Abstract
Background
Remnant cholesterol (RC) is an important marker for assessing the risk of metabolic syndrome. However, the correlation between RC and hyperuricemia (HUA) remains unclear. This study aimed to explore the correlation between RC and HUA in American adults.
Methods
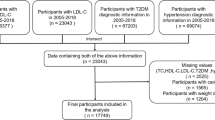
A total of 9089 participants from the 2013–2020 National Health and Nutrition Examination Survey were investigated. The correlation between RC and the odds of HUA was evaluated using multivariate logistic regression analysis. The nonlinear correlation was described using fitted smoothed curves. The correlation in subgroups was analyzed based on race, gender, alcohol consumption, age, body mass index, waist circumference, diabetes and moderate physical activities.
Results
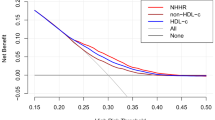
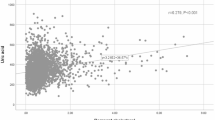
RC was correlated with uric acid (Spearman’s correlation coefficient = 0.208 in males and 0.215 in females; all P < 0.001). Multiple logistic regression analysis indicated a positive correlation between RC and the risk of HUA (odds ratio = 1.022 in males and 1.031 in females; all P < 0.001). Subgroup analysis revealed that the correlation was stronger in females, participants aged < 50 years, and those without diabetes. Furthermore, the generalized smooth curve fitting demonstrated a linear correlation between RC and HUA, without threshold or saturation effects.
Conclusion
Elevated RC significantly and positively correlated with HUA in American adults. This correlation was stronger among females, participants aged < 50 years, and those without diabetes.
Similar content being viewed by others
Introduction
Hyperuricemia (HUA), characterized by the excessive production or inadequate excretion of uric acid, is a significant global health concern closely associated with gout and a variety of other medical conditions, impacting individuals of all genders and ages [1, 2]. HUA can serve as an independent risk factor for numerous systemic diseases, such as cardiovascular diseases, gout, chronic kidney disease and hypertension [3]. As of 2016, the prevalence of HUA has reached 21% worldwide [4], ranging from 14.6 to 20% in the United States [5]. Notably, there is a trend affecting younger individuals, with mean age of 38.6 years old. HUA can impose substantial health burdens on public health infrastructure [43,44,45]. Decreased preglomerular resistance in diabetic patients helps to increase glomerular hyperfiltration, further promotes the excretion of UA, and leads to hypouricemia [46, 47]. Similarly, the islet function of diabetic patients was often damaged, which led to a decrease in insulin secretion in the body, downregulating the expression of renal urate transporters, reducing the reabsorption of UA, and reducing SUA levels [48]. Additionally, dietary irregularities and insufficient exercise in young individuals can lead to excessive fat accumulation, potentially influencing RC [49]. Therefore, HUA usually has a stronger correlation with RC in the younger or without diabetes population.
Several plausible mechanisms can be postulated to elucidate the correlation between RC and the development of HUA. First of all, the elevation of RC levels in body will lead to an induction of heightened production and utilization of free fatty acids, consequently accelerating the catabolism of adenosine triphosphate and resulting in an augmented production of serum uric acid [50]. Secondly, an elevated RC level has been found to be independently associated with a reduced estimated glomerular filtration rate and an increased risk of renal impairment, potentially leading to a diminished excretion of uric acid [41]. Lastly, RC can serve as a surrogate marker for IR [51], and IR is a factor closely related to the pathogenesis of HUA. IR has been shown that it can enhance renal urate reabsorption through the stimulation of URAT1 [52] and/or Nadependent anion co-transporter in brush border membranes of renal proximal tubule [52, 53]. This finding strengthened on previous studies showed a pathogenesis among hyperuricemia and dyslipidemia [54, 55].
Study strengths and limitations
Notably, the study benefits from a large sample size and the national representativeness of Americans. In addition, various indexes in the model were adjusted to enhance the reliability of the findings. Nonetheless, this study is subject to certain limitations. Firstly, the establishment of a causal correlation between RC and HUA was not feasible through cross-sectional studies. Secondly, the measurement of RC is not currently a standard component of clinical blood lipid testing through direct means, thus only RC levels can be calculated. Thirdly, American adults are restricted to the study, necessitating further prospective cohort research to validate and generalize the present results in a broader population.
Conclusion
In summary, elevated RC was independently associated with HUA in a sizable cohort of American adults. This correlation was particularly pronounced among females, those under 50 years old, and those without diabetes. The RC could serve as an effective biomarker for assessing the risk of HUA.
Data availability
The datasets generated and analysis during the current study are available in the NHANES, www.cdc.gov/nchs/NHANEs/.
References
Smith E, Hoy D, Cross M, Merriman TR, Vos T, Buchbinder R, Woolf A, March L. The global burden of gout: estimates from the global burden of Disease 2010 study. Ann Rheum Dis. 2014;73:1470–6.
George C, Leslie SW, Minter DA. Hyperuricemia. In StatPearls Treasure Island (FL) ineligible companies. Disclosure: Stephen Leslie declares no relevant financial relationships with ineligible companies. Disclosure: David Minter declares no relevant financial relationships with ineligible companies.; 2024.
Borghi C, Agabiti-Rosei E, Johnson RJ, Kielstein JT, Lurbe E, Mancia G, Redon J, Stack AG, Tsioufis KP. Hyperuricaemia and gout in cardiovascular, metabolic and kidney disease. Eur J Intern Med. 2020;80:1–11.
Fang XY, Qi LW, Chen HF, Gao P, Zhang Q, Leng RX, Fan YG, Li BZ, Pan HF, Ye DQ. The Interaction between Dietary Fructose and Gut Microbiota in Hyperuricemia and gout. Front Nutr. 2022;9:890730.
Chen-Xu M, Yokose C, Rai SK, Pillinger MH, Choi HK. Contemporary prevalence of gout and Hyperuricemia in the United States and Decadal trends: the National Health and Nutrition Examination Survey, 2007–2016. Arthritis Rheumatol. 2019;71:991–9.
**a Y, Wu Q, Wang H, Zhang S, Jiang Y, Gong T, Xu X, Chang Q, Niu K, Zhao Y. Global, regional and national burden of gout, 1990–2017: a systematic analysis of the global burden of Disease Study. Rheumatology (Oxford). 2020;59:1529–38.
Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384:626–35.
Basu D, Bornfeldt KE. Hypertriglyceridemia and atherosclerosis: using Human Research to Guide mechanistic studies in animal models. Front Endocrinol (Lausanne). 2020;11:504.
Varbo A, Benn M, Tybjaerg-Hansen A, Nordestgaard BG. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation. 2013;128:1298–309.
Nordestgaard BG. Triglyceride-Rich lipoproteins and Atherosclerotic Cardiovascular Disease: New insights from Epidemiology, Genetics, and Biology. Circ Res. 2016;118:547–63.
Ye X, Kong W, Zafar MI, Chen LL. Serum triglycerides as a risk factor for cardiovascular diseases in type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Cardiovasc Diabetol. 2019;18:48.
Nordestgaard BG, Tybjaerg-Hansen A. Genetic determinants of LDL, lipoprotein(a), triglyceride-rich lipoproteins and HDL: concordance and discordance with cardiovascular disease risk. Curr Opin Lipidol. 2011;22:113–22.
Varbo A, Benn M, Nordestgaard BG. Remnant cholesterol as a cause of ischemic heart disease: evidence, definition, measurement, atherogenicity, high risk patients, and present and future treatment. Pharmacol Ther. 2014;141:358–67.
de Graaf J, van der Vleuten GM, ter Avest E, Dallinga-Thie GM, Stalenhoef AF. High plasma level of remnant-like particles cholesterol in familial combined hyperlipidemia. J Clin Endocrinol Metab. 2007;92:1269–75.
Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002;23:201–29.
Arner P, Bernard S, Salehpour M, Possnert G, Liebl J, Steier P, Buchholz BA, Eriksson M, Arner E, Hauner H, et al. Dynamics of human adipose lipid turnover in health and metabolic disease. Nature. 2011;478:110–3.
**ao C, Hsieh J, Adeli K, Lewis GF. Gut-liver interaction in triglyceride-rich lipoprotein metabolism. Am J Physiol Endocrinol Metab. 2011;301:E429–446.
** J, Meng X, Wang D, Han B, Wu T, **e J, Zhang Q, **e D, Zhang Z. Association between ambient temperature and cardiovascular diseases related hospital admissions in Lanzhou, China. Heliyon. 2023;9:e12997.
Rafiullah M, Siddiqui K, Al-Rubeaan K. Association between serum uric acid levels and metabolic markers in patients with type 2 diabetes from a community with high diabetes prevalence. Int J Clin Pract. 2020;74:e13466.
Hou YL, Yang XL, Wang CX, Zhi LX, Yang MJ, You CG. Hypertriglyceridemia and hyperuricemia: a retrospective study of urban residents. Lipids Health Dis. 2019;18:81.
Peng TC, Wang CC, Kao TW, Chan JY, Yang YH, Chang YW, Chen WL. Relationship between hyperuricemia and lipid profiles in US adults. Biomed Res Int 2015, 2015:127596.
Zhang X, Meng Q, Feng J, Liao H, Shi R, Shi D, Renqian L, Langtai Z, Diao Y, Chen X. The prevalence of hyperuricemia and its correlates in Ganzi Tibetan Autonomous Prefecture, Sichuan Province, China. Lipids Health Dis. 2018;17:235.
Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–88.
Liu R, Han C, Wu D, **a X, Gu J, Guan H, Shan Z, Teng W. Prevalence of Hyperuricemia and Gout in Mainland China from 2000 to 2014: A Systematic Review and Meta-Analysis. Biomed Res Int 2015, 2015:762820.
Moura L, Pagotto V, Camargo Pereira C, de Oliveira C, Silveira EA. Does abdominal obesity increase All-Cause, Cardiovascular Disease, and Cancer Mortality risks in older adults? A 10-Year Follow-Up analysis. Nutrients 2022, 14.
Bardin T, Richette P. Impact of comorbidities on gout and hyperuricaemia: an update on prevalence and treatment options. BMC Med. 2017;15:123.
Kumar AUA, Browne LD, Li X, Adeeb F, Perez-Ruiz F, Fraser AD, Stack AG. Temporal trends in hyperuricaemia in the Irish health system from 2006–2014: a cohort study. PLoS ONE. 2018;13:e0198197.
Wang H, Zhang H, Sun L, Guo W. Roles of hyperuricemia in metabolic syndrome and cardiac-kidney-vascular system diseases. Am J Transl Res. 2018;10:2749–63.
Bomback AS, Derebail VK, Shoham DA, Anderson CA, Steffen LM, Rosamond WD, Kshirsagar AV. Sugar-sweetened soda consumption, hyperuricemia, and kidney disease. Kidney Int. 2010;77:609–16.
Nakanishi N, Yoshida H, Nakamura K, Suzuki K, Tatara K. Predictors for development of hyperuricemia: an 8-year longitudinal study in middle-aged Japanese men. Metabolism. 2001;50:621–6.
Jorgensen AB, Frikke-Schmidt R, West AS, Grande P, Nordestgaard BG, Tybjaerg-Hansen A. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur Heart J. 2013;34:1826–33.
Sandesara PB, Virani SS, Fazio S, Shapiro MD. The forgotten lipids: triglycerides, remnant cholesterol, and atherosclerotic Cardiovascular Disease Risk. Endocr Rev. 2019;40:537–57.
Quispe R, Martin SS, Michos ED, Lamba I, Blumenthal RS, Saeed A, Lima J, Puri R, Nomura S, Tsai M, et al. Remnant cholesterol predicts cardiovascular disease beyond LDL and ApoB: a primary prevention study. Eur Heart J. 2021;42:4324–32.
Chen J, Su Y, Su X, Luo F. Remnant cholesterol has a non-linear association with non-alcoholic fatty liver disease. Diabetes Res Clin Pract. 2023;201:110733.
Huang H, **e J, Zeng Y, Liu Z, Miao M, Xu L, Xu C. Remnant cholesterol independently predicts the development of nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2023;108:2907–15.
Szili-Torok T, Sokooti S, Oste MCJ, Gomes-Neto AW, Dullaart RPF, Bakker SJL, Tietge UJF. Remnant lipoprotein cholesterol is associated with incident new onset diabetes after transplantation (NODAT) in renal transplant recipients: results of the TransplantLines Biobank and cohort studies. Cardiovasc Diabetol. 2022;21:41.
Pillinger MH, Bangalore S, Klein AB, Baumgartner S, Morlock R. Cardiovascular Disease and gout: real-world experience evaluating patient characteristics, Treatment Patterns, and Health Care utilization. J Manag Care Spec Pharm. 2017;23:677–83.
Varbo A, Benn M, Tybjaerg-Hansen A, Jorgensen AB, Frikke-Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. 2013;61:427–36.
Zou Y, Lan J, Zhong Y, Yang S, Zhang H, **e G. Association of remnant cholesterol with nonalcoholic fatty liver disease: a general population-based study. Lipids Health Dis. 2021;20:139.
Hu X, Liu Q, Guo X, Wang W, Yu B, Liang B, Zhou Y, Dong H, Lin J. The role of remnant cholesterol beyond low-density lipoprotein cholesterol in diabetes mellitus. Cardiovasc Diabetol. 2022;21:117.
Yan P, Xu Y, Miao Y, Bai X, Wu Y, Tang Q, Zhang Z, Yang J, Wan Q. Association of remnant cholesterol with chronic kidney disease in middle-aged and elderly Chinese: a population-based study. Acta Diabetol. 2021;58:1615–25.
Palmisano BT, Zhu L, Stafford JM. Role of Estrogens in the regulation of liver lipid metabolism. Adv Exp Med Biol. 2017;1043:227–56.
Okada M, Ueda K, Omae T, Takeshita M, Hirota Y. The relationship of serum uric acid to hypertension and ischemic heart disease in Hisayama population, Japan. J Chronic Dis. 1982;35:173–8.
Tuomilehto J, Zimmet P, Wolf E, Taylor R, Ram P, King H. Plasma uric acid level and its association with diabetes mellitus and some biologic parameters in a biracial population of Fiji. Am J Epidemiol. 1988;127:321–36.
Li H, Sun M, Huang C, Wang J, Huang Y. Association between Glycosylated Hemoglobin and Serum Uric Acid: A US NHANES 2011–2020. Int J Endocrinol 2024, 2024:5341646.
Cortinovis M, Perico N, Ruggenenti P, Remuzzi A, Remuzzi G. Glomerular hyperfiltration. Nat Rev Nephrol. 2022;18:435–51.
Golik A, Weissgarten J, Cotariu D, Cohen N, Zaidenstein R, Ramot Y, Averbukh Z, Modai D. Renal uric acid handling in non-insulin-dependent diabetic patients with elevated glomerular filtration rates. Clin Sci (Lond). 1993;85:713–6.
Toyoki D, Shibata S, Kuribayashi-Okuma E, Xu N, Ishizawa K, Hosoyamada M, Uchida S. Insulin stimulates uric acid reabsorption via regulating urate transporter 1 and ATP-binding cassette subfamily G member 2. Am J Physiol Ren Physiol. 2017;313:F826–34.
Correa-Rodriguez M, Gonzalez-Jimenez E, Fernandez-Aparicio A, Luis Gomez-Urquiza J, Schmidt-RioValle J, Rueda-Medina B. Dietary Energy Density is Associated with Body Mass Index and Fat Mass in Early Adulthood. Clin Nurs Res. 2021;30:591–8.
Balasubramanian T. Uric acid or 1-methyl uric acid in the urinary bladder increases serum glucose, insulin, true triglyceride, and total cholesterol levels in Wistar rats. ScientificWorldJournal. 2003;3:930–6.
Fukushima M, Taniguchi A, Nakai Y, Sakai M, Doi K, Nin K, Oguma T, Nagasaka S, Tokuyama K, Seino Y. Remnant-like particle cholesterol and insulin resistance in nonobese nonhypertensive Japanese glucose-tolerant relatives of type 2 diabetic patients. Diabetes Care. 2001;24:1691–4.
Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, Hosoyamada M, Takeda M, Sekine T, Igarashi T, et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002;417:447–52.
Perez-Ruiz F, Aniel-Quiroga MA, Herrero-Beites AM, Chinchilla SP, Erauskin GG, Merriman T. Renal clearance of uric acid is linked to insulin resistance and lower excretion of sodium in gout patients. Rheumatol Int. 2015;35:1519–24.
Lu W, Song K, Wang Y, Zhang Q, Li W, Jiao H, Wang G, Huang G. Relationship between serum uric acid and metabolic syndrome: an analysis by structural equation modeling. J Clin Lipidol. 2012;6:159–67.
Conen D, Wietlisbach V, Bovet P, Shamlaye C, Riesen W, Paccaud F, Burnier M. Prevalence of hyperuricemia and relation of serum uric acid with cardiovascular risk factors in a develo** country. BMC Public Health. 2004;4:9.
Acknowledgements
We would like to thank the NHANES database for providing the data source for this study.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
WXW designed the study; JX collected biochemical data; XHZ and XLW drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The National Center for Health Statistics Ethics Review Board has approved the implementation of NHANES.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, X., Weng, X., Xu, J. et al. Correlation between remnant cholesterol and hyperuricemia in American adults. Lipids Health Dis 23, 176 (2024). https://doi.org/10.1186/s12944-024-02167-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-024-02167-0