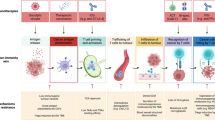
Abstract
Immune escape is a hallmark of cancer. The dynamic and heterogeneous tumour microenvironment (TME) causes insufficient infiltration and poor efficacy of natural killer (NK) cell-based immunotherapy, which becomes a key factor triggering tumour progression. Understanding the crosstalk between NK cells and the TME provides new insights for optimising NK cell-based immunotherapy. Here, we present new advances in direct or indirect crosstalk between NK cells and 9 specialised TMEs, including immune, metabolic, innervated niche, mechanical, and microbial microenvironments, summarise TME-mediated mechanisms of NK cell function inhibition, and highlight potential targeted therapies for NK-TME crosstalk. Importantly, we discuss novel strategies to overcome the inhibitory TME and provide an attractive outlook for the future.
Similar content being viewed by others
Introduction
Cancer is a dynamic and complex disease, and the development, progression and treatment of cancer require the involvement of the entire organism. Tumour formation is the result of the interaction of cancer cells with infiltrating immune cells, stromal cells, blood vessels, the extracellular matrix (ECM), secretory products (cytokines, chemokines, metabolites) and specific environmental conditions (e.g. hypoxia) [77]. NK cells appear to regulate the TME in a dynamic fashion [78].
Notably, the production of prostaglandin E2 (PGE2) in cancer cells can reduce the accumulation of cDCs by impairing NK cell viability and chemokine production, causing immune evasion [71]. Similarly, the production of IL-6 and IL-10 in cancer cells and immune cells in the TME also causes DC dysfunction [79]. Upregulation of CTLA-4 expression on NK cells has a negative impact on DC maturation in human NSCLC [80]. Studies have also shown that DCs may impair NK cell function [81].
In conclusion, the crosstalk between NK cells and DCs is critical for regulating antitumour immunity and may be a promising target for effective antitumour therapy [82]. For example, in an orthotopic liver tumour model, CD47 blockade enhanced antitumour efficacy by triggering the DC- NK cell axis [184]. Although cancer cells can respond to extracellular acidosis and redox reactions by regulating the expression of monocarboxylate transporters (MCT) to adapt to survival and proliferation, exposure to a high lactate environment damages the effector function of NK cells. Preliminary evidence suggests that lactate impairs NK cell function and survival, thereby causing immune evasion and tumour progression [185]. Similarly, the accumulation of lactate in the TME leads to mitochondrial dysfunction and apoptosis of liver-resident NK cells, and the depletion of liver-resident NK cells leads to CRC liver metastases [186]. Tumour-derived lactate can inhibit the cytolytic function of NK cells through direct or indirect pathways involving decreased expression of NK cell perforin, granzyme, and NKp46 and increased abundance of MDSCs [187]. Lactate accumulated in the TME has been considered a potential antitumor target, but this discovery faces significant challenges in translating into clinical therapy [188]. Comprehensive analysis of lactate metabolic signalling and the interaction of lactate with other components in TME, especially NK cells, may be a promising strategy to overcome the limitations of immunotherapy, and more studies are needed.
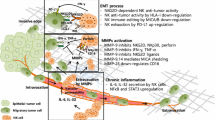
Innervated niche
The "innervated niche" is an emerging concept that aims to describe a specific biological niche "governed by nerves" that is established by the crosstalk between nerves and tumours. There are four main types of cancer-nerve interaction: electrochemical interactions, paracrine interactions, systemic neural-cancer interactions, and cancer therapy-nervous system interactions [189]. Neurological modulation of the immune system and cancer immunotherapy effects on the nervous system represent different mechanisms of neural-cancer crosstalk.
Environmental enrichment (EE) is an established stress model for studying neurogenesis and brain plasticity. Increasing evidence shows that EE can affect the phenotype and function of NK cells. For example, EE positively regulates the maturation, proliferation, cytotoxicity, and tumour infiltration capacity of NK cells, and mice exposed to EE exhibited an antitumour phenotype [190]. EE exposure selectively upregulates hypothalamic brain-derived neurotrophic factor (BDNF) and other brain mediators, induces sympathetic nervous system (SNS) and hypothalamic–pituitary–adrenal axis (HPA) activation, and directly targets NK cells. Moreover, EE acts on adipose tissue and other endocrine organs, which regulate NK cells by secreting leptin, adiponectin, cytokines, and hormones [191]. These mechanisms contribute to the role of NK cells as key mediators in EE-tumour crosstalk and emphasise the significance of a positive stress response and positive emotions for improving immune function and possible antitumour effects (Fig. 4). In contrast, negative stress responses (forced swimming, abdominal surgery) induce inhibition of NK cell activity, which is a major mediator of tumour progression in stress patterns [192]. In population studies, psychological stressors (such as divorce) have also been associated with reduced NK cell activity and increased risk of cancer [193].
Mechanisms of innervated niche crosstalk with NK cells. Positive stressors enhance the antitumor effects of NK cells via the hypothalamic–pituitary–adrenal axis or the sympathetic nervous system. Likewise, NK cells have positive effects on the nervous system. IFN-γ, Interferon-γ. IL-10/17, Interleukin-10/17. BDNF, Brain-derived neurotrophic factor. Adapted from “Hypothalamic-Pituitary-Organ Axis with Cellular Effect (Layout)”, by BioRender.com (2022). Retrieved from https://app.biorender.com/biorender-templates
The effects of NK cells on the nervous system are also worth exploring. Under the recruitment of chemokines (CX3CL1, CCL2, and CXCL10) secreted by central nervous system (CNS) resident cells (microglia, astrocytes, and neurons), circulating NK cells enter the CNS through the blood–brain barrier (BBB) and the choroid plexus, settle in the CNS parenchyma, and become a small proportion of the CNS immune cells [194]. There is growing evidence that NK cells ameliorate a variety of neurodegenerative diseases, including Alzheimer’s, Parkinson’s, and multiple sclerosis. Degradation and internalization of neurotoxic aggregates such as alpha-synuclein (α-syn) protein by NK cells [195], activation of microglia with neuroprotective effects by IFN-γ secreted by NK cells [196], suppression of myelin-reactive Th17 cells [197], and inhibition of inflammatory cells by IL-10 are among the mechanisms involved. NK cells have demonstrated neurotoxic effects via mechanisms such as IFN-γ-mediated cytotoxic T cells, DC recruitment and priming, inflammatory cells promotion via cytokines such as granulocyte–macrophage colony stimulating factor (GM-CSF), and direct release of perforin and granzyme [198]. These findings highlight the elusive dual role of NK cells in CNS homeostasis. It is unclear whether NK cells contribute to the CNS disease pathogenesis or outcome, and this dual role is more likely to be related to individual disease stages and CNS-resident NK cell phenotypes. Overall, the immunomodulatory role of NK cells in mediating cancer-neural crosstalk is critical for cancer progression, and more research is needed to further characterise neural-NK cell-cancer regulatory networks.
Mechanical microenvironment
The TME, as a dynamic and heterogeneous integrated environment, consists mainly of biochemical and mechanical microenvironments. In recent years, researchers have begun to focus on the regulation of the mechanical microenvironment in the TME. Recent studies have shown that the biological behaviour of NK cells in the TME is influenced by mechanical signals, including adhesion, migration, tissue infiltration, and cytotoxic functions [199].
The infiltration of NK cells is accompanied by dramatic changes in the mechanical properties of the stroma and target cells, including cancer cell solid stress, ECM stiffness, topography, and fluid stress [200]. Altered mechanical properties affect the cytotoxic effects of NK cells. It was observed that the softness or hardness of the substrate did not affect the extent of NK cell degranulation; however, exposure to hard substrates enhanced the cytotoxic effect of NK cells, suggesting that mechanosensitivity mediates the cytotoxic effect of NK cells [201]. The decreased rigidity of invasive cells leads to the inability of NK cells to fully respond, which represents a novel mechanism by which cancer cells evade NK cell surveillance [201].
Dynamic actin-mediated mechanical signals are essential to maintain the effector function of NK cells. Evidence suggests that conformational changes in SH2-domain-containing protein tyrosine phosphatase-1 (SHP-1) mediated by actomyosin retrograde flow (ARF) regulate NK cell cytotoxicity. NK cell-activating receptors cause rapid actin flow, which prevents SHP-1 from binding to the actin network, and SHP-1 maintains an inactive conformation and initiates cancer cell death. Conversely, inhibitory receptors slow actin flow, and SHP-1/actin binding activates the SHP-1 conformation, thereby inhibiting NK cell activation [202]. Similarly, actin associated with cytoskeletal conformational rearrangements is actively involved in the formation of lytic IS in NK cells [203]. In conclusion, an understanding of NK cell mechanical signal regulation is necessary for the construction of in vitro mechanical microenvironment models, which are essential for evaluating future NK cell therapies.
Microbial microenvironment
The microbial microenvironment, which has been an underestimated part of the TME for a long time, has now been shown to form a multidimensional tumour-immune-microbe biological network and to be a potential area for improving tumour immunotherapy [204]. Cumulative data suggest that multiple probiotics in the gut enhance NK cell activity and function by promoting the expression of inflammatory cytokines by NK cells [205]. The microbe-mediated immunomodulatory effects of NK cells are also being investigated in the TME.
Microbial regulation of NK cells can alter tumour progression. In a carcinogen-induced in situ mouse model of CRC, colonisation of Helicobacter hepaticus (Hep) resulted in increased infiltration of NK cells and reduced tumour load [206]. Interestingly, Lam et al. found that microbiota could switch the tumorigenic preferences of the microenvironment. A favourable microbiota (intact microbiota, high-fibre diet, responder patients) triggers the production of IFN-I by intratumoral monocytes and regulates macrophage polarisation as well as DC-NK cell crosstalk to form an antitumour microenvironment. In contrast, when the microbiota are adversely disrupted, the monocyte-IFN-I-NK cell-DC cascade is disrupted, and macrophages display the protumour phenotype, creating a protumour microenvironment [207].
The regulation of NK cells by microbiota can affect tumour metastasis. It has been observed that the growth of melanoma cells in bone triggers the proliferation of intestinal NK and Th1 cells and their homing to tumour-bearing bone to inhibit melanoma bone metastasis. This trigger is microbially dependent, and it is weakened by microbiome depletion, thus increasing the progression of bone metastasis [208]. Similarly, Yin et al. found that Fusobacterium nucleatum promotes CRC liver metastasis by increasing the accumulation of MDSCs and Tregs in the liver of a CRC mouse model and reducing the infiltration of NK cells to inhibit the immune niche of the liver [209]. Consistently, the results of Gur et al. showed that Fusobacterium nucleatum adhering to various cancer cells inhibited NK cell cytotoxicity by binding to the NK cell inhibitory receptor TIGIT [210].
Although the beneficial effect of microbiota on NK cell activity still needs further exploration, it represents a new immunomodulatory mechanism.
NK cells as potential participants in tumour metastasis: interaction of NK cells with the metastatic niche
Metastasis is a multistage process that comprises three phases: dissemination, dormancy, and colonization. Metastasis is initiated and maintained by a subpopulation of cancer cells with a stem cell-like phenotype and immune evasion properties, called metastasis-initiating cells (MICs). During propagation and dormancy, MICs are in dynamic balance with host immunity. Once immune surveillance fails, metastasis and organ colonisation will occur [211]. The primary tumour and distant premetastatic sites form a metastasis-friendly microenvironment, termed the premetastatic niche (PMN), which has been recognised as an important factor in immune surveillance failure [212]. NK cells have long been characterised as powerful mediators of cell death that directly eliminate cancer cells and inhibit tumour metastasis. However, recent studies have shown that NK cells can exhibit a prometastatic state [213]. Understanding how PMN promote the prometastatic state of NK cells will aid in the refinement and optimization of NK cell antitumour strategies.
Hematogenous metastasis is the main route of metastasis for most tumours. Primary cancer cells invade and intravasate into new capillaries, shedding circulating tumour cells (CTCs), which travel from the circulation into a new host parenchyma to complete the metastasis of the primary tumour to regional and distal sites. In the circulation, cancer cells must overcome shear stress, oxidative stress, and immune cell attack [211]. PMN remodel the vascular state to aid CTC colonisation during this process. A single CTC in the intravasation cycle is often cleared by NK cells, while CTC clusters are protected from NK cell attack by platelet coats and platelet/neutrophil-associated clusters [214, 215]. This observation underscores the unique role of platelets in protecting cancer cells from NK cell attack, and a significant decrease in NK cell-mediated tumour cell survival in Galphaq (a G protein essential for platelet activation) deficient mice has also been observed [216]. Lo et al. reported the mechanism by which CTC cluster resistance to NK cell immunosurveillance in a follow-up study. The metastatic advantage of CTC clusters is associated with their alteration of cell adhesion and epithelial-mesenchymal properties [217].
Metastatic dormancy is the balance between cancer cell cycle arrest, attempted proliferation, and colonization. Dormant cancer cells adapt to niches and establish complex interactions with immune cells. Massagué summarised that dormant MIC metastasis necessitates not only overcoming systemic immunity of NK cells, resident immunity, and reactive stroma, but also adapting to the phenotype required for dormancy, which includes immune suppression adaptations such as perivascular niches, immune evasive dormancy, TGF-β inhibition, metabolic adaptation, and reactive stroma blockers [211]. It has been observed that MICs can initiate a dormancy programme themselves by autocrine signalling of DKK1 to inhibit WNT signalling and bring MICs into a quiescent state. Quiescent MICs downregulate the expression of NK cell-activating ligands to evade NK cell-mediated clearance [218]. In a latent MICs model, re-entry of latent MIC cells into a proliferative state triggered the expression of NKG2D, and latent MIC cells were cleared by NK cells. Similarly, in a NK cell depletion model, depletion of NK cells to allow progressive growth of metastatic lesions was observed [219]. Interestingly, NK cells can "chew off" a portion of the cell membrane carrying a surface molecule (such as PD-1) of the donor cell membrane from an antigen-presenting cell through trogocytosis, thus entering a dormant state and relieving the anticancer activity [220]. In addition, Correia et al. found in dormant disseminated tumour cells (DTCs) that decreased numbers of NK cells in the liver caused a large proportion of cancer cells to metastasize to the liver and that amplification of NK cells with IL-15 caused the cancer cells to enter a dormant state where metastasis was prevented [221].
Although the interaction between NK cells and the metastatic niche complicates the exploration of how cancer cells in dormancy evade NK cell-mediated cell death, the role of NK cells in maintaining tumour dormancy is gaining attention and represents a promising prospect for antimetastatic drug development.
Current therapeutic strategies for overcoming the TME
CAR-NK
The concept of chimeric antigen receptors (CAR) was pioneered by Gross and his colleagues in 1989. After decades of development, CAR-T cell therapy has made many breakthroughs, and the design of CAR has been gradually optimized. Compared with T cells, NK cells are more advantageous in the development of a CAR engineering platform due to their broad-spectrum antitumour activity, relative safety, broad accessibility, and allogeneic use [7]. Based on the unique advantages of CAR-NK technology, many preclinical and clinical trials have been conducted to evaluate the therapeutic value, safety, and manufacturing process of CAR-NK technology and to actively summarise the shortcomings and challenges of CAR-NK cell therapy.
Various strategies have been developed to enable CAR-NK cell therapy to deregulate the suppressive TME. In recent years, combinations of cytokines have been widely used to stimulate NK cell proliferation and improve persistence, such as the simultaneous expression of IL-15 and other molecules on CAR-NK cells [222]. CAR design targeting TME products (e.g., lactate, adenosine, ROS, etc.) and the hypoxic microenvironment represents an innovative CAR construction concept that may be a new weapon to enhance CAR-NK immunotherapy in solid tumours. For example, a genetic modification strategy that involves terminating the release of immunosuppressive metabolites (e.g., ROS) in solid tumours was used to enhance the resistance and efficacy of CAR-NK cells against solid tumours [223]. A CAR incorporating the oxygen-sensitive structural domain of HIF-1α (HIF-CAR) can generate CAR constructs responsive to hypoxic environments [224]. Depolarized or repolarized immunosuppressive cells such as CAFs, TAMs, and MDSCs have also shown convincing efficacy in preclinical models of CAR-T cells [225,226,227]. It is also an attractive option to enhance CAR-NK immunotherapy in the future.
NK cell engagers
Unlike CAR-NK cell therapy, which expresses CAR on NK cells to mediate the targeted killing of tumour cells, NK cell engagers (NKCEs) consist of a single-chain fragment variable (scFv) of antibodies against NK cell activation receptors (e.g., CD16, NKp30, NKp46, and NKG2D) and one (Bispecific killer engagers, BiKEs) scFv of antibodies against different tumour antigens [228]. This "two-pronged" strategy allows the effective binding of NK cells and cancer cells together, forming IS and conferring specific killing activity to NK cells [229]. The introduction of more scFvs or the insertion of IL-15 as a linker, assembled into tri-or even tetra-specific killer cell engagers (TriKEs and TetraKEs), further enhanced NK cell proliferation and survival [230]. In preclinical studies, NKCEs have been used effectively in CD33+ acute myeloid leukaemia [231] and myelodysplastic syndromes [232], epidermal growth factor receptor (EGFR) in multiple cancers [233], B cell maturation antigen (BCMA) in multiple myeloma [234], etc.
Although most NKCEs are currently in the preclinical stage and their safety and efficacy need further evaluation, it is undeniable that they are emerging as highly promising tumour therapeutic strategies and have the potential to overcome the suppressive TME due to their target specificity [235]. It has been observed that 161,533 TriKE exhibits excellent antitumour activity and promotes NK cell persistence in vivo [236]. Clinical trials applying NKCEs to a variety of hematologic malignancies have demonstrated potential antitumour effects (NCT01221571, NCT01221571) or are recruiting (NCT03214666, NCT04101331).
Immune checkpoint inhibitors
Antitumour immunity of NK cells is a "gambling" process of dynamic balance between NK cells and cancer cells, in which the complex interactions of multiple activating or inhibiting receptors or ligands form the basis of NK cell recognition and activation [237]. Cancer cells exploit NK cells' inhibitory receptors for immune escape, and immune checkpoint inhibitors (ICIs) reactivate NK cells by relieving this inhibition.
A variety of NK cell checkpoint receptors are becoming popular targets for tumour immunotherapy [238] and have demonstrated success in terms of safety, tolerability, and survival [239, 240]. However, the efficacy of immune checkpoints in the TME in patients with solid tumours needs further evaluation, and single checkpoint receptor blockade may not be sufficient to completely rescue NK cells within the TME, which express multiple immune checkpoint ligands [241]. Multiple combination strategies need to be tried to overcome the TME, such as ICIs combined with surgery, chemotherapy, radiotherapy, targeted therapy, and other therapies, which are gradually becoming a popular research direction.
Nanoparticles
Accumulated evidence has shown the great potential of nanoparticles in enhancing NK cell-mediated antitumour immunity, making it possible to unlock numerous innovative approaches to inhibit the microenvironment in the TME. For example, nanoscale liposomes and nanoemulsion system could be used to deliver TGF-β inhibitors and modify TME [132, 242]. Nanoparticle strategies have also been developed to improve the homing and infiltration properties of NK cells, such as liposomes loaded with TUSC2 or nanocomposite microspheres encapsulated with IFN-γ to induce a significant increase in NK cell infiltration [243, 244]. The application of an external magnetic field also helps to guide NK cells bound to magnetic nanoparticles into the tumour, and increase NK cell infiltration [245]. There are also cases of applying nanocarriers to silence NK cell inhibitory signals to release NK cell activity and using multi-targeted nano-junction platforms to promote NK cell recruitment and activation [246, 247].
Small molecules
Small molecules' ability to easily cross cell membranes to access intracellular targets makes them more permeable to the TME, giving them an inherent advantage in overcoming the TME. Zhong et al. reviewed the current application of small molecules in interrupting the hypoxic, acidic, and inflammatory environments in the TME as well as the aberrant ECM network, stating that small molecules can be an attractive strategy for targeting TME to enhance tumour therapy [248].
It has been proven that a variety of small molecules can regulate the antitumour function of NK cells by changing the balance of NK cell activation and inhibition signals or by participating in the expansion, activation, differentiation, and maturation of NK cells [249]. In conclusion, the application of NK cell immunotherapy using small molecules is worthy of anticipation and further research.
Outlooks and directions
In the previous chapters, we discussed the interaction between NK cells and specialised TME, which formed an intricate crosstalk network. Given that targeting TME-NK cell crosstalk represents a promising direction for tumour therapy, a number of questions have been raised.
First, higher-resolution methods are needed to characterise the heterogeneous subsets, spatial distribution, and functional status of NK cells in TME (Fig. 5). Single-cell RNA sequencing (scRNA-seq) makes it possible to characterise specific cell populations and cell subset states, and is a powerful tool for dissecting heterogeneous TME [250, 251]. An unanswered question, however, is why scRNA-seq does not fully map the spatial location of cells in involuted TME. The technology that combines scRNA-seq with spatial transcriptomics (ST) emerges as a solution to this problem [252]. ST can realise the spatial visualisation of transcriptome, and it is worth looking forward to applying it to the TME cell-to-cell crosstalk. To surmount the limitations of single omics analysis, scRNA-seq methods combined with multi-omics (transcriptomics, proteomics, epigenomics, and metabolomics) methods were developed [253]. Integrative analysis of single-cell multi-omics data reveals cellular heterogeneity in the TME across multiple molecular dimensions, providing more novel insights into the identification of specific cell subsets, molecular features, and underlying mechanisms that mediate changes in cellular functional state.
Directions and outlooks for overcoming inhibitory microenvironments. ST, Spatial transcriptomics. Adapted from “Components of Tissue Engineering”, by BioRender.com (2022). Retrieved from https://app.biorender.com/biorender-templates
Second, in vitro research models need to be established to simulate and decipher specific interactions between specializsed TME and NK cells (Fig. 5). Microfluidic in vitro models have been shown to be a simple alternative tool for studying cancer-NK cell interactions, with the benefits of real-time monitoring of NK cell infiltration and simulating the direct contact between NK cells and cancer cells [254, 255]. Three-dimensional (3D) organoids and 3D bioprinting have advantages in displaying multicellular structures and complex positions of cells in the TME and have been developed for 3D reconstruction of complex microenvironments [200, 256, 257]. The above 3D bionic system allows for the in vitro reconstruct for the complex microenvironment, which is a dynamic crosstalk with NK cells.
Last but not least, it is necessary to visualise the functional status of NK cells at different clinical stages and under different experimental characteristics (Fig. 5). In the panoramic analysis of TME and the characterization of cell spatial location, traditional protein techniques, including immunohistochemistry and immunofluorescence, have exposed some limitations. Spatial protein techniques, including multiplex immunohistochemistry (mIHC), multiplex immunofluorescence (mIF), cytometry by time of flight (CyTOF), and multiplex digital spatial profiling (DSP), allow for the simultaneous assessment of multiple protein markers and contribute to the acquisition of a more comprehensive microenvironment map [258]. Live or intravital imaging techniques, such as confocal microscopy, two-photon microscopy, and fluorescence-based biosensors, have shown attractive potential in subcellular dynamic tracking [259, 260], allowing 3D monitoring and visualisation of NK cell interactions in the TME.
Conclusion
NK cells naturally recognise self and non-self, and kill cancer cells through multiple lytic pathways, representing a powerful tool for cancer immunotherapy. Recognizing that TME is often an important driver of NK cell dysfunction as well as the immune escape of cancer cells, a deep understanding of the logic of TME-NK cell crosstalk is necessary to propose new therapeutic strategies.
Here, we systematically discuss TME-mediated changes in NK cell functions from different classifications of TME based on multiple perspectives. Despite the progress made, our characterization of the functional state of NK cells in TME is poorly understood or even lacking. It is difficult to define whether NK cells are friends or foes. NK cells cooperate with or fight against other participants in the TME, and together they form a complex TME ecosystem. Just as NK cells can shift from a tumour killer to a pro-tumour metastatic state, a more plausible explanation is that NK cells switch roles at different stages of cancer. Despite representing therapeutic potential, lack of evidence from clinical trials remains the greatest challenge in targeting TME-NK cell crosstalk.
Availability of data and materials
Not applicable.
Abbreviations
- ECM:
-
Extracellular matrix
- TME:
-
Tumour microenvironment
- CAFs:
-
Cancer-associated fibroblasts
- MSCs:
-
Mesenchymal stem cells
- CSCs:
-
Cancer stem cells
- NK:
-
Natural killer
- ADCC:
-
Antibody-dependent cell-mediated cytotoxicity
- FasL:
-
Fas ligand
- TRAIL:
-
TNF-related apoptosis-inducing ligand
- KIRs:
-
Killer immunoglobulin-like receptors
- LIRs/ILTs:
-
Leukocyte Ig-like receptors
- MHC:
-
Major histocompatibility complex
- NCR:
-
Natural cytotoxic receptors
- DNAM-1:
-
DNAX accessory molecule 1
- NKG2D:
-
Natural killer group 2 member D
- GJ:
-
Gap junctions
- Cx:
-
Connexins
- ATP:
-
Adenosine triphosphate
- cAMP:
-
Cyclic adenosine 3',5'-monophosphate
- IP3:
-
Inositol triphosphate
- IS:
-
Immune synapses
- dNK:
-
Decidual NK
- TDEs:
-
Tumour-derived exosomes
- NK-Exos:
-
NK cell-derived exosomes
- IL:
-
Interleukin
- BC:
-
Breast cancer
- cDC1:
-
Conventional type 1 dendritic cells
- IFN-γ:
-
Interferon-γ
- Th1:
-
T helper cell type 1
- HNC:
-
Head and neck cancer
- MICA/MICB:
-
MHC class I chain-related protein A/B
- Tregs:
-
Regulatory T cells
- NSCLC:
-
Non-small-cell lung cancer
- A2AR:
-
A2A adenosine receptor
- CTLA-4:
-
Cytotoxic T-lymphocyte antigen 4
- PD-1:
-
Programmed death-1
- CCR4:
-
C-C chemokine receptor type 4
- STAT3:
-
Signal transducer and activator of transcription 3
- DCs:
-
Dendritic cells
- FLT3L:
-
FMS-related tyrosine kinase 3 ligand
- cDC:
-
Conventional DC
- CXCL9/10:
-
Chemokine (C-X-C motif) ligand 9/10
- TNF-α:
-
Tumour necrosis factor α
- PGE2:
-
Prostaglandin E2
- EVs:
-
Extracellular vesicles
- ROS:
-
Reactive oxygen species
- NETs:
-
Neutrophil extracellular traps
- TAMs:
-
Tumour-associated macrophages
- pTA-NK:
-
Peripheral blood tumour associated circulating NK
- pCAFs:
-
Cancer-promoting CAFs
- rCAFs:
-
Cancer-restraining CAFs
- IDO:
-
Indoleamine 2,3-dioxygenase
- PVR/CD155:
-
Poliovirus receptor
- HLA:
-
Human leukocyte antigen
- HIFs:
-
Hypoxia induced transcription factors
- OXPHOS:
-
Oxidative phosphorylation
- TCA:
-
Tricarboxylic acid
- MCT:
-
Monocarboxylate transporters
- MDSCs:
-
Myeloid-derived suppressor cells
- EE:
-
Environmental enrichment
- BDNF:
-
Brain-derived neurotrophic factor
- TGF:
-
Transforming growth factor
- SNS:
-
Sympathetic nervous system
- HPA:
-
Hypothalamic-pituitary-adrenal axis
- CNS:
-
Central nervous system
- α-syn:
-
Alpha-synuclein
- GM-CSF:
-
Granulocyte-macrophage colony stimulating factor
- SHP-1:
-
SH2-domain-containing protein tyrosine phosphatase-1
- ARF:
-
Actomyosin retrograde flow
- CRC:
-
Colorectal cancer
- Hep:
-
Helicobacter hepaticus
- MICs:
-
Metastasis initiating cells
- PMN:
-
Pre-metastatic niche
- CTC:
-
Circulating tumour cells
- DTCs:
-
Disseminated tumour cells
- CAR:
-
Chimeric antigen receptors
- NKCE:
-
NK cell engagers
- scFv:
-
Single-chain fragment variable
- BiKEs/TriKEs/TetraKEs:
-
Bispecific/trispecific/tetraspecific killer engagers
- EGFR:
-
Epidermal growth factor receptor
- BCMA:
-
B cell maturation antigen
- ICIs:
-
Immune checkpoint inhibitors
- scRNA-seq:
-
Single-cell RNA sequencing
- ST:
-
Spatial transcriptomics
- 3D:
-
Three-dimensional
- mIHC:
-
Multiplex immunohistochemistry
- mIF:
-
Multiplex immunofluorescence
- CyTOF:
-
Cytometry by time of flight
- DSP:
-
Multiplex digital spatial profiling
References
**ao Y, Yu D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther. 2021;221:107753.
Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–37.
Bejarano L, Jordāo MJC, Joyce JA. Therapeutic Targeting of the Tumor Microenvironment. Cancer Discov. 2021;11:933–59.
Li W-N, Zhang S-J, Feng J-Q, ** W-L. Repurposing Vitamin C for Cancer Treatment: Focus on Targeting the Tumor Microenvironment. Cancers. 2022;14:2608.
** MZ, ** WL. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct Target Ther. 2020;5:166.
Wu SY, Fu T, Jiang YZ, Shao ZM. Natural killer cells in cancer biology and therapy. Mol Cancer. 2020;19:120.
Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, Nassif Kerbauy L, Overman B, Thall P, Kaplan M, et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N Engl J Med. 2020;382:545–53.
Wang J, Matosevic S. Functional and metabolic targeting of natural killer cells to solid tumors. Cell Oncol (Dordr). 2020;43:577–600.
Amand M, Iserentant G, Poli A, Sleiman M, Fievez V, Sanchez IP, Sauvageot N, Michel T, Aouali N, Janji B, et al. Human CD56dimCD16dim Cells As an Individualized Natural Killer Cell Subset. Front Immunol. 2017;8:699.
Fortes-Andrade T, Almeida JS, Sousa LM, Santos-Rosa M, Freitas-Tavares P, Casanova JM, Rodrigues-Santos P. The Role of Natural Killer Cells in Soft Tissue Sarcoma: Prospects for Immunotherapy. Cancers (Basel). 2021;13:3685.
Bruno A, Ferlazzo G, Albini A, Noonan DM. A think tank of TINK/TANKs: tumor-infiltrating/tumor-associated natural killer cells in tumor progression and angiogenesis. J Natl Cancer Inst. 2014;106:200.
Crinier A, Narni-Mancinelli E, Ugolini S, Vivier E. SnapShot: Natural Killer Cells. Cell. 2020;180(1280–1280):e1281.
Romagnani C, Juelke K, Falco M, Morandi B, D’Agostino A, Costa R, Ratto G, Forte G, Carrega P, Lui G, et al. CD56brightCD16- killer Ig-like receptor- NK cells display longer telomeres and acquire features of CD56dim NK cells upon activation. J Immunol. 2007;178:4947–55.
Bradley M, Zeytun A, Rafi-Janajreh A, Nagarkatti PS, Nagarkatti M. Role of spontaneous and interleukin-2-induced natural killer cell activity in the cytotoxicity and rejection of Fas+ and Fas- tumor cells. Blood. 1998;92:4248–55.
Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol. 2013;31:227–58.
Sullivan LC, Berry R, Sosnin N, Widjaja JM, Deuss FA, Balaji GR, LaGruta NL, Mirams M, Trapani JA, Rossjohn J, et al. Recognition of the Major Histocompatibility Complex (MHC) Class Ib Molecule H2–Q10 by the Natural Killer Cell Receptor Ly49C. J Biol Chem. 2016;291:18740–52.
Boudreau JE, Liu XR, Zhao Z, Zhang A, Shultz LD, Greiner DL, Dupont B, Hsu KC. Cell-Extrinsic MHC Class I Molecule Engagement Augments Human NK Cell Education Programmed by Cell-Intrinsic MHC Class I. Immunity. 2016;45:280–91.
Boudreau JE, Hsu KC. Natural Killer Cell Education and the Response to Infection and Cancer Therapy: Stay Tuned. Trends Immunol. 2018;39:222–39.
Rezvani K, Rouce R, Liu E, Shpall E. Engineering Natural Killer Cells for Cancer Immunotherapy. Mol Ther. 2017;25:1769–81.
Delvaeye T, Vandenabeele P, Bultynck G, Leybaert L, Krysko DV. Therapeutic Targeting of Connexin Channels: New Views and Challenges. Trends Mol Med. 2018;24:1036–53.
Tittarelli A, Mendoza-Naranjo A, Farías M, Guerrero I, Ihara F, Wennerberg E, Riquelme S, Gleisner A, Kalergis A, Lundqvist A, et al. Gap junction intercellular communications regulate NK cell activation and modulate NK cytotoxic capacity. J Immunol. 2014;192:1313–9.
Rousalova I, Krepela E. Granzyme B-induced apoptosis in cancer cells and its regulation (review). Int J Oncol. 2010;37:1361–78.
Tittarelli A, Janji B, Van Moer K, Noman MZ, Chouaib S. The Selective Degradation of Synaptic Connexin 43 Protein by Hypoxia-induced Autophagy Impairs Natural Killer Cell-mediated Tumor Cell Killing. J Biol Chem. 2015;290:23670–9.
Chauveau A, Aucher A, Eissmann P, Vivier E, Davis DM. Membrane nanotubes facilitate long-distance interactions between natural killer cells and target cells. Proc Natl Acad Sci U S A. 2010;107:5545–50.
Crespo ÂC, Mulik S, Dotiwala F, Ansara JA, Sen Santara S, Ingersoll K, Ovies C, Junqueira C, Tilburgs T, Strominger JL, Lieberman J. Decidual NK Cells Transfer Granulysin to Selectively Kill Bacteria in Trophoblasts. Cell. 2020;182:1125-1139.e1118.
Comerci CJ, Mace EM, Banerjee PP, Orange JS. CD2 promotes human natural killer cell membrane nanotube formation. PLoS ONE. 2012;7:e47664.
van Niel G, Carter DRF, Clayton A, Lambert DW, Raposo G, Vader P. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat Rev Mol Cell Biol. 2022;23:369–82.
Batista IA, Quintas ST, Melo SA. The Interplay of Exosomes and NK Cells in Cancer Biology. Cancers (Basel). 2021;13:473.
Hosseini R, Sarvnaz H, Arabpour M, Ramshe SM, Asef-Kabiri L, Yousefi H, Akbari ME, Eskandari N. Cancer exosomes and natural killer cells dysfunction: biological roles, clinical significance and implications for immunotherapy. Mol Cancer. 2022;21:15.
Lv LH, Wan YL, Lin Y, Zhang W, Yang M, Li GL, Lin HM, Shang CZ, Chen YJ, Min J. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J Biol Chem. 2012;287:15874–85.
Borrelli C, Ricci B, Vulpis E, Fionda C, Ricciardi MR, Petrucci MT, Masuelli L, Peri A, Cippitelli M, Zingoni A, et al. Drug-Induced Senescent Multiple Myeloma Cells Elicit NK Cell Proliferation by Direct or Exosome-Mediated IL15 Trans-Presentation. Cancer Immunol Res. 2018;6:860–9.
Jong AY, Wu CH, Li J, Sun J, Fabbri M, Wayne AS, Seeger RC. Large-scale isolation and cytotoxicity of extracellular vesicles derived from activated human natural killer cells. J Extracell Vesicles. 2017;6:1294368.
Zhu L, Kalimuthu S, Gangadaran P, Oh JM, Lee HW, Baek SH, Jeong SY, Lee SW, Lee J, Ahn BC. Exosomes Derived From Natural Killer Cells Exert Therapeutic Effect in Melanoma. Theranostics. 2017;7:2732–45.
Pace AL Di, Tumino N, Besi F, Alicata C, Conti LA, Munari E, Maggi E, Vacca P, Moretta L. Characterization of human nk cell-derived exosomes: role of dnam1 receptor in exosome-mediated cytotoxicity against tumor. Cancers (Basel). 2020;12:661.
Neviani P, Wise PM, Murtadha M, Liu CW, Wu CH, Jong AY, Seeger RC, Fabbri M. Natural Killer-Derived Exosomal miR-186 Inhibits Neuroblastoma Growth and Immune Escape Mechanisms. Cancer Res. 2019;79:1151–64.
Jiang Y, Jiang H, Wang K, Liu C, Man X, Fu Q. Hypoxia enhances the production and antitumor effect of exosomes derived from natural killer cells. Ann Transl Med. 2021;9:473.
Kaban K, Hinterleitner C, Zhou Y, Salva E, Kantarci AG, Salih HR, Märklin M. Therapeutic Silencing of BCL-2 Using NK Cell-derived exosomes as a novel therapeutic approach in breast cancer. Cancers (Basel). 2021;13:2397.
Wang G, Hu W, Chen H, Shou X, Ye T, Xu Y. Cocktail Strategy Based on NK Cell-Derived Exosomes and Their Biomimetic Nanoparticles for Dual Tumor Therapy. Cancers (Basel). 2019;11:1560.
Huntington ND, Cursons J, Rautela J. The cancer-natural killer cell immunity cycle. Nat Rev Cancer. 2020;20:437–54.
Myers JA, Miller JS. Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol. 2021;18:85–100.
Agaugué S, Marcenaro E, Ferranti B, Moretta L, Moretta A. Human natural killer cells exposed to IL-2, IL-12, IL-18, or IL-4 differently modulate priming of naive T cells by monocyte-derived dendritic cells. Blood. 2008;112:1776–83.
Zingoni A, Sornasse T, Cocks BG, Tanaka Y, Santoni A, Lanier LL. Cross-talk between activated human NK cells and CD4+ T cells via OX40-OX40 ligand interactions. J Immunol. 2004;173:3716–24.
Horowitz A, Newman KC, Evans JH, Korbel DS, Davis DM, Riley EM. Cross-talk between T cells and NK cells generates rapid effector responses to Plasmodium falciparum-infected erythrocytes. J Immunol. 2010;184:6043–52.
Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, Caligiuri MA. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–7.
Ardolino M, Zingoni A, Cerboni C, Cecere F, Soriani A, Iannitto ML, Santoni A. DNAM-1 ligand expression on Ag-stimulated T lymphocytes is mediated by ROS-dependent activation of DNA-damage response: relevance for NK-T cell interaction. Blood. 2011;117:4778–86.
Mocikat R, Braumüller H, Gumy A, Egeter O, Ziegler H, Reusch U, Bubeck A, Louis J, Mailhammer R, Riethmüller G, et al. Natural killer cells activated by MHC class I(low) targets prime dendritic cells to induce protective CD8 T cell responses. Immunity. 2003;19:561–9.
Adam C, King S, Allgeier T, Braumüller H, Lüking C, Mysliwietz J, Kriegeskorte A, Busch DH, Röcken M, Mocikat R. DC-NK cell cross talk as a novel CD4+ T-cell-independent pathway for antitumor CTL induction. Blood. 2005;106:338–44.
Peng P, Lou Y, Wang S, Wang J, Zhang Z, Du P, Zheng J, Liu P, Xu LX. Activated NK cells reprogram MDSCs via NKG2D-NKG2DL and IFN-γ to modulate antitumor T-cell response after cryo-thermal therapy. J Immunother Cancer. 2022;10:e005769.
Malhotra A, Shanker A. NK cells: immune cross-talk and therapeutic implications. Immunotherapy. 2011;3:1143–66.
Sconocchia G, Eppenberger S, Spagnoli GC, Tornillo L, Droeser R, Caratelli S, Ferrelli F, Coppola A, Arriga R, Lauro D, et al. NK cells and T cells cooperate during the clinical course of colorectal cancer. Oncoimmunology. 2014;3:e952197.
Shanker A, Verdeil G, Buferne M, Inderberg-Suso EM, Puthier D, Joly F, Nguyen C, Leserman L, Auphan-Anezin N, Schmitt-Verhulst AM. CD8 T cell help for innate antitumor immunity. J Immunol. 2007;179:6651–62.
Srivastava RM, Lee SC, Andrade Filho PA, Lord CA, Jie HB, Davidson HC, Lopez-Albaitero A, Gibson SP, Gooding WE, Ferrone S, Ferris RL. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin Cancer Res. 2013;19:1858–72.
Badrinath S, Dellacherie MO, Li A, Zheng S, Zhang X, Sobral M, Pyrdol JW, Smith KL, Lu Y, Haag S, et al. A vaccine targeting resistant tumours by dual T cell plus NK cell attack. Nat. 2022;606:992–8.
Wolf NK, Blaj C, Picton LK, Snyder G, Zhang L, Nicolai CJ, Ndubaku CO, McWhirter SM, Garcia KC, Raulet DH. Synergy of a STING agonist and an IL-2 superkine in cancer immunotherapy against MHC I-deficient and MHC I+ tumors. Proc Natl Acad Sci U S A. 2022;119:e2200568119.
Bozward AG, Warricker F, Oo YH, Khakoo SI. Natural Killer Cells and Regulatory T Cells Cross Talk in Hepatocellular Carcinoma: Exploring Therapeutic Options for the Next Decade. Front Immunol. 2021;12:643310.
Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–8.
Trzonkowski P, Szmit E, Myśliwska J, Dobyszuk A, Myśliwski A. CD4+CD25+ T regulatory cells inhibit cytotoxic activity of T CD8+ and NK lymphocytes in the direct cell-to-cell interaction. Clin Immunol. 2004;112:258–67.
Ghiringhelli F, Ménard C, Terme M, Flament C, Taieb J, Chaput N, Puig PE, Novault S, Escudier B, Vivier E, et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med. 2005;202:1075–85.
Liu W, Wei X, Li L, Wu X, Yan J, Yang H, Song F. CCR4 mediated chemotaxis of regulatory T cells suppress the activation of T cells and NK cells via TGF-β pathway in human non-small cell lung cancer. Biochem Biophys Res Commun. 2017;488:196–203.
Smyth MJ, Teng MW, Swann J, Kyparissoudis K, Godfrey DI, Hayakawa Y. CD4+CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J Immunol. 2006;176:1582–7.
Sarhan D, Hippen KL, Lemire A, Hying S, Luo X, Lenvik T, Curtsinger J, Davis Z, Zhang B, Cooley S, et al. Adaptive NK Cells Resist Regulatory T-cell Suppression Driven by IL37. Cancer Immunol Res. 2018;6:766–75.
Littwitz-Salomon E, Akhmetzyanova I, Vallet C, Francois S, Dittmer U, Gibbert K. Activated regulatory T cells suppress effector NK cell responses by an IL-2-mediated mechanism during an acute retroviral infection. Retrovirology. 2015;12:66.
Dean JW, Peters LD, Fuhrman CA, Seay HR, Posgai AL, Stimpson SE, Brusko MA, Perry DJ, Yeh WI, Newby BN, et al. Innate inflammation drives NK cell activation to impair Treg activity. J Autoimmun. 2020;108:102417.
Jie HB, Schuler PJ, Lee SC, Srivastava RM, Argiris A, Ferrone S, Whiteside TL, Ferris RL. CTLA-4+ Regulatory T Cells Increased in Cetuximab-Treated Head and Neck Cancer Patients Suppress NK Cell Cytotoxicity and Correlate with Poor Prognosis. Cancer Res. 2015;75:2200–10.
Doi T, Muro K, Ishii H, Kato T, Tsushima T, Takenoyama M, Oizumi S, Gemmoto K, Suna H, Enokitani K, et al. A Phase I Study of the Anti-CC Chemokine Receptor 4 Antibody, Mogamulizumab, in Combination with Nivolumab in Patients with Advanced or Metastatic Solid Tumors. Clin Cancer Res. 2019;25:6614–22.
Piper M, Van Court B, Mueller A, Watanabe S, Bickett T, Bhatia S, Darragh LB, Mayeda M, Nguyen D, Gadwa J, et al. Targeting Treg-Expressed STAT3 Enhances NK-Mediated Surveillance of Metastasis and Improves Therapeutic Response in Pancreatic Adenocarcinoma. Clin Cancer Res. 2022;28:1013–26.
Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020;20:7–24.
Fernandez NC, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, Perricaudet M, Tursz T, Maraskovsky E, Zitvogel L. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405–11.
Bosch NC, Martin LM, Voskens CJ, Berking C, Seliger B, Schuler G, Schaft N, Dörrie J. A Chimeric IL-15/IL-15Rα Molecule Expressed on NFκB-Activated Dendritic Cells Supports Their Capability to Activate Natural Killer Cells. Int J Mol Sci. 2021;22:10227.
Sköld AE, Mathan TSM, van Beek JJP, Flórez-Grau G, van den Beukel MD, Sittig SP, Wimmers F, Bakdash G, Schreibelt G, de Vries IJM. Naturally produced type I IFNs enhance human myeloid dendritic cell maturation and IL-12p70 production and mediate elevated effector functions in innate and adaptive immune cells. Cancer Immunol Immunother. 2018;67:1425–36.
Bottcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, Rogers NC, Sahai E, Zelenay S. Reis e Sousa C: NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell. 2018;172(1022–1037):e1014.
Allen F, Bobanga ID, Rauhe P, Barkauskas D, Teich N, Tong C, Myers J, Huang AY. CCL3 augments tumor rejection and enhances CD8+ T cell infiltration through NK and CD103+ dendritic cell recruitment via IFNγ. Oncoimmunology. 2018;7:e1393598.
Holmes TD, Wilson EB, Black EV, Benest AV, Vaz C, Tan B, Tanavde VM, Cook GP. Licensed human natural killer cells aid dendritic cell maturation via TNFSF14/LIGHT. Proc Natl Acad Sci U S A. 2014;111:E5688-5696.
Salmon H, Idoyaga J, Rahman A, Leboeuf M, Remark R, Jordan S, Casanova-Acebes M, Khudoynazarova M, Agudo J, Tung N, et al. Expansion and Activation of CD103(+) Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity. 2016;44:924–38.
Barry KC, Hsu J, Broz ML, Cueto FJ, Binnewies M, Combes AJ, Nelson AE, Loo K, Kumar R, Rosenblum MD, et al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat Med. 2018;24:1178–91.
Cazzetta V, Franzese S, Carenza C, Della Bella S, Mikulak J, Mavilio D. Natural Killer-Dendritic Cell Interactions in Liver Cancer: Implications for Immunotherapy. Cancers (Basel). 2021;13:2184.
Flommersfeld S, Bottcher JP, Ersching J, Flossdorf M, Meiser P, Pachmayr LO, Leube J, Hensel I, Jarosch S, Zhang Q, et al. Fate map** of single NK cells identifies a type 1 innate lymphoid-like lineage that bridges innate and adaptive recognition of viral infection. Immunity. 2021;54(2288–2304):e2287.
Uzhachenko RV, Shanker A. CD8(+) T Lymphocyte and NK Cell Network: Circuitry in the Cytotoxic Domain of Immunity. Front Immunol. 1906;2019:10.
Tang M, Diao J, Gu H, Khatri I, Zhao J, Cattral MS. Toll-like Receptor 2 Activation Promotes Tumor Dendritic Cell Dysfunction by Regulating IL-6 and IL-10 Receptor Signaling. Cell Rep. 2015;13:2851–64.
Russick J, Joubert PE, Gillard-Bocquet M, Torset C, Meylan M, Petitprez F, Dragon-Durey MA, Marmier S, Varthaman A, Josseaume N, et al. Natural killer cells in the human lung tumor microenvironment display immune inhibitory functions. J Immunother Cancer. 2020;8:e001054.
Perez-Martinez A, Iyengar R, Gan K, Chotsampancharoen T, Rooney B, Holladay M, Ramírez M, Leung W. Blood dendritic cells suppress NK cell function and increase the risk of leukemia relapse after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17:598–607.
Cancel JC, Crozat K, Dalod M, Mattiuz R. Are Conventional Type 1 Dendritic Cells Critical for Protective Antitumor Immunity and How? Front Immunol. 2019;10:9.
Wang S, Wu Q, Chen T, Su R, Pan C, Qian J, Huang H, Yin S, **e H, Zhou L, Zheng S. Blocking CD47 promotes antitumour immunity through CD103+ dendritic cell-NK cell axis in murine hepatocellular carcinoma model. J Hepatol. 2022;77:467–78.
Singh R, Gupta U, Srivastava P, Paladhi A, Sk UH, Hira SK, Manna PP. γc cytokine-aided crosstalk between dendritic cells and natural killer cells together with doxorubicin induces a healer response in experimental lymphoma by downregulating FOXP3 and programmed cell death protein 1. Cytotherapy. 2022;24:1232–44.
Schmidt A, Weber OF. In memoriam of Rudolf virchow: a historical retrospective including aspects of inflammation, infection and neoplasia. Contrib Microbiol. 2006;13:1–15.
Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–9.
Carnevale S, Ghasemi S, Rigatelli A, Jaillon S. The complexity of neutrophils in health and disease: Focus on cancer. Semin Immunol. 2020;48:101409.
Costantini C, Calzetti F, Perbellini O, Micheletti A, Scarponi C, Lonardi S, Pelletier M, Schakel K, Pizzolo G, Facchetti F, et al. Human neutrophils interact with both 6-sulfo LacNAc+ DC and NK cells to amplify NK-derived IFN{gamma}: role of CD18, ICAM-1, and ICAM-3. Blood. 2011;117:1677–86.
Wculek SK, Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature. 2015;528:413–7.
Cui C, Chakraborty K, Tang XA, Zhou G, Schoenfelt KQ, Becker KM, Hoffman A, Chang YF, Blank A, Reardon CA, et al. Neutrophil elastase selectively kills cancer cells and attenuates tumorigenesis. Cell. 2021;184:3163-3177.e3121.
Li P, Lu M, Shi J, Hua L, Gong Z, Li Q, Shultz LD, Ren G. Dual roles of neutrophils in metastatic colonization are governed by the host NK cell status. Nat Commun. 2020;11:4387.
Ogura K, Sato-Matsushita M, Yamamoto S, Hori T, Sasahara M, Iwakura Y, Saiki I, Tahara H, Hayakawa Y. NK Cells Control Tumor-Promoting Function of Neutrophils in Mice. Cancer Immunol Res. 2018;6:348–57.
Scapini P, Cassatella MA. Social networking of human neutrophils within the immune system. Blood. 2014;124:710–9.
Sun R, **ong Y, Liu H, Gao C, Su L, Weng J, Yuan X, Zhang D, Feng J. Tumor-associated neutrophils suppress antitumor immunity of NK cells through the PD-L1/PD-1 axis. Transl Oncol. 2020;13:100825.
Valayer A, Brea D, Lajoie L, Avezard L, Combes-Soia L, Labas V, Korkmaz B, Thibault G, Baranek T, Si-Tahar M. Neutrophils can disarm NK cell response through cleavage of NKp46. J Leukoc Biol. 2017;101:253–9.
Romero AI, Thorén FB, Brune M, Hellstrand K. NKp46 and NKG2D receptor expression in NK cells with CD56dim and CD56bright phenotype: regulation by histamine and reactive oxygen species. Br J Haematol. 2006;132:91–8.
Pliyev BK, Kalintseva MV, Abdulaeva SV, Yarygin KN, Savchenko VG. Neutrophil microparticles modulate cytokine production by natural killer cells. Cytokine. 2014;65:126–9.
Spiegel A, Brooks MW, Houshyar S, Reinhardt F, Ardolino M, Fessler E, Chen MB, Krall JA, DeCock J, Zervantonakis IK, et al. Neutrophils Suppress Intraluminal NK Cell-Mediated Tumor Cell Clearance and Enhance Extravasation of Disseminated Carcinoma Cells. Cancer Discov. 2016;6:630–49.
Costantini C, Micheletti A, Calzetti F, Perbellini O, Pizzolo G, Cassatella MA. Neutrophil activation and survival are modulated by interaction with NK cells. Int Immunol. 2010;22:827–38.
Oberlies J, Watzl C, Giese T, Luckner C, Kropf P, Muller I, Ho AD, Munder M. Regulation of NK cell function by human granulocyte arginase. J Immunol. 2009;182:5259–67.
Palano MT, Gallazzi M, Cucchiara M, De Lerma Barbaro A, Gallo D, Bassani B, Bruno A, Mortara L. Neutrophil and Natural Killer Cell Interactions in Cancers: Dangerous Liaisons Instructing Immunosuppression and Angiogenesis. Vaccines (Basel). 2021;9:1488.
Chan L, Mehrani Y, Wood GA, Bridle BW, Karimi K. Dendritic Cell-Based Vaccines Recruit Neutrophils to the Local Draining Lymph Nodes to Prime Natural Killer Cell Responses. Cells. 2022;12:121.
Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5.
Zhang Y, Chandra V, Riquelme Sanchez E, Dutta P, Quesada PR, Rakoski A, Zoltan M, Arora N, Baydogan S, Horne W, et al. Interleukin-17-induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. J Exp Med. 2020;217:e20190354.
Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, Ferri L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013;123:3446–58.
Teijeira A, Garasa S, Gato M, Alfaro C, Migueliz I, Cirella A, de Andrea C, Ochoa MC, Otano I, Etxeberria I, et al. CXCR1 and CXCR2 Chemokine Receptor Agonists Produced by Tumors Induce Neutrophil Extracellular Traps that Interfere with Immune Cytotoxicity. Immunity. 2020;52(856–871):e858.
Cassetta L, Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov. 2018;17:887–904.
Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14:399–416.
DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19:369–82.
Li C, Xu X, Wei S, Jiang P, Xue L, Wang J. Tumor-associated macrophages: potential therapeutic strategies and future prospects in cancer J Immunother. Cancer. 2021;9:e001341.
Zhou J, Zhang S, Guo C. Crosstalk between macrophages and natural killer cells in the tumor microenvironment. Int Immunopharmacol. 2021;101:108374.
Gaggero S, Witt K, Carlsten M, Mitra S. Cytokines Orchestrating the Natural Killer-Myeloid Cell Crosstalk in the Tumor Microenvironment: Implications for Natural Killer Cell-Based Cancer Immunotherapy. Front Immunol. 2020;11:621225.
Castriconi R, Cantoni C, Della Chiesa M, Vitale M, Marcenaro E, Conte R, Biassoni R, Bottino C, Moretta L, Moretta A. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci U S A. 2003;100:4120–5.
Regis S, Caliendo F, Dondero A, Casu B, Romano F, Loiacono F, Moretta A, Bottino C, Castriconi R. TGF-β1 Downregulates the Expression of CX(3)CR1 by Inducing miR-27a-5p in Primary Human NK Cells. Front Immunol. 2017;8:868.
Nuñez SY, Ziblat A, Secchiari F, Torres NI, Sierra JM, Raffo Iraolagoitia XL, Araya RE, Domaica CI, Fuertes MB, Zwirner NW. Human M2 Macrophages Limit NK Cell Effector Functions through Secretion of TGF-β and Engagement of CD85j. J Immunol. 2018;200:1008–15.
Gao Y, Souza-Fonseca-Guimaraes F, Bald T, Ng SS, Young A, Ngiow SF, Rautela J, Straube J, Waddell N, Blake SJ, et al. Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nat Immunol. 2017;18:1004–15.
Mannino MH, Zhu Z, **ao H, Bai Q, Wakefield MR, Fang Y. The paradoxical role of IL-10 in immunity and cancer. Cancer Lett. 2015;367:103–7.
Park JY, Lee SH, Yoon SR, Park YJ, Jung H, Kim TD, Choi I. IL-15-induced IL-10 increases the cytolytic activity of human natural killer cells. Mol Cells. 2011;32:265–72.
Cai G, Kastelein RA, Hunter CA. IL-10 enhances NK cell proliferation, cytotoxicity and production of IFN-gamma when combined with IL-18. Eur J Immunol. 1999;29:2658–65.
O’Sullivan T, Saddawi-Konefka R, Vermi W, Koebel CM, Arthur C, White JM, Uppaluri R, Andrews DM, Ngiow SF, Teng MW, et al. Cancer immunoediting by the innate immune system in the absence of adaptive immunity. J Exp Med. 2012;209:1869–82.
Gallazzi M, Baci D, Mortara L, Bosi A, Buono G, Naselli A, Guarneri A, Deho F, Capogrosso P, Albini A, et al. Prostate Cancer Peripheral Blood NK Cells Show Enhanced CD9, CD49a, CXCR4, CXCL8, MMP-9 Production and Secrete Monocyte-Recruiting and Polarizing Factors. Front Immunol. 2020;11:586126.
Eisinger S, Sarhan D, Boura VF, Ibarlucea-Benitez I, Tyystjärvi S, Oliynyk G, Arsenian-Henriksson M, Lane D, Wikström SL, Kiessling R, et al. Targeting a scavenger receptor on tumor-associated macrophages activates tumor cell killing by natural killer cells. Proc Natl Acad Sci U S A. 2020;117:32005–16.
Wu F, Yang J, Liu J, Wang Y, Mu J, Zeng Q, Deng S, Zhou H. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct Target Ther. 2021;6:218.
Mao X, Xu J, Wang W, Liang C, Hua J, Liu J, Zhang B, Meng Q, Yu X, Shi S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer. 2021;20:131.
Kanzaki R, Pietras K. Heterogeneity of cancer-associated fibroblasts: Opportunities for precision medicine. Cancer Sci. 2020;111:2708–17.
Li T, Yang Y, Hua X, Wang G, Liu W, Jia C, Tai Y, Zhang Q, Chen G. Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer Lett. 2012;318:154–61.
Inoue T, Adachi K, Kawana K, Taguchi A, Nagamatsu T, Fujimoto A, Tomio K, Yamashita A, Eguchi S, Nishida H, et al. Cancer-associated fibroblast suppresses killing activity of natural killer cells through downregulation of poliovirus receptor (PVR/CD155), a ligand of activating NK receptor. Int J Oncol. 2016;49:1297–304.
Zhang R, Qi F, Zhao F, Li G, Shao S, Zhang X, Yuan L, Feng Y. Cancer-associated fibroblasts enhance tumor-associated macrophages enrichment and suppress NK cells function in colorectal cancer. Cell Death Dis. 2019;10:273.
Trotta R, Dal Col J, Yu J, Ciarlariello D, Thomas B, Zhang X, Allard J 2nd, Wei M, Mao H, Byrd JC, et al. TGF-beta utilizes SMAD3 to inhibit CD16-mediated IFN-gamma production and antibody-dependent cellular cytotoxicity in human NK cells. J Immunol. 2008;181:3784–92.
Viel S, Marçais A, Guimaraes FS, Loftus R, Rabilloud J, Grau M, Degouve S, Djebali S, Sanlaville A, Charrier E, et al. TGF-β inhibits the activation and functions of NK cells by repressing the mTOR pathway. Sci Signal. 2016;9:19.
Lian GY, Wan Y, Mak TS, Wang QM, Zhang J, Chen J, Wang ZY, Li M, Tang PM, Huang XR, et al. Self-carried nanodrug (SCND-SIS3): A targeted therapy for lung cancer with superior biocompatibility and immune boosting effects. Biomaterials. 2022;288:121730.
Liu C, Lai H, Chen T. Boosting Natural Killer Cell-Based Cancer Immunotherapy with Selenocystine/Transforming Growth Factor-Beta Inhibitor-Encapsulated Nanoemulsion. ACS Nano. 2020;14:11067–82.
Otegbeye F, Ojo E, Moreton S, Mackowski N, Lee DA, de Lima M, Wald DN. Inhibiting TGF-beta signaling preserves the function of highly activated, in vitro expanded natural killer cells in AML and colon cancer models. PLoS ONE. 2018;13:e0191358.
Ireland L, Luckett T, Schmid MC, Mielgo A. Blockade of Stromal Gas6 Alters Cancer Cell Plasticity, Activates NK Cells, and Inhibits Pancreatic Cancer Metastasis. Front Immunol. 2020;11:297.
Costa D, Venè R, Benelli R, Romairone E, Scabini S, Catellani S, Rebesco B, Mastracci L, Grillo F, Minghelli S, et al. Targeting the Epidermal Growth Factor Receptor Can Counteract the Inhibition of Natural Killer Cell Function Exerted by Colorectal Tumor-Associated Fibroblasts. Front Immunol. 2018;9:1150.
Timaner M, Tsai KK, Shaked Y. The multifaceted role of mesenchymal stem cells in cancer. Semin Cancer Biol. 2020;60:225–37.
Gao F, Chiu SM, Motan DA, Zhang Z, Chen L, Ji HL, Tse HF, Fu QL, Lian Q. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 2016;7:e2062.
Moloudizargari M, Govahi A, Fallah M, Rezvanfar MA, Asghari MH, Abdollahi M. The mechanisms of cellular crosstalk between mesenchymal stem cells and natural killer cells: Therapeutic implications. J Cell Physiol. 2021;236:2413–29.
Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–33.
Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74–85.
Fu Q, Man X, Yu M, Chu Y, Luan X, Piao H, Xue J, ** C. Human decidua mesenchymal stem cells regulate decidual natural killer cell function via interactions between collagen and leukocyte-associated immunoglobulin-like receptor 1. Mol Med Rep. 2017;16:2791–8.
Li Y, Wei J, Wu YF, Wang XP, Huang K, Lin YC, Huang SL, Fang JP. Effect of mesenchymal stem cells on expression of CD69 in cord blood CIK/NK cells and quantity ratio of T regulatory cells in CIK/NK cell culture. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2009;17:1301–6.
Giuliani M, Oudrhiri N, Noman ZM, Vernochet A, Chouaib S, Azzarone B, Durrbach A, Bennaceur-Griscelli A. Human mesenchymal stem cells derived from induced pluripotent stem cells down-regulate NK-cell cytolytic machinery. Blood. 2011;118:3254–62.
Thomas H, Jäger M, Mauel K, Brandau S, Lask S, Flohé SB. Interaction with mesenchymal stem cells provokes natural killer cells for enhanced IL-12/IL-18-induced interferon-gamma secretion. Mediators Inflamm. 2014;2014:143463.
Wang HF, Shi YJ, Ren HY. Bone marrow-derived mesenchymal stem cells regulate the proliferation and activity of natural killer cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2012;20:438–42.
Abbasi B, Shamsasenjan K, Ahmadi M, Beheshti SA, Saleh M. Mesenchymal stem cells and natural killer cells interaction mechanisms and potential clinical applications. Stem Cell Res Ther. 2022;13:97.
Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS ONE. 2010;5:e10088.
Hu C-HD, Kosaka Y, Marcus P, Rashedi I, Keating A. Differential Immunomodulatory Effects of Human Bone Marrow-Derived Mesenchymal Stromal Cells on Natural Killer Cells. Stem Cells and Dev. 2019;28:933–43.
Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107:1484–90.
Crop MJ, Korevaar SS, de Kuiper R. JN IJ, van Besouw NM, Baan CC, Weimar W, Hoogduijn MJ: Human mesenchymal stem cells are susceptible to lysis by CD8(+) T cells and NK cells. Cell Transplant. 2011;20:1547–59.
Giuliani M, Bennaceur-Griscelli A, Nanbakhsh A, Oudrhiri N, Chouaib S, Azzarone B, Durrbach A, Lataillade JJ. TLR ligands stimulation protects MSC from NK killing. Stem Cells. 2014;32:290–300.
Jewett A, Arasteh A, Tseng HC, Behel A, Arasteh H, Yang W, Cacalano NA, Paranjpe A. Strategies to rescue mesenchymal stem cells (MSCs) and dental pulp stem cells (DPSCs) from NK cell mediated cytotoxicity. PLoS ONE. 2010;5: e9874.
Desai A, Yan Y, Gerson SL. Concise Reviews: Cancer Stem Cell Targeted Therapies: Toward Clinical Success. Stem Cells Transl Med. 2019;8:75–81.
Jain S, Annett SL, Morgan MP, Robson T. The Cancer Stem Cell Niche in Ovarian Cancer and Its Impact on Immune Surveillance. Int J Mol Sci. 2021;22:4091.
Bayik D, Lathia JD. Cancer stem cell-immune cell crosstalk in tumour progression. Nat Rev Cancer. 2021;21:526–36.
Shokouhifar A, Firouzi J, Nouri M, Sarab GA, Ebrahimi M. NK cell upraise in the dark world of cancer stem cells. Cancer Cell Int. 2021;21:682.
Castriconi R, Daga A, Dondero A, Zona G, Poliani PL, Melotti A, Griffero F, Marubbi D, Spaziante R, Bellora F, et al. NK cells recognize and kill human glioblastoma cells with stem cell-like properties. J Immunol. 2009;182:3530–9.
Tallerico R, Todaro M, Di Franco S, Maccalli C, Garofalo C, Sottile R, Palmieri C, Tirinato L, Pangigadde PN, La Rocca R, et al. Human NK cells selective targeting of colon cancer-initiating cells: a role for natural cytotoxicity receptors and MHC class I molecules. J Immunol. 2013;190:2381–90.
Yin T, Wang G, He S, Liu Q, Sun J, Wang Y. Human cancer cells with stem cell-like phenotype exhibit enhanced sensitivity to the cytotoxicity of IL-2 and IL-15 activated natural killer cells. Cell Immunol. 2016;300:41–5.
Ames E, Canter RJ, Grossenbacher SK, Mac S, Chen M, Smith RC, Hagino T, Perez-Cunningham J, Sckisel GD, Urayama S, et al. NK Cells Preferentially Target Tumor Cells with a Cancer Stem Cell Phenotype. J Immunol. 2015;195:4010–9.
Li M, Knight DA, Smyth MJ, Stewart TJ. Sensitivity of a novel model of mammary cancer stem cell-like cells to TNF-related death pathways. Cancer Immunol Immunother. 2012;61:1255–68.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8.
Akhter MZ, Sharawat SK, Kumar V, Kochat V, Equbal Z, Ramakrishnan M, Kumar U, Mathur S, Kumar L, Mukhopadhyay A. Aggressive serous epithelial ovarian cancer is potentially propagated by EpCAM+CD45+ phenotype. Oncogene. 2018;37:2089–103.
Tsuchiya H, Shiota G. Immune evasion by cancer stem cells. Regen Ther. 2021;17:20–33.
Wang B, Wang Q, Wang Z, Jiang J, Yu SC, ** YF, Yang J, Xu SL, Ye XZ, Xu C, et al. Metastatic consequences of immune escape from NK cell cytotoxicity by human breast cancer stem cells. Cancer Res. 2014;74:5746–57.
Jewett A, Kos J, Fong Y, Ko MW, Safaei T, Perišić Nanut M, Kaur K. NK cells shape pancreatic and oral tumor microenvironments; role in inhibition of tumor growth and metastasis. Semin Cancer Biol. 2018;53:178–88.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 2014;74:665–74.
Parodi M, Raggi F, Cangelosi D, Manzini C, Balsamo M, Blengio F, Eva A, Varesio L, Pietra G, Moretta L, et al. Hypoxia Modifies the Transcriptome of Human NK Cells, Modulates Their Immunoregulatory Profile, and Influences NK Cell Subset Migration. Front Immunol. 2018;9:2358.
Terren I, Orrantia A, Vitalle J, Astarloa-Pando G, Zenarruzabeitia O, Borrego F. Modulating NK cell metabolism for cancer immunotherapy. Semin Hematol. 2020;57:213–24.
Balsamo M, Manzini C, Pietra G, Raggi F, Blengio F, Mingari MC, Varesio L, Moretta L, Bosco MC, Vitale M. Hypoxia downregulates the expression of activating receptors involved in NK-cell-mediated target cell killing without affecting ADCC. Eur J Immunol. 2013;43:2756–64.
Zheng X, Qian Y, Fu B, Jiao D, Jiang Y, Chen P, Shen Y, Zhang H, Sun R, Tian Z, Wei H. Mitochondrial fragmentation limits NK cell-based tumor immunosurveillance. Nat Immunol. 2019;20:1656–67.
Sebestyén A, Kopper L, Dankó T, Tímár J. Hypoxia Signaling in Cancer: From Basics to Clinical Practice. Pathol Oncol Res. 2021;27:1609802.
Ni J, Wang X, Stojanovic A, Zhang Q, Wincher M, Bühler L, Arnold A, Correia MP, Winkler M, Koch PS, et al. Single-Cell RNA Sequencing of Tumor-Infiltrating NK Cells Reveals that Inhibition of Transcription Factor HIF-1α Unleashes NK Cell Activity. Immunity. 2020;52:1075-1087.e1078.
Krzywinska E, Kantari-Mimoun C, Kerdiles Y, Sobecki M, Isagawa T, Gotthardt D, Castells M, Haubold J, Millien C, Viel T, et al. Loss of HIF-1α in natural killer cells inhibits tumour growth by stimulating non-productive angiogenesis. Nat Commun. 2017;8:1597.
Poznanski SM, Ashkar AA. What Defines NK Cell Functional Fate: Phenotype or Metabolism? Front Immunol. 2019;10:1414.
Schafer JR, Salzillo TC, Chakravarti N, Kararoudi MN, Trikha P, Foltz JA, Wang R, Li S, Lee DA. Education-dependent activation of glycolysis promotes the cytolytic potency of licensed human natural killer cells. J Allergy Clin Immunol. 2019;143:346-358.e346.
Wu T, Ke Y, Tang H, Liao C, Li J, Wang L. Fidarestat induces glycolysis of NK cells through decreasing AKR1B10 expression to inhibit hepatocellular carcinoma. Mol Ther Oncolytics. 2021;23:420–31.
Sheppard S, Santosa EK, Lau CM, Violante S, Giovanelli P, Kim H, Cross JR, Li MO, Sun JC. Lactate dehydrogenase A-dependent aerobic glycolysis promotes natural killer cell anti-viral and anti-tumor function. Cell Rep. 2021;35:109210.
Lian X, Yang K, Li R, Li M, Zuo J, Zheng B, Wang W, Wang P, Zhou S. Immunometabolic rewiring in tumorigenesis and anti-tumor immunotherapy. Mol Cancer. 2022;21:27.
Chang CH, Qiu J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJ, et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell. 2015;162:1229–41.
Renner K, Singer K, Koehl GE, Geissler EK, Peter K, Siska PJ, Kreutz M. Metabolic Hallmarks of Tumor and Immune Cells in the Tumor Microenvironment. Front Immunol. 2017;8:248.
Nakajima EC, Van Houten B. Metabolic symbiosis in cancer: refocusing the Warburg lens. Mol Carcinog. 2013;52:329–37.
Rabinowitz JD, Enerbäck S. Lactate: the ugly duckling of energy metabolism. Nat Metab. 2020;2:566–71.
Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, Matos C, Bruss C, Klobuch S, Peter K, et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab. 2016;24:657–71.
Harmon C, Robinson MW, Hand F, Almuaili D, Mentor K, Houlihan DD, Hoti E, Lynch L, Geoghegan J, O’Farrelly C. Lactate-Mediated Acidification of Tumor Microenvironment Induces Apoptosis of Liver-Resident NK Cells in Colorectal Liver Metastasis. Cancer Immunol Res. 2019;7:335–46.
Husain Z, Huang Y, Seth P, Sukhatme VP. Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J Immunol. 2013;191:1486–95.
** M, Cao W, Chen B, **ong M, Cao G. Tumor-Derived Lactate Creates a Favorable Niche for Tumor via Supplying Energy Source for Tumor and Modulating the Tumor Microenvironment. Front Cell Dev Biol. 2022;10:808859.
Monje M, Borniger JC, D’Silva NJ, Deneen B, Dirks PB, Fattahi F, Frenette PS, Garzia L, Gutmann DH, Hanahan D, et al. Roadmap for the Emerging Field of Cancer Neuroscience. Cell. 2020;181:219–22.
Garofalo S, D’Alessandro G, Chece G, Brau F, Maggi L, Rosa A, Porzia A, Mainiero F, Esposito V, Lauro C, et al. Enriched environment reduces glioma growth through immune and non-immune mechanisms in mice. Nat Commun. 2015;6:6623.
**ao R, Ali S, Caligiuri MA, Cao L. Enhancing Effects of Environmental Enrichment on the Functions of Natural Killer Cells in Mice. Front Immunol. 2021;12:695859.
Ben-Eliyahu S, Page GG, Yirmiya R, Shakhar G. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int J Cancer. 1999;80:880–8.
Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5:617–25.
Balatsoukas A, Rossignoli F, Shah K. NK cells in the brain: implications for brain tumor development and therapy. Trends Mol Med. 2022;28:194–209.
Earls RH, Menees KB, Chung J, Gutekunst CA, Lee HJ, Hazim MG, Rada B, Wood LB, Lee JK. NK cells clear α-synuclein and the depletion of NK cells exacerbates synuclein pathology in a mouse model of α-synucleinopathy. Proc Natl Acad Sci U S A. 2020;117:1762–71.
Baron R, Nemirovsky A, Harpaz I, Cohen H, Owens T, Monsonego A. IFN-gamma enhances neurogenesis in wild-type mice and in a mouse model of Alzheimer’s disease. Faseb j. 2008;22:2843–52.
Hao J, Liu R, Piao W, Zhou Q, Vollmer TL, Campagnolo DI, **ang R, La Cava A, Van Kaer L, Shi FD. Central nervous system (CNS)-resident natural killer cells suppress Th17 responses and CNS autoimmune pathology. J Exp Med. 2010;207:1907–21.
Poli A, Kmiecik J, Domingues O, Hentges F, Bléry M, Chekenya M, Boucraut J, Zimmer J. NK cells in central nervous system disorders. J Immunol. 2013;190:5355–62.
Santoni G, Amantini C, Santoni M, Maggi F, Morelli MB, Santoni A. Mechanosensation and Mechanotransduction in Natural Killer Cells. Front Immunol. 2021;12:688918.
Zhou H, Wang M, Zhang Y, Su Q, **e Z, Chen X, Yan R, Li P, Li T, Qin X, et al. Functions and clinical significance of mechanical tumor microenvironment: cancer cell sensing, mechanobiology and metastasis. Cancer Commun (Lond). 2022;42:374–400.
Friedman D, Simmonds P, Hale A, Bere L, Hodson NW, White MRH, Davis DM. Natural killer cell immune synapse formation and cytotoxicity are controlled by tension of the target interface. J Cell Sci. 2021;134:jcs258570.
Matalon O, Ben-Shmuel A, Kivelevitz J, Sabag B, Fried S, Joseph N, Noy E, Biber G, Barda-Saad M. Actin retrograde flow controls natural killer cell response by regulating the conformation state of SHP-1. Embo j. 2018;37:e96264.
Ben-Shmuel A, Sabag B, Biber G, Barda-Saad M. The Role of the Cytoskeleton in Regulating the Natural Killer Cell Immune Response in Health and Disease: From Signaling Dynamics to Function. Front Cell Dev Biol. 2021;9:609532.
Ma J, Huang L, Hu D, Zeng S, Han Y, Shen H. The role of the tumor microbe microenvironment in the tumor immune microenvironment: bystander, activator, or inhibitor? J Exp Clin Cancer Res. 2021;40:327.
Aziz N, Bonavida B. Activation of Natural Killer Cells by Probiotics. For Immunopathol Dis Therap. 2016;7:41–55.
Overacre-Delgoffe AE, Bumgarner HJ, Cillo AR, Burr AHP, Tometich JT, Bhattacharjee A, Bruno TC, Vignali DAA, Hand TW. Microbiota-specific T follicular helper cells drive tertiary lymphoid structures and anti-tumor immunity against colorectal cancer. Immunity. 2021;54:2812-2824.e2814.
Lam KC, Araya RE, Huang A, Chen Q, Di Modica M, Rodrigues RR, Lopès A, Johnson SB, Schwarz B, Bohrnsen E, et al. Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment. Cell. 2021;184:5338-5356.e5321.
Pal S, Perrien DS, Yumoto T, Faccio R, Stoica A, Adams J, Coopersmith CM, Jones RM, Weitzmann MN, Pacifici R. The microbiome restrains melanoma bone growth by promoting intestinal NK and Th1 cell homing to bone. J Clin Invest. 2022;132:e157340.
Yin H, Miao Z, Wang L, Su B, Liu C, ** Y, Wu B, Han H, Yuan X. Fusobacterium nucleatum promotes liver metastasis in colorectal cancer by regulating the hepatic immune niche and altering gut microbiota. Aging (Albany NY). 2022;14:1941–58.
Gur C, Ibrahim Y, Isaacson B, Yamin R, Abed J, Gamliel M, Enk J, Bar-On Y, Stanietsky-Kaynan N, Coppenhagen-Glazer S, et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity. 2015;42:344–55.
Massagué J, Ganesh K. Metastasis-Initiating Cells and Ecosystems. Cancer Discov. 2021;11:971–94.
Liu Y, Cao X. Characteristics and Significance of the Pre-metastatic Niche. Cancer Cell. 2016;30:668–81.
Chan IS, Knútsdóttir H, Ramakrishnan G, Padmanaban V, Warrier M, Ramirez JC, Dunworth M, Zhang H, Jaffee EM, Bader JS, Ewald AJ. Cancer cells educate natural killer cells to a metastasis-promoting cell state. J Cell Biol. 2020;219:e202001134.
Dianat-Moghadam H, Mahari A, Heidarifard M, Parnianfard N, Pourmousavi-Kh L, Rahbarghazi R, Amoozgar Z. NK cells-directed therapies target circulating tumor cells and metastasis. Cancer Lett. 2021;497:41–53.
Nieswandt B, Hafner M, Echtenacher B, Männel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59:1295–300.
Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Jirousková M, Degen JL. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105:178–85.
Lo HC, Xu Z, Kim IS, **el B, Aguirre S, Kodali S, Liu J, Zhang W, Muscarella AM, Hein SM, et al. Resistance to natural killer cell immunosurveillance confers a selective advantage to polyclonal metastasis. Nat Cancer. 2020;1:709–22.
Malladi S, Macalinao DG, ** X, He L, Basnet H, Zou Y, de Stanchina E, Massagué J. Metastatic Latency and Immune Evasion through Autocrine Inhibition of WNT. Cell. 2016;165:45–60.
Er EE, Valiente M, Ganesh K, Zou Y, Agrawal S, Hu J, Griscom B, Rosenblum M, Boire A, Brogi E, et al. Pericyte-like spreading by disseminated cancer cells activates YAP and MRTF for metastatic colonization. Nat Cell Biol. 2018;20:966–78.
Hasim MS, Marotel M, Hodgins JJ, Vulpis E, Makinson OJ, Asif S, Shih HY, Scheer AK, MacMillan O, Alonso FG, et al. When killers become thieves: Trogocytosed PD-1 inhibits NK cells in cancer. Sci Adv. 2022;8:eabj3286.
Correia AL, Guimaraes JC, Auf der Maur P, De Silva D, Trefny MP, Okamoto R, Bruno S, Schmidt A, Mertz K, Volkmann K, et al. Hepatic stellate cells suppress NK cell-sustained breast cancer dormancy. Nature. 2021;594:566–71.
Christodoulou I, Ho WJ, Marple A, Ravich JW, Tam A, Rahnama R, Fearnow A, Rietberg C, Yanik S, Solomou EE, et al. Engineering CAR-NK cells to secrete IL-15 sustains their anti-AML functionality but is associated with systemic toxicities. J Immunother Cancer. 2021;9:e003894.
Klopotowska M, Bajor M, Graczyk-Jarzynka A, Kraft A, Pilch Z, Zhylko A, Firczuk M, Baranowska I, Lazniewski M, Plewczynski D, et al. PRDX-1 Supports the Survival and Antitumor Activity of Primary and CAR-Modified NK Cells under Oxidative Stress. Cancer Immunol Res. 2022;10:228–44.
Juillerat A, Marechal A, Filhol JM, Valogne Y, Valton J, Duclert A, Duchateau P, Poirot L. An oxygen sensitive self-decision making engineered CAR T-cell. Sci Rep. 2017;7:39833.
Lo A, Wang LS, Scholler J, Monslow J, Avery D, Newick K, O’Brien S, Evans RA, Bajor DJ, Clendenin C, et al. Tumor-Promoting Desmoplasia Is Disrupted by Depleting FAP-Expressing Stromal Cells. Cancer Res. 2015;75:2800–10.
Nalawade SA, Shafer P, Bajgain P, McKenna MK, Ali A, Kelly L, Joubert J, Gottschalk S, Watanabe N, Leen A, et al. Selectively targeting myeloid-derived suppressor cells through TRAIL receptor 2 to enhance the efficacy of CAR T cell therapy for treatment of breast cancer. J Immunother Cancer. 2021;9:e003237.
Luo W, Napoleon JV, Zhang F, Lee YG, Wang B, Putt KS, Low PS. Repolarization of Tumor-Infiltrating Myeloid Cells for Augmentation of CAR T Cell Therapies. Front Immunol. 2022;13:816761.
Phung SK, Miller JS, Felices M. Bi-specific and Tri-specific NK Cell Engagers: The New Avenue of Targeted NK Cell Immunotherapy. Mol Diagn Ther. 2021;25:577–92.
Hodgins JJ, Khan ST, Park MM, Auer RC, Ardolino M. Killers 2.0: NK cell therapies at the forefront of cancer control. J Clin Invest. 2019;129:3499–510.
Arvindam US, van Hauten PMM, Schirm D, Schaap N, Hobo W, Blazar BR, Vallera DA, Dolstra H, Felices M, Miller JS. A trispecific killer engager molecule against CLEC12A effectively induces NK-cell mediated killing of AML cells. Leukemia. 2021;35:1586–96.
Wiernik A, Foley B, Zhang B, Verneris MR, Warlick E, Gleason MK, Ross JA, Luo X, Weisdorf DJ, Walcheck B, et al. Targeting natural killer cells to acute myeloid leukemia in vitro with a CD16 x 33 bispecific killer cell engager and ADAM17 inhibition. Clin Cancer Res. 2013;19:3844–55.
Gleason MK, Ross JA, Warlick ED, Lund TC, Verneris MR, Wiernik A, Spellman S, Haagenson MD, Lenvik AJ, Litzow MR, et al. CD16xCD33 bispecific killer cell engager (BiKE) activates NK cells against primary MDS and MDSC CD33+ targets. Blood. 2014;123:3016–26.
Wingert S, Reusch U, Knackmuss S, Kluge M, Damrat M, Pahl J, Schniegler-Mattox U, Mueller T, Fucek I, Ellwanger K, et al. Preclinical evaluation of AFM24, a novel CD16A-specific innate immune cell engager targeting EGFR-positive tumors. MAbs. 2021;13:1950264.
Kakiuchi-Kiyota S, Ross T, Wallweber HA, Kiefer JR, Schutten MM, Adedeji AO, Cai H, Hendricks R, Cohen S, Myneni S, et al. A BCMA/CD16A bispecific innate cell engager for the treatment of multiple myeloma. Leukemia. 2022;36:1006–14.
Kaminski MF, Bendzick L, Hopps R, Kauffman M, Kodal B, Soignier Y, Hinderlie P, Walker JT, Lenvik TR, Geller MA, et al. TEM8 Tri-specific Killer Engager binds both tumor and tumor stroma to specifically engage natural killer cell anti-tumor activity. J Immunother Cancer. 2022;10:e004725.
Vallera DA, Felices M, McElmurry R, McCullar V, Zhou X, Schmohl JU, Zhang B, Lenvik AJ, Panoskaltsis-Mortari A, Verneris MR, et al. IL15 Trispecific Killer Engagers (TriKE) Make Natural Killer Cells Specific to CD33+ Targets While Also Inducing Persistence, In Vivo Expansion, and Enhanced Function. Clin Cancer Res. 2016;22:3440–50.
Bald T, Krummel MF, Smyth MJ, Barry KC. The NK cell-cancer cycle: advances and new challenges in NK cell-based immunotherapies. Nat Immunol. 2020;21:835–47.
Khan M, Arooj S, Wang H. NK Cell-Based Immune Checkpoint Inhibition. Front Immunol. 2020;11:167.
André P, Denis C, Soulas C, Bourbon-Caillet C, Lopez J, Arnoux T, Bléry M, Bonnafous C, Gauthier L, Morel A, et al. Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells. Cell. 2018;175:1731-1743.e1713.
Lin M, Luo H, Liang S, Chen J, Liu A, Niu L, Jiang Y. Pembrolizumab plus allogeneic NK cells in advanced non-small cell lung cancer patients. J Clin Invest. 2020;130:2560–9.
Navin I, Lam MT, Parihar R. Design and Implementation of NK Cell-Based Immunotherapy to Overcome the Solid Tumor Microenvironment. Cancers (Basel). 2020;12:3871.
Park J, Wrzesinski SH, Stern E, Look M, Criscione J, Ragheb R, Jay SM, Demento SL, Agawu A, Licona Limon P, et al. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat Mater. 2012;11:895–905.
Meraz IM, Majidi M, Cao X, Lin H, Li L, Wang J, Baladandayuthapani V, Rice D, Sepesi B, Ji L, Roth JA. TUSC2 Immunogene Therapy Synergizes with Anti-PD-1 through Enhanced Proliferation and Infiltration of Natural Killer Cells in Syngeneic Kras-Mutant Mouse Lung Cancer Models. Cancer Immunol Res. 2018;6:163–77.
Park W, Gordon AC, Cho S, Huang X, Harris KR, Larson AC, Kim DH. Immunomodulatory Magnetic Microspheres for Augmenting Tumor-Specific Infiltration of Natural Killer (NK) Cells. ACS Appl Mater Interfaces. 2017;9:13819–24.
Jang ES, Shin JH, Ren G, Park MJ, Cheng K, Chen X, Wu JC, Sunwoo JB, Cheng Z. The manipulation of natural killer cells to target tumor sites using magnetic nanoparticles. Biomaterials. 2012;33:5584–92.
Au KM, Park SI, Wang AZ. Trispecific natural killer cell nanoengagers for targeted chemoimmunotherapy. Sci Adv. 2020;6:eaba8564.
Biber G, Sabag B, Raiff A, Ben-Shmuel A, Puthenveetil A, Benichou JIC, Jubany T, Levy M, Killner S, Barda-Saad M. Modulation of intrinsic inhibitory checkpoints using nano-carriers to unleash NK cell activity. EMBO Mol Med. 2022;14:e14073.
Zhong S, Jeong JH, Chen Z, Chen Z, Luo JL. Targeting Tumor Microenvironment by Small-Molecule Inhibitors. Transl Oncol. 2020;13:57–69.
Miyazato K, Hayakawa Y. Pharmacological targeting of natural killer cells for cancer immunotherapy. Cancer Sci. 2020;111:1869–75.
McFarland AP, Yalin A, Wang SY, Cortez VS, Landsberger T, Sudan R, Peng V, Miller HL, Ricci B, David E, et al. Multi-tissue single-cell analysis deconstructs the complex programs of mouse natural killer and type 1 innate lymphoid cells in tissues and circulation. Immunity. 2021;54:1320-1337.e1324.
Moreno-Nieves UY, Tay JK, Saumyaa S, Horowitz NB, Shin JH, Mohammad IA, Luca B, Mundy DC, Gulati GS, Bedi N, et al. Landscape of innate lymphoid cells in human head and neck cancer reveals divergent NK cell states in the tumor microenvironment. Proc Natl Acad Sci U S A. 2021;118:e2101169118.
Fawkner-Corbett D, Antanaviciute A, Parikh K, Jagielowicz M, Gerós AS, Gupta T, Ashley N, Khamis D, Fowler D, Morrissey E, et al. Spatiotemporal analysis of human intestinal development at single-cell resolution. Cell. 2021;184:810-826.e823.
Carter B, Zhao K. The epigenetic basis of cellular heterogeneity. Nat Rev Genet. 2021;22:235–50.
Song J, Choi H, Koh SK, Park D, Yu J, Kang H, Kim Y, Cho D, Jeon NL. High-Throughput 3D In Vitro Tumor Vasculature Model for Real-Time Monitoring of Immune Cell Infiltration and Cytotoxicity. Front Immunol. 2021;12:733317.
Ke LY, Kuo ZK, Chen YS, Yeh TY, Dong M, Tseng HW, Liu CH. Cancer immunotherapy μ-environment LabChip: taking advantage of optoelectronic tweezers. Lab Chip. 2017;18:106–14.
Neal JT, Li X, Zhu J, Giangarra V, Grzeskowiak CL, Ju J, Liu IH, Chiou SH, Salahudeen AA, Smith AR, et al. Organoid Modeling of the Tumor Immune Microenvironment. Cell. 2018;175:1972-1988.e1916.
Kingsley DM, Roberge CL, Rudkouskaya A, Faulkner DE, Barroso M, Intes X, Corr DT. Laser-based 3D bioprinting for spatial and size control of tumor spheroids and embryoid bodies. Acta Biomater. 2019;95:357–70.
Lewis SM, Asselin-Labat ML, Nguyen Q, Berthelet J, Tan X, Wimmer VC, Merino D, Rogers KL, Naik SH. Spatial omics and multiplexed imaging to explore cancer biology. Nat Methods. 2021;18:997–1012.
Murphy KJ, Reed DA, Trpceski M, Herrmann D, Timpson P. Quantifying and visualising the nuances of cellular dynamics in vivo using intravital imaging. Curr Opin Cell Biol. 2021;72:41–53.
Boulch M, Grandjean CL, Cazaux M, Bousso P. Tumor Immunosurveillance and Immunotherapies: A Fresh Look from Intravital Imaging. Trends Immunol. 2019;40:1022–34.
Acknowledgements
Special thanks to Wei-Lin ** for his suggestions on the conceptualization and revision of the manuscript. We apologize to those colleagues whose important work could not be cited due to space constraints.
Funding
This work was funded through the regional project of National Natural Science Foundation of China (82060119) and the special fund for basic scientific research operation expenses of central universities of Lanzhou University (lzujbky-2021-kb37).
Author information
Authors and Affiliations
Contributions
Conceptualization: YZ and XL; original manuscript preparation: YZ and LL; revision and proofreading: YZ, LC and XL; visualization: YZ and LL; funding acquisition: XL. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent to publication
All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, Y., Cheng, L., Liu, L. et al. NK cells are never alone: crosstalk and communication in tumour microenvironments. Mol Cancer 22, 34 (2023). https://doi.org/10.1186/s12943-023-01737-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12943-023-01737-7