Abstract
Immunotherapeutic interventions represent a promising approach to treating cancer, with strategies such as immune checkpoint blockade (ICB), immunogenic sonodynamic therapy (SDT), and immune adjuvant T cell delivery having exhibited clinical promise. In this report, we describe the use of cancer cell membrane-coated triphenylphosphonium (TPP) decorated nano-metal–organic framework (nMOF) constructs [Zr-TCPP(TPP)/R837@M] that were used to generate homologous, mitochondria-targeted platforms with a high rate of sonosensitizer loading. This construct was utilized to simultaneously promote tumor antigen presentation via enhancing SDT while synergistically promoting dendritic cell (DC) maturation through the delivery of the Toll-like receptor agonist R837. In vitro, these functionalized nMOFs were readily internalized by homologous tumor cells in which they were efficiently targeted to the mitochondria, promoting DC activation through the induction of immunogenic cell death (ICD) following ultrasound exposure. Moreover, this nanoplatform was able to achieve in vivo synergy with anti-CTLA-4 ICB to reverse immunosuppression tumor microenvironment (TME), thus achieving more robust antitumor efficacy capable of suppressing metastatic disease progression and facilitating the development of durable antitumor memory responses. Together, these results highlight a promising approach to achieving enhanced SDT activity while overcoming an immunosuppressive TME, thereby achieving more robust antitumor immunity.
Similar content being viewed by others
Cancer immunotherapy strategies leverage host immune responses to target and eliminate malignant neoplasms, and several such approaches have emerged as promising antitumor treatments in recent years [1,2,3,4,5]. Immune checkpoint blockade (ICB) strategies targeting immunosuppressive proteins including cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) have been shown to achieve marked clinical efficacy in a range of tumor types [6,7,8]. In addition to overcoming immunosuppression, the establishment of a robust antitumor immune response is dependent on tumor-associated antigen (TAA) release and the subsequent presentation of these antigens by dendritic cells (DCs) and other antigen-presenting cells (APCs) to T cells [9, 10]. ICB is thought to fail in many treated patients owing to a dearth of TAAs within the tumor microenvironment (TME) and insufficient DC-mediated antigen presentation.
Sonodynamic therapy (SDT) has emerged as a promising approach to enhancing antigen presentation [11, 12]. SDT is an inexpensive and nonionizing therapeutic modality that is easily controlled and can effectively penetrate target tissues, killing cancer cells by generating highly cytotoxic singlet oxygen (1O2) [13,14,15]. Importantly, SDT promotes highly immunogenic cell death (ICD) and the associated release of TAAs and other tumor debris, thus enhancing DC recruitment, activation, and T cell priming [16,17,18]. The efficacy of SDT strategies is thus closely tied to the consequent induction of ICD and associated TAA release. Antigen presentation can also be augmented by stimulating DCs with Toll-like receptor (TLR) agonists [19], including imiquimod (R837), which binds to TLR7 and promotes DC maturation [20, 21]. Despite its immunogenic potential, R837 exhibits poor pharmacokinetic properties and very limited water solubility, constraining its ability to effectively activate DCs in vivo [Full size image
In the Zr-TCPP(TPP)/R837@M UV–vis absorption spectrum, a characteristic peak at ~ 320 nm assigned to R837 in the UV–vis absorption spectrum is consistent with successful R837 encapsulation in these nMOFs (Fig. 1g). Zr-TCPP(TPP) XPS spectra indicated that O, N, C, Zr, and P were present within prepared samples (Fig. 1h). We further confirmed the elements distribution of Zr-TCPP(TPP) via STEM-based elemental map** (Fig. 1i), with P and Zr respectively corresponding to TPP and Zr-MOF, confirming the connection of TPP.
Next, NP uptake by 4T1 cells was assessed in vitro by combining these cells with Zr-TCPP(TPP)/R837@M NPs for a range of time periods (0, 1, 4, 8, 12, and 24 h) and then assessing cells via flow cytometry. Within a 12 h incubation period, substantial Zr-TCPP(TPP)/R837@M NP uptake was detectable within 4T1 cells (Additional file 1: Figure S1a). When a range of Zr-TCPP(TPP)/R837@M concentrations (up to 200 µg/mL) were used to treat 4T1 cells for 24 h, these NPs were found to induce only limited cytotoxicity (Additional file 1: Figure S1b).
To assess the homologous targeting activity of Zr-TCPP(TPP)/R837@M, these NPs were incubated with 4T1 murine breast cancer cells or with other tumor cell lines including the MDA-MB-468 human breast cancer cell line, the Hepa1-6 murine hepatocellular carcinoma cell line, and the Bxpc-3 human pancreatic cancer cell line. Of these four cell lines, Zr-TCPP(TPP)/R837@M uptake was significantly higher in 4T1 cells, while the uptake of Zr-TCPP(TPP)/R837 in 4T1 cells didn’t show any increase compared with other cell lines, confirming the specificity with which Zr-TCPP(TPP)/R837@M particles can bind to homologous 4T1 cells (Fig. 2a, b; Additional file 1: Figure S2). The prepared Zr-TCPP(TPP)/R837@M samples were then utilized to facilitate mitochondrial-targeted SDT in target 4T1 cells. Relative to control treatment, Zr-TCPP/R837@M was found to efficiently enter into these tumor cells even in the absence of specific mitochondria targeting (Fig. 2c), with a mitochondrial colocalization coefficient Pearson’s R value (no threshold): 0.17. In contrast, Zr-TCPP(TPP)/R837@M was both efficiently internalized by these 4T1 cells and preferentially accumulated in the mitochondria, with a mitochondrial colocalization coefficient Pearson's R value (no threshold): 0.62.
In vitro uptake and stimulation of in vitro immune response. a Homotypic effect of Zr-TCPP(TPP)/R837@M on four cell lines, namely 4T1, MDA-MB-468, Hepa1-6, and Bxpc-3, examined by flow cytometry. b Statistical analysis of four cell lines after 8 h incubation with Zr-TCPP(TPP)/R837@M or Zr-TCPP(TPP)/R837. c CLSM images to show subcellular localization of Zr-TCPP(TPP)/R837@M, and Zr-TCPP/R837@M. Scale bar: 25 μm. d Time related SOSG (5 μM) fluorescence changes of Zr-TCPP(TPP)/R837@M under US irradiation. e CLSM images of 4T1 cells after treated with of Zr-TCPP(TPP)/R837@M with or without US irradiation. DCFH-DA (10 μM) was employed as the intracellular ROS sensor. Scale bar: 25 μm. f CRT exposure and HMGB1 release in 4T1 cells treated with different formulations, following by CLSM. Scale bar: 25 μm. g Flow cytometry analysis of CRT exposure in different groups. h HMGB1 release in cell supernatant after various treatments. i ATP secretion in different groups. Data are expressed as means ± SD (n = 3). Statistical significances were calculated via one-way ANOVA Tukey's multiple comparisons test, *P < 0.05, **P < 0.01, ***P < 0.001
To evaluate Zr-TCPP(TPP)/R837@M-induced ROS yields, a singlet oxygen sensor green (SOSG; 1 μM) reagent was selected to trap and detect 1O2. With the prolongation of US exposure time, SOSG fluorescent intensity rose substantially (Fig. 2d). DCFH-DA was then chosen as a fluorescent indicator to gauge intracellular ROS levels. After Zr-TCPP(TPP)/R837@M + US exposure and irradiation for 1 min, a bright green fluorescent signal was evident in these cells, while this signal was less robust in cells from the US or Zr-TCPP(TPP)/R837@M treatment groups (Fig. 2e).
We additionally examined the impact of Zr-TCPP(TPP)/R837@M-based SDT on the induction of ICD by quantifying calreticulin (CRT) exposure, the release of high mobility group box 1 (HMGB1), and the secretion of ATP. CLSM imaging revealed Zr-TCPP(TPP)/R837@M to effectively promote the exposure of CRT on 4T1 cell surfaces when Dil was used for membrane staining (Fig. 2f). The release of HMGB1 from the nuclei of these cells was apparent in the Zr-TCPP(TPP)@M, Zr-TCPP(TPP)/R837@M and Zr-TCPP(TPP)@M groups within 1 min following US exposure, especially in the Zr-TCPP(TPP)/R837@M + US group, which almost invisible fluorescence. The result of HMGB1 was also confirmed by the ELISA test (Fig. 2h). Flow cytometry analyses further demonstrated that Zr-TCPP(TPP)/R837@M-treated cell exposure to US induced CRT exposure to an extent greater than that observed in other treatment groups (Fig. 2g; Additional file 1: Figure S3). Owing to the ROS-mediated induction of mitochondrial damage, Zr-TCPP(TPP)/R837@M + US treatment was associated with significantly increased ATP secretion as compared to other treatments (Fig. 2i). In conclusion, these data confirmed that Zr-TCPP(TPP)/R837@M-based SDT was able to effectively induce in vitro tumor cell ICD.
When Zr-TCPP(TPP)/R837 and Zr-TCPP(TPP)/R837@M biodistributions were assessed in BALB/c mice bearing 4T1 tumors using an IVIS system, Zr-TCPP(TPP)/R837@M accumulation within tumors was evident within 24 h of treatment followed by gradual reductions in these levels over time, whereas Zr-TCPP(TPP)/R837 exhibited reduced levels within these 4T1 tumors at all tested time points (Fig. 3a, b). Ex vivo fluorescent analyses indicated that Zr-TCPP(TPP)/R837 and Zr-TCPP(TPP)/R837@M were readily metabolized within 24 h following injection in most assessed organs, with Zr-TCPP(TPP)/R837@M treatment being associated with higher fluorescence intensity values as compared to Zr-TCPP(TPP)/R837 treatment (Additional file 1: Figure S4a, b). Together, these results suggested that Zr-TCPP(TPP)/R837@M was able to more readily accumulate within homologous tumor cells owing to the tumor-targeting activity of the 4T1 cell membranes used for NP encapsulation.
In vivo evaluation of tumor targeting and immune responses. a In vivo fluorescence images to reveal the biodistribution of Zr-TCPP(TPP)/R837 and Zr-TCPP(TPP)/R837@M post i.v. injection into 4T1-tumour-bearing mice at the indicated time points. White circles indicate tumours; b the accumulation curve of Zr-TCPP(TPP)/R837 and Zr-TCPP(TPP)/R837@M in the tumour tissue by measuring the fluorescence intensity of tumours at different time points post i.v. injection, error bars are based on SD (n = 3); c, d DC maturation in the tumour-draining lymph nodes induced by various treatments on mice bearing 4T1 tumours, as assessed by flow cytometry after staining with CD11c, CD80, CD86 and live dead; e–g cytokine levels of TNF-α, IL-6 and IL-12p70 in sera from mice after different treatment. h, i Flow cytometry plots and the corresponding quantification showing percentages (gated on CD4+ cells) of CD4+Foxp3+ Treg cells in tumours tumors after various treatments indicated. Data are expressed as means ± SD (n = 3). Statistical significances were calculated via one-way ANOVA Tukey's multiple comparisons test, *P < 0.05, **P < 0.01, ***P < 0.001
Several different therapeutic modalities including photodynamic therapy, photothermal therapy, and SDT have been shown to promote antitumor immunity by inducing ICD and consequent TAA release. These TAAs, in turn, can function in a vaccine-like manner when an immune adjuvant induces DC maturation and consequent cytokine production and cytolytic cell killing. SDT treatment in the Zr-TCPP(TPP)/R837@M + US treatment group was associated with clear increases in lymph node DC maturation (9.36 ± 0.82%) to levels above those in other groups including the Zr-TCPP(TPP)/R837@M only group (6.19 ± 1.16%) or the US + Zr-TCPP(TPP)@M (7.33 ± 0.71%) treatment group in which R837 was absent. Moreover, R837 alone induced only very low levels of DC maturation (4.67 ± 1.48%). Zr-TCPP(TPP)/R837@M + US treatment also induced more robust DC maturation than did non-mitochondria-targeting Zr-TCPP/R837@M + US treatment (6.15 ± 1.49%), consistent with the beneficial effects of mitochondria targeting (Fig. 3c, d). In line with the results of DC maturation assays, tumor cell debris release following Zr-TCPP(TPP)/R837@M-enhanced SDT treatment was sufficient to augment the production of key immune-related cytokines including IL-6, IL-12p40, and TNF-α by DCs, further confirming the ability of R837 to function as an immune adjuvant in this assay context, enhancing overall immune response induction (Fig. 3e–g). While IL-6 and IL-12 are important mediators of the activation of innate immune NK cells, TNF-α plays a central role in the induction of adaptive cell-mediated immunity necessary for a robust tumor immunotherapy response. As such, TAAs released from tumor cell debris following SDT, together with immunostimulatory adjuvants, were able to effectively trigger the maturation of DCs.
While eliciting ICD can benefit antitumor immunity, intratumoral feedback mechanisms can suppress immune activation and thereby compromise the effective induction of a robust or durable immune response [39, 40]. Regulatory T cells (Tregs) are frequently present at high levels within tumors wherein they function to suppress immune responses and associated cytolytic tumor cell killing [41, 42]. Flow cytometry analyses revealed clear increases in the levels of Tregs (CD4+ Foxp3+) following Zr-TCPP(TPP)/R837@M-enhanced SDT treatment (Fig. 3h, i). As such, we posit that the antitumor immune responses that were induced as a result of SDT-induced ICD may, in the absence of other treatments, be suppressed by the post-SDT recruitment of Tregs into the TME.
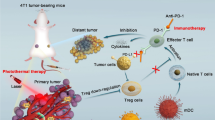
The antibody-mediated blockade of the immunosuppressive immune checkpoint protein CTLA-4 can suppress Treg activity. In addition, simultaneous CTLA-4 ICB in combination with other antitumor treatments can readily induce tumor-specific immune responses, thereby eliminating residual tumor cells that may be present at the site of tumor metastasis. We next evaluated the therapeutic efficacy of combining Zr-TCPP(TPP)/R837@M-enhanced SDT with anti-CTLA-4 treatment. For these analyses, primary 4T1 tumors were implanted on the right side of each mouse, with an artificial metastatic tumor then being established 1 week later on the left side of these same mice (Fig. 4a). Tumor growth in these animals was then monitored to evaluate treatment outcomes in primary and secondary tumors (Fig. 4b–e; Additional file 1: Figure S5a). US treatment in the absence of other therapeutic interventions failed to inhibit the growth of primary or distant tumors. While Zr-TCPP(TPP)/R837@M-enhanced SDT treatment was sufficient to inhibit primary tumor growth, it failed to impact the growth of metastasis-mimicking distant tumors, and its antitumor activity was enhanced in the context of anti-CTLA-4 combination treatment. Importantly, Zr-TCPP/R837@M-augmented SDT together with anti-CTLA-4 blockade was sufficient to eradicate primary 4T1 tumors (volume reduction rate, VRR, 59.5%) while also markedly suppressing distant tumor growth (VRR, 61.4%). Consistent with the above results, Zr-TCPP(TPP)/R837@M-enhanced SDT + anti-CTLA-4 combination treatment was more readily able to inhibit tumor growth than was an equivalent therapeutic strategy lacking mitochondria targeting activity, consistent with the therapeutic benefits of this intervention. When assessing the biosafety and toxicology of Zr-TCPP(TPP)/R837@M treatment in vivo, the hemolysis rate was found to be < 5% following red blood cell treatment with a range of Zr-TCPP(TPP)/R837@M concentrations (Additional file 1: Figure S6). These NPs are thus highly biocompatible and unlikely to induce systemic toxicity. Consistently, no abnormalities or organ damage were visible upon the hematoxylin and eosin (H&E) staining major organs (heart, lungs, liver, kidneys, and spleen) of treated mice (Additional file 1: Figure S7).
Antitumor immune effects of US in combination with checkpoint blockade. a Schematic illustration of our experiment design. Mice with 4T1 tumors on both sides were used. Tumors on the right side were designated as “primary tumors” for US, and those on the left side were designated as “distant tumors” without direct exposure to US. Growth curves and excised tumors at the end of treatments for b, c primary tumors and d, e distant tumors on mice after various treatments indicated. (n = 5). Statistical significances were calculated via one-way ANOVA Tukey's multiple comparisons test, *P < 0.05, **P < 0.01, ***P < 0.001. f, g Representative flow cytometry plots showing different types of T cells in secondary tumors from different groups of mice, and h, i proportions of tumor-infiltrating CD4 + and CD8 + T cells among all cancer cells. Proportions of Foxp3 + Tregs among CD4 + T cells. (n = 3) Statistical significance was calculated by one-way ANOVA Dunnett's multiple comparisons test. P-value: *, P < 0.05;**, P < 0.01; ***, P < 0.001. j In vivo apoptosis and/or necrosis of the tumour induced by different treatment, as shown by H&E staining and TUNEL assay; representative CLSM images of the mimic distant tumours after immunofluorescence staining (scale bar = 50 μm)
To explore the mechanisms whereby Zr-TCPP(TPP)/R837@M-enhanced SDT + anti-CTLA-4 treatment achieves robust therapeutic efficacy, distant tumors were collected from mice on day 7 post-treatment and assessed via flow cytometry (Fig. 4f–i). The frequency of intratumoral tumor-infiltrating lymphocytes (TILs, CD3+) rose following Zr-TCPP(TPP)/R837@M-enhanced SDT + anti-CTLA-4 treatment, with a particularly marked increase in intratumoral CD8+ T cell infiltration (Additional file 1: Figure S5b). The absolute CD8+ T cell frequency in the combination treatment group was 61.53 ± 0.81%, which was almost 2.9-fold higher than that in the PBS treatment group (21.10 ± 1.61%), and was also significantly higher than that observed in the group without mitochondrial targeting (48.27 ± 13.65%, P = 0.0592) (Fig. 4f, h). Together, these results indicated that Zr-TCPP(TPP)/R837@M-enhanced SDT + anti-CTLA-4 treatment promotes the immunosuppressive TME more conducive to the development of a productive immune response owing to the recruitment of many immune cells to target tumors.
Assessing the presence of Foxp3+ Tregs within human breast tumors can offer valuable insight regarding disease progression and prognosis, making these cells a valuable target for therapeutic intervention owing to their ability to suppress T cell proliferation and effector cytokine production [43]. When leukocytes were collected from distant tumors in our assay system and examined for CD3, CD4, and Foxp3 expression, no significant changes in CD4+T cell frequencies were observed in distant tumors, whereas the frequency of immunosuppressive Tregs declined in these distant tumors following Zr-TCPP(TPP)/R837@M-enhanced SDT treatment, suggesting that anti-CTLA-4 treatment effectively suppressed the activity of Tregs recruited into these distant tumors following primary tumor SDT treatment (Fig. 4g–i).
Histological and immunofluorescent staining of tumor tissue sections was next conducted (Fig. 4j). TUNEL staining indicated that the greatest levels of apoptotic and necrotic cell death were evident in the Zr-TCPP(TPP)/R837@M-enhanced SDT + anti-CTLA-4 treatment group, consistent with the superior combinatory efficacy of this therapeutic modality. Immunofluorescent staining demonstrated that this combined therapeutic approach enhanced CD3+T cell infiltration into distant tumors, whereas such infiltration was largely absent in animals subjected to PBS treatment. The majority of these tumor-infiltrating T cells were CD8+, further confirming the ability of Zr-TCPP(TPP)/R837@M-enhanced SDT + anti-CTLA-4 treatment to markedly enhance intratumoral CD8+T cell infiltration.
Given the promising efficacy observed in our orthotopic tumor model system, we additionally examined the ability of this combination therapeutic regimen to achieve therapeutic benefit in a model that more closely mimics aggressive metastatic tumor growth. Tumor growth and metastasis were monitored via in vivo bioluminescent imaging in different treatment groups (Fig. 5a). For mice in which primary breast tumors had been surgically removed, a clear bioluminescent signal consistent with tumor metastasis was evident on day 14 following intravenous fLuc-4T1 cell injection, even in animals that underwent anti-CTLA-4 treatment or combination Zr-TCPP(TPP)/R837@M-augmented SDT (Fig. 5b). When animals were instead subjected to US treatment following injection with nMOFs lacking mitochondrial targeting activity together with anti-CTLA-4 treatment, only a slight delay of metastatic progression was evident, while Zr-TCPP(TPP)/R837@M-augmented SDT + anti-CTLA-4 strongly suppressed such metastasis (Fig. 5b). When dissected lung nodules were assessed in these mice, this analysis further confirmed the therapeutic benefits of combination therapy (Fig. 5c). When murine survival following treatment was monitored (n = 5 mice/group), 40% of mice in the combination Zr-TCPP(TPP)/R837 @M-enhanced SDT + anti-CTLA-4 treatment group survived for 50 days, whereas no mice in the other groups (Fig. 5d). The data further indicate that combining SDT, Zr-TCPP(TPP)/R837@M, and CTLA-4 ICB can effectively induce systemic antitumor immunity conducive to preventing tumor metastasis and prolonging survival in a murine model of metastatic disease.
Tumor metastasis inhibition by SDT with Zr-TCPP(TPP)/R837@M combined anti-CTLA-4 therapy on the 4T1 orthotopic breast tumor metastasis model. a Schematic illustration of our experimental design by using Zr-TCPP(TPP)/R837@M-based SDT combined anti-CTLA-4 therapy to inhibit orthotopic cancer metastasis. FLuc-4T1 cells were inoculated into the breast pad of each mouse to establish the orthotopic murine breast cancer. Six days later, fLuc-4T1 cells were i.v. injected into each mouse to trigger whole-body spreading of cancer cells. b In vivo bioluminescence images to track the spreading and growth of fLuc-4T1 cancer cells in different groups of mice after various treatments to eliminate their primary orthotopic tumors. c Representative photographs to show the gross appearance of tumour nodules in the lungs. d Morbidity free survival of different groups of mice with metastatic 4T1 tumors after various treatments indicated to eliminate their primary orthotopic tumors (n = 5). Statistical significance was calculated by Log-rank (Mantel-Cox) test. P-value: *, P < 0.05;**, P < 0.01
Immune-mediated memory responses can protect against antigens that have been previously encountered through the rapid induction of recall responses [44]. CTLA-4 blockade can enhance the expansion and effector activity of memory CD8+T cells [45]. To assess the immunological memory induction following combination SDT + Zr-TCPP(TPP)/R837@M NP treatment, we subjected mice in which primary tumors had been eliminated to tumor rechallenge on day 40 post-surgery. Animals were then separated into four treatment groups, with five mice per group: (1) surgery; (2) surgery + anti-CTLA-4; (3) surgery + Zr-TCPP(TPP)/R837@M + US + anti-CTLA-4; and (4) surgery + Zr-TCPP/R837@M + US + anti-CTLA-4. Appropriate mice were treated with anti-CTLA-4 (10 μg/mouse/injection) on days 7, 9, and 11 following initial tumor removal (Fig. 6a). Images of 4T1 tumor-bearing mice were obtained since the second tumor inculation. (Additional file 1: Figure S7a) In animals that had undergone surgical primary tumor removal, secondary tumor growth was not significantly inhibited by anti-CTLA-4 treatment. However, for mice in the Zr-TCPP(TPP)/R837@M + US + anti-CTLA-4 treatment group, these secondary tumors grew very slowly, while in the photograph and the weight of excised rechallenged tumors in the Zr-TCPP/R837@M + US + anti-CTLA-4 treatment group were evidently smaller than other groups (Fig. 6b; Additional file 1: Figure S8b, c). These results thus indicated that SDT + Zr-TCPP(TPP)/R837@M + anti-CTLA-4 combination therapy was conducive to the establishment of durable antitumor immunity.
Long-term immune-memory effects induced by Zr-TCPP(TPP)/R837@M-based SDT. a Schematic illustration of the experiment design to assess the anti-tumour immune responses against mimic distant tumours and the immunological memory response triggered by Zr-TCPP(TPP)/R837@M -augmented SDT and anti-CTLA-4 combination to inhibit cancer relapse. b Tumor growth curves of rechallenged tumors inoculated 40 days post eliminated of their first tumors (n = 5) by different treatment. c Representative flow-cytometry plots of Lymph nodes cells of 4T1 tumour-bearing mice treated with the combined immunotherapy at day 40 right before mice rechallenged with the secondary tumours; d, e Proportions of CD44+CD62L−TEM and CD44+CD62L+TCM among CD4+ and CD8+ T cells. (n = 3). f–h Cytokine levels in sera from mice isolated at day 40 right before rechallenging mice with secondary tumors. (n = 3) Error bars indicate s.e.m. Statistical significance was calculated by one-way ANOVA Tukey's multiple comparisons test. P-value: *, P < 0.05; **, P < 0.01; ***, P < 0.001
To assess immunological memory in this 4T1 mouse model system, lymph nodes were collected on day 40 prior to tumor rechallenge to assess the central memory and effector T cell (TCM and TEM, respectively) populations present therein. Memory T cells are able to mediate specific and efficient responses to pathogens or other sources of target antigens as they are present at high numbers, undergo persistent homeostatic population maintenance that is antigen-independent, and can rapidly expand when they encounter their cognate antigen. While TCMs begin rapidly expanding and differentiating following initial antigen stimulation, TEMs exhibit more robust and efficient acquisition of effector functions including cytokine production and cytolytic activity relative to TCMs [46, 47].
In both the CD4+ or CD8+ compartments, the frequencies of CD62L−CD44+ TEMs were significantly increased following Zr-TCPP(TPP)/R837@M-based SDT + anti-CTLA-4 treatment as compared to those frequencies observed in mice that underwent surgery with or without anti-CTLA-4 treatment (Fig. 6c–e). On day 40, serum cytokine levels in mice in these different groups were assessed via ELISA, revealing significant increases in the innate immunity-related IL-12p70 and the adaptive immunity-related TNF-α and IL-6 at this time point for mice that underwent combination Zr-TCPP(TPP)/R837@M-based SDT + anti-CTLA-4 treatment relative to the levels observed in mice that underwent surgery with or without CTLA-4 blockage. Relatively low expression levels were also evident when using NPs lacking mitochondria targeting activity (Fig. 6f–h). As such, combining Zr-TCPP(TPP)/R837@M and anti-CTLA-4 treatment can robustly enhance immunological memory to specifically prevent tumor recurrence.