Abstract
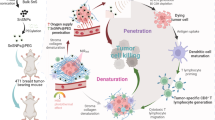
Ultrasound with deep penetration depth and high security could be adopted in sonodynamic therapy (SDT) by activating sonosensitizers to generate cytotoxic reactive oxygen species (ROS). Herein, two-dimensional (2D) coordination nanosheets composed of Zn2+ and Tetrakis(4-carboxyphenyl) porphyrin (TCPP) are fabricated. While exhibiting greatly enhanced ultrasound-triggered ROS generation useful for noninvasive SDT, such Zn-TCPP 2D nanosheets show high loading capacity of oligodeoxynucleotides such as cytosine-phosphorothioate-guanine (CpG), which is a potent toll like receptor 9 (TLR9) agonist useful in activating immune responses. Highly effective SDT of primary tumors could release tumor-associated antigens, which working together with Zn-TCPP/CpG adjuvant nanosheets could function like whole-tumor-cell vaccines and trigger tumor-specific immune responses. Interestingly, ultrasound itself could strengthen anti-tumor immune responses by improving the tumor-infiltration of T cells and limiting regulatory T cells in the tumor microenvironment. Thus, SDT using Zn-TCPP/CpG nanosheets after destruction of primary tumors could induce potent antitumor immune responses to inhibit distant abscopal tumors without direct SDT treatment. Moreover, SDT with Zn-TCPP/CpG could trigger strong immunological memory effects to inhibit cancer recurrence after elimination of primary tumors. Therefore, the 2D coordination nanosheet may be a promising platform to deliver potent SDT-triggered immunotherapy for highly effective cancer treatment.

Similar content being viewed by others
References
ter Haar, G. Therapeutic ultrasound. Eur. J. Ultrasound 1999, 9, 3–9.
Ferrara, K.; Pollard, R.; Borden, M. Ultrasound microbubble contrast agents: Fundamentals and application to gene and drug delivery. Annu. Rev. Biomed. Eng. 2007, 9, 415–447.
Mitragotri, S. Healing sound: The use of ultrasound in drug delivery and other therapeutic applications. Nat. Rev. Drug Discov. 2005, 4, 255–260.
Kost, J.; Mitragotri, S.; Gabbay, R. A.; Pishko, M.; Langer, R. Transdermal monitoring of glucose and other analytes using ultrasound. Nat. Med. 2000, 6, 347–350.
Tezel, A.; Paliwal, S.; Shen, Z. C.; Mitragotri, S. Low-frequency ultrasound as a transcutaneous immunization adjuvant. Vaccine 2005, 23, 3800–3807.
Schoellhammer, C. M.; Blankschtein, D.; Langer, R. Skin permeabilization for transdermal drug delivery: Recent advances and future prospects. Expert Opin. Drug Deliv. 2014, 11, 393–407.
Oberli, M. A.; Schoellhammer, C. M.; Langer, R.; Blankschtein, D. Ultrasound-enhanced transdermal delivery: Recent advances and future challenges. Ther. Deliv. 2014, 5, 843–857.
Peek, M. C. L.; Ahmed, M.; Scudder, J.; Baker, R.; Pinder, S. E.; Douek, M. High intensity focused ultrasound in the treatment of breast fibroadenomata: Results of the HIFU-F trial. Int. J. Hyperth. 2016, 32, 881–888.
Hijnen, N. M.; Heijman, E.; Köhler, M. O.; Ylihautala, M.; Ehnholm, G. J.; Simonetti, A. W.; Grüll, H. Tumour hyperthermia and ablation in rats using a clinical MR-HIFU system equipped with a dedicated small animal set-up. Int. J. Hyperth. 2012, 28, 141–155.
Trendowski, M. The promise of sonodynamic therapy. Cancer Metastasis Rev. 2014, 33, 143–160.
Costley, D.; McEwan, C.; Fowley, C.; McHale, A. P.; Atchison, J.; Nomikou, N.; Callan, J. F. Treating cancer with sonodynamic therapy: A review. Int. J. Hyperth. 2015, 31, 107–117.
McEwan, C.; Owen, J.; Stride, E.; Fowley, C.; Nesbitt, H.; Cochrane, D.; Coussios, C. C.; Borden, M.; Nomikou, N.; McHale, A. P. et al. Oxygen carrying microbubbles for enhanced sonodynamic therapy of hypoxic tumours. J. Control. Release 2015, 203, 51–56.
Gong, F.; Cheng, L.; Yang, N. L.; Betzer, O.; Feng, L. Z.; Zhou, Q.; Li, Y. G.; Chen, R. H.; Popovtzer, R.; Liu, Z. Ultrasmall oxygen-deficient bimetallic oxide MnWOX nanoparticles for depletion of endogenous GSH and enhanced sonodynamic cancer therapy. Adv. Mater. 2019, 31, 1900730.
Yumita, N.; Umemura, S. Sonodynamic therapy with photofrin II on AH130 solid tumor. Cancer Chemother. Pharmacol. 2003, 51, 174–178.
Rosenthal, I.; Sostaric, J. Z.; Riesz, P. Sonodynamic therapy-a review of the synergistic effects of drugs and ultrasound. Ultrason. Sonochem. 2004, 11, 349–363.
Su, X. M.; Wang, P.; Yang, S.; Zhang, K.; Liu, Q. H.; Wang, X. B. Sonodynamic therapy induces the interplay between apoptosis and autophagy in K562 cells through ROS. Int. J. Biochem. Cell Biol. 2015, 60, 82–92.
Cheng, J. L.; Sun, X.; Guo, S. Y.; Cao, W.; Chen, H. B.; **, Y. H.; Li, B.; Li, Q. N.; Wang, H.; Wang, Z. et al. Effects of 5-aminolevulinic acid-mediated sonodynamic therapy on macrophages. Int. J. Nanomedicine 2013, 8, 669–676.
Jiang, Y. Q.; Kou, J. Y.; Han, X. B.; Li, X. S.; Zhong, Z. Y.; Liu, Z. N.; Zheng, Y. H.; Tian, Y.; Yang, L. M. ROS-dependent activation of autophagy through the PI3K/Akt/mTOR pathway is induced by hydroxysafflor yellow a-sonodynamic therapy in THP-1 macrophages. Oxid. Med. Cell. Longev. 2017, 2017, 8519169.
Su, X. M.; Wang, P.; Wang, X. B.; Guo, L.; Li, S. L.; Liu, Q. H. Involvement of MAPK activation and ROS generation in human leukemia U937 cells undergoing apoptosis in response to sonodynamic therapy. Int. J. Radiat. Biol. 2013, 89, 915–927.
Zhang, Q. Y.; Bao, C. X.; Cai, X. J.; **, L. W.; Sun, L. L.; Lang, Y. H.; Li, L. B. Sonodynamic therapy-assisted immunotherapy: A novel modality for cancer treatment. Cancer Sci. 2018, 109, 1330–1345.
Zhao, H. J.; Zhao, B. B.; Li, L.; Ding, K. L.; **ao, H. F.; Zheng, C. X.; Sun, L. L.; Zhang, Z. Z.; Wang, L. Biomimetic decoy inhibits tumor growth and lung metastasis by reversing the drawbacks of sonodynamic therapy. Adv. Healthc. Mater. 2020, 9, 1901335.
Yue, W. W.; Chen, L.; Yu, L. D.; Zhou, B. G.; Yin, H. H.; Ren, W. W.; Liu, C.; Guo, L. H.; Zhang, Y. F.; Sun, L. P. et al. Checkpoint blockade and nanosonosensitizer-augmented noninvasive sonodynamic therapy combination reduces tumour growth and metastases in mice. Nat. Commun. 2019, 10, 2025.
Chen, Q.; Hu, Q. Y.; Dukhovlinova, E.; Chen, G. J.; Ahn, S.; Wang, C.; Ogunnaike, E. A.; Ligler, F. S.; Dotti, G.; Gu, Z. Photothermal therapy: Photothermal therapy promotes tumor infiltration and antitumor activity of CAR T cells (Adv. Mater. 23/2019). Adv. Mater. 2019, 31, 1970166.
Mehrad, H.; Farhoudi, M. Investigation of protoporphyrin IX-mediated sonodynamic therapy on intermediate stage atherosclerosis using a new computerized B-mode ultrasound analyzing method. Atherosclerosis 2016, 252, E192.
Yao, J. T.; Gao, W. W.; Wang, Y.; Wang, L.; Diabakte, K.; Li, J. Y.; Yang, J. M.; Jiang, Y. X.; Liu, Y. R.; Guo, S. Y. et al. Sonodynamic therapy suppresses neovascularization in atherosclerotic plaques via macrophage apoptosis-induced endothelial cell apoptosis. JACC Basic Transl. Sci. 2020, 5, 53–65.
Tang, C. H.; Lu, D. Y.; Tan, T. W.; Fu, W. M.; Yang, R. S. Ultrasound induces hypoxia-inducible factor-1 activation and inducible nitric-oxide synthase expression through the integrin/integrin-linked kinase/Akt/mammalian target of rapamycin pathway in osteoblasts. J. Biol. Chem. 2007, 282, 25406–25415.
Peng, Y.; Jia, L. M.; Wang, S.; Cao, W. W.; Zheng, J. H. Sonodynamic therapy improves anti-tumor immune effect by increasing the infiltration of CD8+ T cells and altering tumor blood vessels in murine B16F10 melanoma xenograft. Oncol. Rep. 2018, 40, 2163–2170.
Cheng, L.; Wang, X. W.; Gong, F.; Liu, T.; Liu, Z. 2D nanomaterials for cancer theranostic applications. Adv. Mater. 2020, 32, e1902333.
Zhang, H. Ultrathin two-dimensional nanomaterials. ACS Nano 2015, 9, 9451–9469.
Martín, C.; Kostarelos, K.; Prato, M.; Bianco, A. Biocompatibility and biodegradability of 2D materials: Graphene and beyond. Chem. Commun. 2019, 55, 5540–5546.
Manzeli, S.; Ovchinnikov, D.; Pasquier, D.; Yazyev, O. V.; Kis, A. 2D transition metal dichalcogenides. Nat. Rev. Mater. 2017, 2, 17033.
Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M. W. Two-dimensional transition metal carbides. ACS Nano 2012, 6, 1322–1331.
Lan, G. X.; Ni, K. Y.; Xu, R. Y.; Lu, K. D.; Lin, Z. K.; Chan, C.; Lin, W. B. Nanoscale metal-organic layers for deeply penetrating X-ray-induced photodynamic therapy. Angew. Chem. 2017, 129, 12270–12274.
Zhao, M. T.; Wang, Y. X.; Ma, Q. L.; Huang, Y.; Zhang, X.; **, J. F.; Zhang, Z. C.; Lu, Q. P.; Yu, Y. F.; Xu, H. et al. Ultrathin 2D metal-organic framework nanosheets. Adv. Mater. 2015, 27, 7372–7378.
Wan, S. S.; Cheng, Q.; Zeng, X.; Zhang, X. Z. A Mn(III)-sealed metal-organic framework nanosystem for redox-unlocked tumor theranostics. ACS Nano 2019, 13, 6561–6571.
Krieg, A. M. Antitumor applications of stimulating toll-like receptor 9 with CpG oligodeoxynucleotides. Curr. Oncol. Rep. 2004, 6, 88–95.
Akca, S.; Foroughi, A.; Frochtzwajg, D.; Postma, H. W. C. Competing interactions in DNA assembly on graphene. PLoS One 2011, 6, e18442.
Johnson, A. T. C.; Khamis, S. M.; Preti, G.; Kwak, J.; Gelperin, A. DNA-coated nanosensors for breath analysis. IEEE Sens. J. 2010, 10, 159–166.
Mignon, P.; Loverix, S.; Steyaert, J.; Geerlings, P. Influence of the π-π interaction on the hydrogen bonding capacity of stacked DNA/RNA bases. Nucleic Acids Res. 2005, 33, 1779–1789.
Zhu, C. F.; Zeng, Z. Y.; Li, H.; Li, F.; Fan, C. H.; Zhang, H. Single-layer MoS2-based nanoprobes for homogeneous detection of biomolecules. J. Am. Chem. Soc. 2013, 135, 5998–6001.
Zhu, W. J.; Yang, Y.; **, Q. T.; Chao, Y.; Tian, L. L.; Liu, J. J.; Dong, Z. L.; Liu, Z. Two-dimensional metal-organic-framework as a unique theranostic nano-platform for nuclear imaging and chemophotodynamic cancer therapy. Nano Res. 2019, 12, 1307–1312.
Ni, K. Y.; Luo, T. K.; Culbert, A.; Kaufmann, M.; Jiang, X. M.; Lin, W. B. Nanoscale metal-organic framework Co-delivers TLR-7 agonists and Anti-CD47 antibodies to modulate macrophages and orchestrate cancer immunotherapy. J. Am. Chem. Soc. 2020, 142, 12579–12584.
Lu, K. D.; He, C. B.; Lin, W. B. Nanoscale metal-organic framework for highly effective photodynamic therapy of resistant head and neck cancer. J. Am. Chem. Soc. 2014, 136, 16712–16715.
Rosenkranz, A. R.; Schmaldienst, S.; Stuhlmeier, K. M.; Chen, W. J.; Knapp, W.; Zlabinger, G. J. A microplate assay for the detection of oxidative products using 2′,7′-dichlorofluorescin-diacetate. J. Immunol. Methods 1992, 156, 39–45.
Campana, L.; Bosurgi, L.; Rovere-Querini, P. HMGB1: A two-headed signal regulating tumor progression and immunity. Curr. Opin. Immunol. 2008, 20, 518–523.
Obeid, M.; Tesniere, A.; Ghiringhelli, F.; Fimia, G. M.; Apetoh, L.; Perfettini, J. L.; Castedo, M.; Mignot, G.; Panaretakis, T.; Casares, N. et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 2007, 13, 54–61.
Tesniere, A.; Apetoh, L.; Ghiringhelli, F.; Joza, N.; Panaretakis, T.; Kepp, O.; Schlemmer, F.; Zitvogel, L.; Kroemer, G. Immunogenic cancer cell death: A key-lock paradigm. Curr. Opin. Immunol. 2008, 20, 504–511.
**, Q.; Zhou, D. M.; Kan, Y. Y.; Ge, J.; Wu, Z. K.; Yu, R. Q.; Jiang, J. H. Highly sensitive and selective strategy for MicroRNA detection based on WS2 nanosheet mediated fluorescence quenching and duplex-specific nuclease signal amplification. Anal. Chem. 2014, 86, 1361–1365.
Ge, J.; Ou, E. C.; Yu, R. Q.; Chu, X. A novel aptameric nanobiosensor based on the self-assembled DNA-MoS2 nanosheet architecture for biomolecule detection. J. Mater. Chem. B 2014, 2, 625–628.
Zhang, H. Y.; Ruan, Y. J.; Lin, L.; Lin, M. G.; Zeng, X. X.; **, Z. M.; Fu, F. F. A turn-off fluorescent biosensor for the rapid and sensitive detection of uranyl ion based on molybdenum disulfide nanosheets and specific DNAzyme. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 146, 1–6.
Müiz, C.; Steinman, R. M.; Fujii, S. Dendritic cell maturation by innate lymphocytes. J. Exp. Med. 2005, 202, 203–207.
Chen, Q.; Xu, L. G.; Liang, C.; Wang, C.; Peng, R.; Liu, Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat. Commun. 2016, 7, 13193.
Semenza, G. L. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci. STKE 2007, 407, cm8.
Schlesinger, M.; Bendas, G. Vascular cell adhesion molecule-1 (VCAM-1) — An increasing insight into its role in tumorigenicity and metastasis. Int. J. Cancer 2015, 136, 2504–2514.
Yoong, K. F.; McNab, G.; Hübscher, S. G.; Adams, D. H. Vascular adhesion protein-1 and ICAM-1 support the adhesion of tumor-infiltrating lymphocytes to tumor endothelium in human hepatocellular carcinoma. J. Immunol. 1998, 160, 3978–3988.
Chen, Q.; Chen, M. C.; Liu, Z. Local biomaterials-assisted cancer immunotherapy to trigger systemic antitumor responses. Chem. Soc. Rev. 2019, 48, 5506–5526.
Nam, J.; Son, S.; Ochyl, L. J.; Kuai, R.; Schwendeman, A.; Moon, J. J. Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer. Nat. Commun. 2018, 9, 1074.
Chen, Q.; Xu, L. G.; Chen, J. W.; Yang, Z. J.; Liang, C.; Yang, Y.; Liu, Z. Tumor vasculature normalization by orally fed erlotinib to modulate the tumor microenvironment for enhanced cancer nanomedicine and immunotherapy. Biomaterials 2017, 148, 69–80.
Chao, Y.; Xu, L. G.; Liang, C.; Feng, L. Z.; Xu, J.; Dong, Z. L.; Tian, L. L.; Yi, X.; Yang, K.; Liu, Z. Combined local immunostimulatory radioisotope therapy and systemic immune checkpoint blockade imparts potent antitumour responses. Nat. Biomed. Eng. 2018, 2, 611–621.
Chen, Q.; Chen, J. W.; Yang, Z. J.; Xu, J.; Xu, L. G.; Liang, C.; Han, X.; Liu, Z. Nanoparticle-enhanced radiotherapy to trigger robust cancer immunotherapy. Adv. Mater. 2019, 31, 1802228.
Chao, Y.; Chen, G. B.; Liang, C.; Xu, J.; Dong, Z. L.; Han, X.; Wang, C.; Liu, Z. Iron nanoparticles for low-power local magnetic hyperthermia in combination with immune checkpoint blockade for systemic antitumor therapy. Nano Lett. 2019, 19, 4287–4296.
Acknowledgements
This article was partially supported by the National Research Programs of China (No. 2016YFA0201200), the National Natural Science Foundation of China (Nos. 91959104, 21927803, 51903182, and 51525203,), the Natural Science Foundation of Jiangsu Province (No. BK20190826), Collaborative Innovation Center of Suzhou Nano Science and Technology, and the 111 Program from the Ministry of Education of China.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2020_3070_MOESM1_ESM.pdf
Sonodynamic therapy with immune modulatable two-dimensional coordination nanosheets for enhanced anti-tumor immunotherapy
Rights and permissions
About this article
Cite this article
Zhu, W., Chen, Q., **, Q. et al. Sonodynamic therapy with immune modulatable two-dimensional coordination nanosheets for enhanced anti-tumor immunotherapy. Nano Res. 14, 212–221 (2021). https://doi.org/10.1007/s12274-020-3070-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-020-3070-8