–44.
Garafalo AV, Cideciyan AV, Héon E, Sheplock R, Pearson A, Yu CW, Sumaroka A, Aguirre GD, Jacobson SG. Progress in treating inherited retinal diseases: early subretinal gene therapy clinical trials and candidates for future initiatives. Prog Retin Eye Res. 2019;77:100827.
Xu CL, Cho GY, Sengillo JD, Park KS, Mahajan VB, Tsang SH. Translation of CRISPR genome surgery to the bedside for retinal diseases. Front Cell Dev Biol. 2018;6:46.
Bondy-Denomy J, Pawluk A, Maxwell KL, Davidson AR. Bacteriophage genes that inactivate the CRISPR/Cas bacterial immune system. Nature. 2013;493:429–32.
Ford K, McDonald D, Mali P. Functional genomics via CRISPR-Cas. J Mol Biol. 2019;431:48–65.
Kim J, Koo B-K, Knoblich JA. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. 2020;21:571–84.
Acknowledgements
This study was funded by Ministry of Science and Technology (MOST) (MOST 108-2320-B-010 -019 -MY3; MOST 109-2327-B-010-007), Ministry of Health and Welfare (MOHW)(MOHW108-TDU-B-211-133001, MOHW109-TDU-B-211-114001), VGH, NTUH Joint Research Program (VN109-16), VGH, TSGH, NDMC, AS Joint Research Program (VTA107-V1-5-1, VTA108-V1-5-3, VTA109-V1-4-1), Academia Sinica (AS-TM-110-02-02), -AS Clinical Research Center (IBMS-CRC109-P04), the “Cancer Progression Research Center, National Yang-Ming University” from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan, and the Ministry of Education through the SPROUT Project- Center For Intelligent Drug Systems and Smart Bio-devices (IDS2B) of National Chiao Tung University and, Taiwan.
Author information
Authors and Affiliations
Contributions
Conceptualization-YC, YPY, SHC. Software-TYL, WYL, YC. Validation-MSL, YYL, TWL, DKH, TCL, SJC. writing—original draft preparation YC, YJH; writing—review and editing, YC, YJH, AAY; visualization, TYL, YC, YJH, AAY; supervision, YPY, SJC, YC, SHC. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors have no Conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chien, Y., Hsiao, YJ., Chou, SJ. et al. Nanoparticles-mediated CRISPR-Cas9 gene therapy in inherited retinal diseases: applications, challenges, and emerging opportunities. J Nanobiotechnol 20, 511 (2022). https://doi.org/10.1186/s12951-022-01717-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-022-01717-x