Abstract
Background
Proteus mirabilis is an opportunistic pathogen that has been held responsible for numerous nosocomial and community-acquired infections which are difficult to be controlled because of its diverse antimicrobial resistance mechanisms.
Methods
Antimicrobial susceptibility patterns of P. mirabilis isolates collected from different clinical sources in Mansoura University Hospitals, Egypt was determined. Moreover, the underlying resistance mechanisms and genetic relatedness between isolates were investigated.
Results
Antimicrobial susceptibility testing indicated elevated levels of resistance to different classes of antimicrobials among the tested P. mirabilis clinical isolates (n = 66). ERIC-PCR showed great diversity among the tested isolates. Six isolates (9.1%) were XDR while all the remaining isolates were MDR. ESBLs and AmpCs were detected in 57.6% and 21.2% of the isolates, respectively, where blaTEM, blaSHV, blaCTX−M, blaCIT−M and blaAmpC were detected. Carbapenemases and MBLs were detected in 10.6 and 9.1% of the isolates, respectively, where blaOXA−48 and blaNDM−1 genes were detected. Quinolone resistant isolates (75.8%) harbored acc(6')-Ib-cr, qnrD, qnrA, and qnrS genes. Resistance to aminoglycosides, trimethoprim-sulfamethoxazole and chloramphenicol exceeded 80%. Fosfomycin was the most active drug against the tested isolates as only 22.7% were resistant. Class I or II integrons were detected in 86.4% of the isolates. Among class I integron positive isolates, four different gene cassette arrays (dfrA17- aadA5, aadB-aadA2, aadA2-lnuF, and dfrA14-arr-3-blaOXA−10-aadA15) and two gene cassettes (dfrA7 and aadA1) were detected. While class II integron positive isolates carried four different gene cassette arrays (dfrA1-sat1-aadA1, estXVr-sat2-aadA1, lnuF- dfrA1-aadA1, and dfrA1-sat2).
Conclusion
P. Mirabilis ability to acquire resistance determinants via integrons may be held responsible for the elevated rates of antimicrobial resistance and emergence of XDR or even PDR strains limiting the available therapeutic options for management of infections caused by those strains.
Similar content being viewed by others
Background
Proteus mirabilis is Gram-Negative, facultative anaerobe that belongs to family Morganellaceae. It is ubiquitous in nature and a member of the gastrointestinal flora of animals and human. However, it is held responsible for many nosocomial and community acquired outbreaks all over the world including urinary and respiratory tract infections, foot ulcers of the diabetic patients, and wide range of other infections [1].
Misuse or non-specific use of antibiotics has led to increased levels of drug resistance and wide spread of various resistance genes among clinical P. mirabilis isolates. Besides, P. mirabilis is characterized by intrinsic resistance to tetracycline, tigecycline, and polymyxins [2]. β-lactam antibiotics, including penicillins and cephalosporins, and carbapenems is considered the first choice for treatment of infections caused by P. mirabilis. One of the most common resistance mechanisms is the enzymatic hydrolysis of β-lactam antibiotics [3]. Structural and functional classification of β-lactamases have a critical role in the adequate choice of appropriate antimicrobial agent [4]. Prevalence of carbapenem resistance is relatively low, although it is increasing with time [5].
Recently, elevated levels of resistance to quinolones and aminoglycosides were reported worldwide [6, 7]. In addition, folate pathway inhibitors, nitrofurans, and even fosfomycin resistance are increasingly reported [8,9,10].
Most of resistance determinants are carried on integron’s that can be transferred by plasmids, transposons and other mobile genetic elements. Therefore, it is considered a major cause for the transfer of drug resistance traits among different bacterial pathogens, especially in family Enterobacteriaceae. More than 130 integron’s gene cassette arrays of various resistance genes to different classes of antibiotics have been identified [11].
Clinicians may face very limited therapeutic options, due to spread of multidrug-resistant (MDR), emergence of extensive drug resistant (XDR) and even pandrug resistant (PDR) strains [12]. Therefore, the aim of the present study is to assess the prevalence of resistance to different classes of antimicrobial agents among P. mirabilis isolates collected from different clinical sources from Mansoura University Hospitals, Egypt. Moreover, molecular detection of underlying resistance mechanisms and genetic relatedness among collected isolates was unveiled.
Materials and methods
Bacterial isolates
Bacterial isolates were collected from Mansoura University Hospitals from different clinical sources between September 2021 and January 2022. Isolates were identified as P. mirabilis according to standard microbiology and molecular methods [13].
Antimicrobial susceptibility pattern of P. mirabilis clinical isolates
Kirby Bauer disk diffusion method was used to assess P. mirabilis antimicrobial susceptibility profile using Mueller Hinton agar plates [14]. Interpretation of the results was performed according to the recommendations of Clinical and Laboratory Standard Institute [15]. Antimicrobial discs of various antimicrobial categories (Bioanalyze ® products, Turkey) have been used to assess the resistance profile of the tested P. mirabilis clinical isolates (Table S1).
Bacterial isolates were classified into MDR, XDR and PDR. MDR is recognized as being non susceptible to at least one antimicrobial agent in three antimicrobial classes or more. While XDR is being resistant to at least one antimicrobial agent in all antimicrobial classes but susceptible to two or fewer. PDR is recognized as being resistant to all agents in all different antimicrobial classes [16].
Phenotypic detection of β-lactamases
Acidimetric method for β-lactamases detection
β-lactamases hydrolyze β-lactam ring resulting in generating a carboxyl group which acidifies un-buffered systems. The resulting acidity was tested in 96-wells microtiter plates using benzylpenicillin as substrate and phenol red as a pH indicator [17].
Detection of extended spectrum β-lactamases (ESBLs)
Combination disk method (CDM) was used as previously described [15]. The zone of inhibition of cefotaxime (30 µg) and ceftazidime (30 µg) discs alone was measured and compared with that of cefotaxime/clavulanate (30 µg / 10 µg), and ceftazidime/ clavulanate (30 µg / 10 µg) discs, respectively. An increase in the inhibition zone (≥ 5 mm) of either or both anti-microbial agents when combined with clavulanate indicates ESBLs production.
Detection of plasmid mediated cephalosporinases (AmpCs)
Streaking method on MacConkey agar plates was employed as described previously [18]. In brief, sensitive E. coli DH5α strain lawn was inoculated on the surface of MacConkey agar plates. Cefoxitin disc (30 µg) was applied in the center of the plate and the tested isolates were streaked as a line, away from the cefoxitin disc by 5 mm. Isolates that distorted cefoxitin inhibition zone (clover leaf-like shape) was considered AmpC producers.
Detection of carbapenemases
Modified Hodge test (MHT) was used according to CLSI recommendations [15]. Sensitive E. coli DH5α strain lawn culture was inoculated on MacConkey agar plates [19]. Meropenem disc (10 µg) was applied in the center of the plate. The tested isolates were streaked as a line, 5 mm away from the disc. Distortion of the meropenem inhibition zone (clover leaf-like shape) indicates carbapenemase production [20].
Detection of metallo β‑lactamases (MBLs)
Combined disk synergy test (CDST) was used to as described previously. Briefly, two disks of IPM (10 µg) were placed on inoculated Mueller Hinton agar plate and then 2 µL of 0.5 M EDTA was added to one of them [21]. If inhibition zone of IPM/EDTA increased by ≥ 5 mm compared with that of IPM alone, the isolate was considered MBLs producer [15].
Molecular detection of resistance determinants
Extraction of DNA
DNA was extracted by boiling method as previously described [22]. Amplification of UreC gene was used for confirming identification of P. mirabilis isolates (Table 1) [23].
Detection of antimicrobial resistance encoding genes
Prevalence of antimicrobial resistance determinants including β‑lactams [24,25,26,27,28,29,30] and quinolones [31,32,33,34,35,36] resistance encoding genes among the tested P. mirabilis clinical isolates was detected via PCR using DreamTaq™ Green PCR master mix (Thermo Scientific, USA). PCR was performed in ProFlex™ PCR System (Cat. No. 4,484,073, Applied Biosystems™, USA) with programmed cycling conditions: initial denaturation (95 °C / 5 min), followed by 35 cycles of denaturation (95 °C / 30s), annealing (specified temperature (Table 1) / 30s) and extension step (72 °C / 60 s), then final extension (72 °C / 5 min). PCR products were analyzed using agarose gel electrophoresis (1.2%), stained with ethidium bromide, and their sizes were confirmed under ultraviolet light by comparison with appropriate DNA markers (Gene Ruler 100 bp or Gene Ruler 100 bp plus, Thermo Fisher Scientific Tm, UK).
Integrase genes and gene cassettes amplification
Amplification of integrase gene was performed using primers for intI1 and intI2 to detect class I and class II integrons, respectively [37]. Variable regions (gene cassettes) in isolates harboring class I integrons were amplified using 5’CS and 3’CS primers and class II integrons were amplified using attI2 and orfX primers (Table 1) [37]. PCR conditions were as follows; an initial denaturation (95 °C / 5 min), followed by 35 cycles of denaturation (95 °C / 1 min), annealing (58 °C / 1 min), and extension (72 °C / 2 min). Last step was final extension (72 °C / 10 min).
Sequencing and characterization of variable region’s gene cassettes
Amplicons of the same size of the variable region of class I and class II integrons (≥ 800 bp) were compared by restriction analysis with HinfI (TaKaRa, Japan). At least one representative of each type was sequenced using an automated sequencer (ABI Prism 3100), as described previously [38]. BLAST against the GenBank database (http://blast.ncbi.nlm.nih.gov/Blast.cgi) was performed for sequence comparison, and annotation. Nucleotide sequences of integrons’ variable regions were deposited in GenBank.
Molecular ty**
Enterobacterial repetitive intergenic consensus PCR (ERIC-PCR) was used for detecting the genetic relatedness of the tested P. mirabilis clinical isolates using specific primers (Table 1) [39]. PCR was conducted starting with initial denaturation (95 °C/5 min), then 35 cycles of denaturation (95 °C / 1 min), annealing (48 °C / 1 min), and extension step (72 °C / 5 min). Finally, termination of the reaction by a final extension step (72 °C / 5 min) [40]. The amplified products were separated on 2% agarose gel. The resulting banding patterns of ERIC-PCR were analyzed by Gel J software version 2.0 [41]. Unweighted pair-group method with arithmetic mean (UPGMA) and Jaccard’s coefficient were used for similarity clustering analysis. Clinical P. mirabilis isolates with a similarity coefficient ≥ 85% were considered genetically related [42].
Statistical analysis
R Studio (version 1.3.1093) was used for data analysis. Comparisons of frequencies and association phenotypic of and genotypic features were analyzed by contingency tables using the chi-square test (P < 0.05). Heat map was used for data graphing and visualization.
Results
Collection and identification of P. mirabilis clinical isolates
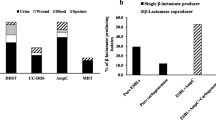
Sixty-six clinical isolates were collected and identified as P. mirabilis according to standard microbiological and molecular procedures (Table S1). Isolates were collected from different clinical sources including urine (24 isolates, 36.4%), diabetic foot lesions (12 isolates, 18.2%), sputum (7 isolates, 10.6%), T-Tube drain (6 isolates, 9.1%), bedsores and wound swabs (6 isolates, 9.1%), burn swab (5 isolates, 7.6%), blood (3 isolates, 4.5%), thigh boils swabs (2 isolates, 3%), and aspirate swab (1 isolate, 1.5%).
Clinical isolates of P. mirabilis appeared as scattered Gram-negative rods with characteristic fishy odor and strong swarming motility on nutrient agar. Identification of P. mirabilis isolates was performed according to their biochemical profile including positive phenyl alanine deaminase test, negative indole test, positive citrate utilization test, positive Voges-Proskauer (VP) and methyl red (MR) tests, and non-lactose fermentation on MacConkey’s agar media. On triple sugar iron agar slants, isolates showed alkaline red slant with acidic butt (indicative of glucose fermentation only) and heavy black precipitate (indicative of hydrogen sulfide production). Moreover, the gene coding for urease enzyme (UreC) was successfully amplified in all P. mirabilis clinical isolates confirming its identification.
Antibiotic sensitivity pattern of P. mirabilis clinical isolates
Antibiotic sensitivity testing of P. mirabilis clinical isolates indicated elevated rates of resistance to ampicillin, amoxicillin-clavulanic acid, cefazolin, cefuroxime, cefepime, trimethoprim-sulfamethoxazole, nitrofurantoin and Chloramphenicol (Fig. 1 and Table S2). Resistance to third generation cephalosporins ranged from 44 to 68%, while resistance to carbapenems was less than 11%. High rates of resistance to fluoroquinolones (53 to 76%) and aminoglycosides (45 to 82%) were observed, while fosfomycin resistance was detected in 23% of the tested isolates. Based on the resistance profile of P. mirabilis clinical isolates against the tested antimicrobials, 60 isolates (90.9%) were MDR, 6 isolates (9.1%) were XDR, and none of the isolates were PDR (Fig. 1 and Table S1).
Dendrogram constructed using ERIC-PCR patterns of P. mirabilis clinical isolates. Banding patterns were analyzed by using Gel J software version 2.0. Analysis of similarity clustering was performed by using UPGMA and Jaccard’s coefficient. The vertical line is a hypothetical line illustrating 85% similarity. Heatmap representing resistance profile of each isolate to different classes of antibiotics (red = resistant, yellow = intermediate, and blue = sensitive), and resistance determinants (red = positive, and yellow = negative) was added for comparison between isolates
Phenotypic detection of β-lactamases
Detection of β-lactamases
Acidimetric test was used for general detection of β-lactamases. The results showed that 38 isolates (57.6%), including all the XDR isolates, were β-lactamase producers (Table S1).
Detection of ESBLs
Based on CDM (Fig. S1), 38 isolates (57.6%) were ESBLs producers (Fig. 1 and Table S1).
Detection of AmpC
Fourteen isolates (21.2%) showed distortion of cefoxitin inhibition zone (clover leaf-like shape, Fig. S1) and were considered positive AmpC producers (Fig. 1 and Table S1).
Detection of MBLs
Six isolates (9.1%) showed increase in inhibition zone of Imipenem/EDTA by ≥ 5 mm compared with that of IPM alone in CDST (Fig. S1) and were considered MBLs producers (Fig. 1 and Table S1).
Detection of carbapenemases
Seven isolates (10.6%) distorted the inhibition zone around meropenem disc (clover leaf like shape, Fig. S1) in MHT indicating carbapenemases production (Fig. 1 and Table S1).
Molecular detection of resistance determinants
Detection of β-lactamases encoding genes
Among the tested P. mirabilis clinical isolates, blaOXA−1−like (class D β-lactamases) was the most predominant as it was detected in 35 isolates (53%) (Fig. 1, Fig. S2 and Table S1). Regarding Class A β-lactamases, blaTEM was detected in 34 isolates (51.5%), blaSHV in 8 isolates (12.1%), blaCTX−M in 12 isolates (18.2%). While blaGES, blaPER, and blaVEB genes were not detected among the tested isolates.
Detection of AmpC encoding genes
Among the tested genes, blaCIT−M and blaAmpC genes were detected in 5 isolates (7.6%) and 2 isolates (3%), respectively (Fig. 1, Fig. S2 and Table S1). While blaACC−1, blaACT−1, blaFox, and blaMOX genes were not detected among the tested isolates.
Detection of carbapenemases and MBLs encoding genes
blaOXA−48−like gene (class D β-lactamases) was detected in all carbapenem resistant P. mirabilis isolates (7 isolates, 10.6%), while blaKPC gene (class A β-lactamases) was not detected (Fig. 1, Fig. S2 and Table S1). Among MBLs encoding genes, blaNDM−1 was detected in 5 MBLs producing isolates (7.6%). While blaVIM−1 and blaIMP genes were not detected.
Detection of quinolone resistance encoding genes
Analysis of plasmid mediated quinolone resistance (PMQR) genes showed that acc (6’)-Ib-cr gene was detected in 38 isolates (57.6%). Regarding qnr encoding genes, qnrD was detected in 26 isolates (39.4%), qnrA in 23 isolates (34.8%), and qnrS in 6 isolates (9.1%) (Fig. 1, Fig. S2 and Table S1). Other qnr encoding genes (qnrB and qnrC) and qepA, oqxA, and oqxB were not detected.
Detection of integrons and their gene cassettes
Integrons Class I and II were screened among all the tested P. mirabilis clinical isolates. Fifty-seven isolates (86.4%) harbored either class I or class II integrons. Fifty-one isolates (77.3%) harbored both class I and II integrons, while six isolates (9.1%) lacked both (Fig. S3 and Table S1). The variable regions of class I and class II integrons were successfully amplified in 42 isolates (73.7%) and 54 isolates (94.7%), respectively. The size of the variable region ranged from 200 to 3000 bp (Fig. S3 and Table S1). Nucleotide sequences of class I integron’s variable region were deposited in GenBank (accessions no. OR567431, and OR573795:OR573799). Four different gene cassette arrays, dfrA17-aadA5 (11 isolates), aadB-aadA2 (4 isolates), aadA2-lnuF (4 isolates), and dfrA14-arr-3-blaOXA−10-aadA15 (3 isolates), and two different gene cassettes, dfrA7 (12 isolates), and aadA1 (4 isolates), were detected. While four different gene cassette arrays, dfrA1-sat1-aadA1 (34 isolates), estXVr-sat2-aadA1 (3 isolates), lnuF-dfrA1-aadA1 (3 isolates), and dfrA1-sat2 (1 isolate), were detected in class II integron’s variable regions (Fig. 2 and Table S1). Nucleotide sequences were deposited in GenBank (accessions no. OR597588, OR597589, OR573800, and OR573801).
Correlation between resistance to aminoglycosides tested and folate pathway inhibitors (trimethoprim/sulfamethoxazole) and their resistance determinants (aadA, sat and dfrA variants) carried by either class I or class II integrons (chi-square test, P < 0.5). Most isolates lacking integrons (class I and class II) were sensitive to the tested aminoglycosides and folate pathway inhibitors.
Molecular ty**
ERIC-PCR ty** method demonstrated enormous diversity among the tested P. mirabilis clinical isolates. Some isolates were considered genetically related as they showed a similarity coefficient higher than 85% (Fig. 1). Moreover, it showed great diversity among isolates that showed XDR profile and were considered genetically unrelated.
Discussion
Management of infectious diseases is of great importance for human health, especially with the continuous increase of MDR and the emergence of XDR or even PDR [5, 22, 43,44,45].
Therefore, evaluation of the local antimicrobial resistance patterns and underlying resistance determinants is fundamental for the implementation of effective stewardship programs in each country/region.
Among Enterobacterales, K. pneumoniae, E. coli and P. mirabilis were held responsible for most of hospital and community-acquired infections. P. mirabilis caused several nosocomial and community-acquired outbreaks in different regions of the world [46]. It does not produce any chromosomally encoded β-lactamases resulting in full susceptibility to all β-lactams for a wild-type phenotype and it is generally susceptible to fluoroquinolones [4]. However, strains resistant to different antibiotics are increasingly reported, which complicates the treatment of infections caused by Proteus spp [12].
In this study, 57.6% of the tested isolates were ESBLs producers which coincide with reports in different regions of the world [47,48,49]. In Egypt, low rate ESBLs production (28.3%) has been reported but a recent study recorded a higher rate (51.7%) [9, 10]. blaTEM, blaCTX−M, and blaSHV genes were detected in the tested isolates. Recent studies indicated similar findings in Egypt [9, 10, 12] and worldwide [3, 50,51,52].
In Croatia, blaTEM and blaPER genes were detected [53, 54], while blaCTX−M2 was the most prevalent ESBLs encoding gene in Japan [55].
AmpC cephalosporinases were held responsible for nosocomial outbreaks and failure of treatment.
AmpC production was detected in 21.2% of the tested isolates which coincide with previous studies [49, 56]. In Egypt, low AmpC production was previously recorded (3.8%), but a recent study reported higher AmpC production (34.5%) [10, 57]. blaCIT−M and blaAmpC genes were detected among the tested isolates as previously reported in Egypt [10, 57]. In Singapore and Bahrain, only blaCIT−M was detected [58, 59], while a recent study in Nigeria showed that 66.7% of the isolates carried blaAmpC gene [60].
Carbapenems remain one of the last resort antibiotics for treatment of severe infection especially those caused by ESBLs producing Enterobacterales. Globally resistance of carbapenem in P. mirabilis is relatively low, although it tends to increase with time [10, 61]. Among the tested isolates, 10.6% were carbapenem resistant and blaOXA−48 and blaNDM−1 genes were detected. A recent study in Egypt recorded blaKPC, and blaNDM−1 [12], while another study recorded blaoxa and blaVIM genes [10]. In general, blaKPC, blaNDM−1, blaVIM and blaOXA−48 are the most predominant in Europe, blaKPC in United States and blaIMP in Japan [62, 63].
Quinolones is a promising, relatively safe substitute to β-lactams in case of their resistance. However, high quinolone resistance (75.8%) was detected among the tested isolates. The prevalence of resistance determinants was studied, where acetylation by acc (6’)-Ib-cr gene was the most predominant mechanism (76%). Among qnr genes, qnrD, qnrA and qnrS genes were detected. qnrD was highly reported in isolates of Proteus and Providencia, therefore, it was hypothesized that it originated in family Morganellaceae then disseminated to other Enterobacterales [64]. Previous studies reported detection of acc (6’)-Ib-cr, qnrD, and qnr A among quinolone resistant proteus isolates [6, 52].
Fosfomycin has attracted a great attention for treating serious systemic infections caused by MDR Enterobacterales. However, resistance to fosfomycin have emerged [65]. Among the tested isolates, (22.7%) were fosfomycin resistant which coincide with recent studies in Brazil and Lebanon [6, 66]. Among the tested isolates, 81.8% were resistant to aminoglycosides. Recent studies in Egypt recorded resistance rate 37.9 to 53.2% [9, 10, 67], while in Ghana, India and Japan, higher rates of resistance (54–100%) were recorded [18, 48, 55, 68]. In addition, 87.9% of the isolates were resistant to trimethoprim/sulfamethoxazole which coincide with recent studies in Egypt [9, 10, 12]. Similarly, elevated rates of resistance were recoded worldwide [3, 18, 52].
Different genetic mechanisms are involved in the acquisition of resistance genes to different antibiotic classes. Horizontal gene transfer, via plasmids, transposons and integrons, is a major cause of the spread of antimicrobial resistance and turn P. mirabilis into MDR, XDR or even PDR resistant [11]. Integrons are not self- transferable elements, however they are frequently located on transposons or plasmids, allowing efficient gene transfer. More than 100 gene cassettes bearing resistance to different classes of antibiotics have been reported [51, 52, 69, 70]. Class I and II integrons were detected in 86.4% of the tested isolates. Sequencing analysis of their variable region revealed that they carried distinctive cassettes encoding aminoglycosides and trimethoprim resistance determinants mainly. Previous reports have also indicated that most integrons-carrying genes are coding for aminoglycosides and trimethoprim resistance [52, 69,70,71].
MDR phenomenon is frequently linked to integrons as they can be transferred, integrated, expressed, and causes distribution of several antimicrobial resistance genes [72].
The high rate of MDR and emergence of XDR among the tested isolates along with their enormous diversity (ERIC-PCR genoty**) could be explained by horizontal transfer of resistance determinants among bacterial isolates in hospitals. Variable rates of MDR (14.5–100%) were reported worldwide among P. mirabilis clinical isolates [3, 52, 69]. Previous studies in Egypt reported MDR (29.3–87.2%) among P. mirabilis clinical isolates [9, 10, 67]. A recent study reported 22.8% MDR, 31.4% XDR, and 8.5% PDR, which is considered the first report of PDR P. mirabilis in Egypt [12].
Conclusion
The elevated rate of MDR and emergence of XDR among P. mirabilis clinical isolates poses a public threat in Egypt limiting the therapeutic options for management of infections caused by these superbugs. Appropriate use of antimicrobial agents in the health setting along with surveillance of antimicrobial resistance profiles and the underlying resistance determinants are highly requested for controlling the spread of antimicrobial resistance and emergence of PDR stains in the future.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
- MDR:
-
Multidrug-resistant
- XDR:
-
Extensive drug resistant
- PDR:
-
Pandrug-resistant
- ESBLs:
-
Extended spectrum β-lactamases
- AmpCs:
-
Plasmid mediated cephalosporinase
- MBLs:
-
Metallo β‑lactamases
- ERIC-PCR:
-
Enterobacterial repetitive intergenic consensus PCR
- MCA:
-
Multiple correspondence analysis
- PMQR:
-
Plasmid mediated quinolone resistance
References
Armbruster CE, Mobley HLT, Pearson MM. Pathogenesis of Proteus mirabilis infection. EcoSal Plus. 2018;8(1).
Aghapour Z, Gholizadeh P, Ganbarov K, Bialvaei AZ, Mahmood SS, Tanomand A, et al. Molecular mechanisms related to colistin resistance in Enterobacteriaceae. Infect drug Resist. 2019;12:965–75.
Mirzaei A, Nasr Esfahani B, Raz A, Ghanadian M, Moghim S. From the Urinary Catheter to the Prevalence of Three Classes of Integrons, β-Lactamase Genes, and Differences in Antimicrobial Susceptibility of Proteus mirabilis and Clonal Relatedness with Rep-PCR. BioMed research international. 2021;2021.
Girlich D, Bonnin RA, Dortet L, Naas T. Genetics of Acquired Antibiotic Resistance genes in Proteus spp. Front Microbiol. 2020;11.
Benmahmod AB, Said HS, Ibrahim RH. Prevalence and mechanisms of carbapenem resistance among Acinetobacter baumannii clinical isolates in Egypt. Microb Drug Resist. 2019;25(4):480–8.
Sanches MS, Silva LC, Silva CRD, Montini VH, Oliva BHD, Guidone GHM et al. Prevalence of Antimicrobial Resistance and Clonal Relationship in ESBL/AmpC-Producing Proteus mirabilis isolated from Meat products and Community-acquired urinary tract infection (UTI-CA) in Southern Brazil. Antibiotics 2023;12(2).
Danilo de Oliveira W, Lopes Barboza MG, Faustino G, Yamanaka Inagaki WT, Sanches MS, Takayama Kobayashi RK et al. Virulence, resistance and clonality of Proteus mirabilis isolated from patients with community-acquired urinary tract infection (CA-UTI) in Brazil. Microb Pathog. 2021;152.
Saiprasad PV, Krishnaprasad K. Exploring the hidden potential of fosfomycin for the fight against severe Gram-negative infections. Ind J Med Microbiol. 2016;34(4):416–20.
Salama LA, Saleh H, Abdel-Rhman S, Barwa R, Hassan R. Phenotypic and genotypic characterization of Extended Spectrum β-lactamases producing Proteus mirabilis isolates. J Records Pharm Biomedical Sci. 2021;5:89–99.
Shaaban M, Elshaer SL, Abd El-Rahman OA. Prevalence of extended-spectrum β-lactamases, AmpC, and carbapenemases in Proteus mirabilis clinical isolates. BMC Microbiol. 2022;22(1).
Partridge SR, Kwong SM, Firth N, Jensen SO. Mobile Genetic Elements Associated with Antimicrobial Resistance. Clin Microbiol Rev. 2018;31(4).
Algammal AM, Hashem HR, Alfifi KJ, Hetta HF, Sheraba NS, Ramadan H et al. atpD gene sequencing, multidrug resistance traits, virulence-determinants, and antimicrobial resistance genes of emerging XDR and MDR-Proteus mirabilis. Sci Rep. 2021;11(1).
Leboffe MJ, Pierce BE. A photographic atlas for the Microbiology Laboratory. 4th ed: Morton Publishing Company; 2011.
Bauer AW, Kirby WMM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45(4):493–6.
CLSI. Performance standards for Antimicrobial susceptibility Testing.CLSI supplement M100. 31th ed. Wayne, United States: Clinical and Laboratory Standards Institute (CLSI); 2021.
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infection: Official Publication Eur Soc Clin Microbiol Infect Dis. 2012;18(3):268–81.
Winn WC, Koneman EW. Koneman’s color atlas and textbook of diagnostic microbiology. 6th ed. Philadelphia: Lippincott Williams and Wilkins; 2006.
Pal N, Hooja S, Sharma R, Maheshwari RK. Phenotypic detection and Antibiogram of β-lactamase-producing Proteus species in a Tertiary Care Hospital, India. Annals Med Health Sci Res. 2016;6(5):267–73.
Lee K, Kim CK, Yong D, Jeong SH, Yum JH, Seo YH, et al. Improved performance of the modified Hodge test with MacConkey agar for screening carbapenemase-producing Gram-negative bacilli. J Microbiol Methods. 2010;83(2):149–52.
Amjad A, Mirza I, Abbasi S, Farwa U, Malik N, Zia F. Modified Hodge test: a simple and effective test for detection of carbapenemase production. Iran J Microbiol. 2011;3(4):189–93.
Panchal CA, Oza SS, Mehta SJ. Comparison of four phenotypic methods for detection of metallo-β-lactamase-producing Gram-negative bacteria in rural teaching hospital. J Lab Physicians. 2017;9(2):81–3.
Said HS, Benmahmod AB, Ibrahim RH. Co-production of AmpC and extended spectrum beta-lactamases in cephalosporin-resistant Acinetobacter baumannii in Egypt. World J Microbiol Biotechnol. 2018;34(12).
Pathirana H, De Silva BCJ, Wimalasena S, Hossain S, Heo GJ. Comparison of virulence genes in Proteus species isolated from human and pet turtle. Iran J Veterinary Res. 2018;19(1):48–52.
Gharrah MM, Mostafa El-Mahdy A, Barwa RF. Association between virulence factors and extended Spectrum Beta-Lactamase producing Klebsiella pneumoniae compared to Nonproducing isolates. Interdiscip Perspect Infect Dis. 2017;2017:7279830.
Pagani L, Dell’Amico E, Migliavacca R, D’Andrea MM, Giacobone E, Amicosante G, et al. Multiple CTX-M-type extended-spectrum beta-lactamases in nosocomial isolates of Enterobacteriaceae from a hospital in northern Italy. J Clin Microbiol. 2003;41(9):4264–9.
Dallenne C, Da Costa A, Decré D, Favier C, Arlet G. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae. J Antimicrob Chemother. 2010;65(3):490–5.
Barwa R, Abdelmegeed E, Abd El Galil K. Occurrence and detection of AmpC β-lactamases among some clinical isolates of Enterobacteriaceae obtained from Mansoura University Hospitals, Egypt. Afr J Microbiol Res. 2012;6(41):6924–30.
Khalil MAF, Elgaml A, El-Mowafy M. Emergence of Multidrug-Resistant New Delhi Metallo-β-Lactamase-1-Producing Klebsiella pneumoniae in Egypt. Microb drug Resist (Larchmont NY). 2017;23(4):480–7.
Barwa R, Shaaban M. Molecular characterization of Klebsiella pneumoniae clinical isolates with elevated resistance to Carbapenems. open Microbiol J. 2017;11:152–9.
Poirel L, Walsh TR, Cuvillier V, Nordmann P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn Microbiol Infect Dis. 2011;70(1):119–23.
Cattoir V, Poirel L, Rotimi V, Soussy CJ, Nordmann P. Multiplex PCR for detection of plasmid-mediated quinolone resistance qnr genes in ESBL-producing enterobacterial isolates. J Antimicrob Chemother. 2007;60(2):394–7.
Cattoir V, Weill FX, Poirel L, Fabre L, Soussy CJ, Nordmann P. Prevalence of qnr genes in Salmonella in France. J Antimicrob Chemother. 2007;59(4):751–4.
Wang M, Guo Q, Xu X, Wang X, Ye X, Wu S, et al. New plasmid-mediated quinolone resistance gene, qnrC, found in a clinical isolate of Proteus mirabilis. Antimicrob Agents Chemother. 2009;53(5):1892–7.
Cattoir V, Poirel L, Nordmann P. Plasmid-mediated quinolone resistance pump QepA2 in an Escherichia coli isolate from France. Antimicrob Agents Chemother. 2008;52(10):3801–4.
Chen X, Zhang W, Pan W, Yin J, Pan Z, Gao S, et al. Prevalence of qnr, aac(6’)-Ib-cr, qepA, and oqxAB in Escherichia coli isolates from humans, animals, and the environment. Antimicrob Agents Chemother. 2012;56(6):3423–7.
Park CH, Robicsek A, Jacoby GA, Sahm D, Hooper DC. Prevalence in the United States of aac(6’)-Ib-cr encoding a ciprofloxacin-modifying enzyme. Antimicrobial agents and chemotherapy. 2006;50(11):3953-5.
Machado E, Cantón R, Baquero F, Galán JC, Rollán A, Peixe L, et al. Integron content of extended-spectrum-beta-lactamase-producing Escherichia coli strains over 12 years in a single hospital in Madrid, Spain. Antimicrob Agents Chemother. 2005;49(5):1823–9.
Wei Q, Jiang X, Li M, Li G, Hu Q, Lu H, et al. Diversity of gene cassette promoter variants of class 1 integrons in uropathogenic Escherichia coli. J Curr Microbiol. 2013;67:543–9.
Versalovic J, Koeuth T, Lupski R. Distribution of repetitive DNA sequences in eubacteria and application to finerpriting of bacterial enomes. Nucleic Acids Res. 1991;19(24):6823–31.
Michelim L, Muller G, Zacaria J, Delamare AP, Costa SO, Echeverrigaray S. Comparison of PCR-based molecular markers for the characterization of Proteus mirabilis clinical isolates. Brazilian J Infect Diseases: Official Publication Brazilian Soc Infect Dis. 2008;12(5):423–9.
Heras J, Dominguez C, Mata E, Pascual V, Lozano C, Torres C, et al. GelJ–a tool for analyzing DNA fingerprint gel images. BMC Bioinformatics. 2015;16:270.
Hunter PR, Gaston MA. Numerical index of the discriminatory ability of ty** systems: an application of Simpson’s index of diversity. J Clin Microbiol. 1988;26(11):2465–6.
Aladarose BE, Said HS, Abdelmegeed ES. Incidence of virulence determinants among Enterococcal Clinical isolates in Egypt and its Association with Biofilm formation. Microbial drug resistance (Larchmont, NY). 2019;25(6):880–9.
El-Baz R, Said HS, Abdelmegeed ES, Barwa R. Characterization of virulence determinants and phylogenetic background of multiple and extensively drug resistant Escherichia coli isolated from different clinical sources in Egypt. Appl Microbiol Biotechnol. 2022;106(3):1279–98.
Said HS, Abdelmegeed ES. Emergence of multidrug resistance and extensive drug resistance among enterococcal clinical isolates in Egypt. Infect drug Resist. 2019;12:1113–25.
Abreu AG, Marques SG, Monteiro-Neto V, Carvalho RM, Gonçalves AG. Nosocomial infection and characterization of extended-spectrum β-lactamases-producing Enterobacteriaceae in Northeast Brazil. Rev Soc Bras Med Trop. 2011;44(4):441–6.
Datta P, Gupta V, Arora S, Garg S, Chander J. Epidemiology of extended-spectrum β-lactamase, AmpC, and carbapenemase production in Proteus mirabilis. Jpn J Infect Dis. 2014;67(1):44–6.
Feglo P, Opoku S. AmpC beta-lactamase production among Pseudomonas aeruginosa and Proteus mirabilis isolates at the Komfo Anokye Teaching Hospital, Kumasi, Ghana. J Microbiol. 2014;6(1):13–20.
Rudresh SM, Nagarathnamma T. Extended spectrum β-lactamase producing Enterobacteriaceae & antibiotic co-resistance. Indian J Med Res. 2011;133(1):116–8.
Pavez M, Troncoso C, Osses I, Salazar R, Illesca V, Reydet P, et al. High prevalence of CTX-M-1 group in ESBL-producing enterobacteriaceae infection in intensive care units in southern Chile. Brazilian J Infect Diseases: Official Publication Brazilian Soc Infect Dis. 2019;23(2):102–10.
Chen CM, Lai CH, Wu HJ, Wu LT. Genetic characteristic of class 1 integrons in proteus mirabilis isolates from urine samples. BioMedicine. 2017;7(2):12–7.
Alabi OS, Mendonça N, Adeleke OE, da Silva GJ. Molecular screening of antibiotic-resistant determinants among multidrug-resistant clinical isolates of Proteus mirabilis from SouthWest Nigeria. Afr Health Sci. 2017;17(2):356–65.
Tonkić M, Mohar B, Šiško-Kraljević K, Meško-Meglič K, Goić-Barišić I, Novak A, et al. High prevalence and molecular characterization of extended-spectrum β-lactamase-producing Proteus mirabilis strains in southern Croatia. J Med Microbiol. 2010;59(10):1185–90.
Bedenić B, Firis N, Elveđi-Gašparović V, Krilanović M, Matanović K, Štimac I, et al. Emergence of multidrug-resistant Proteus mirabilis in a long-term care facility in Croatia. Wiener Klinische Wochenschrift. 2016;128(11–12):404–13.
Kurihara Y, Hitomi S, Oishi T, Kondo T, Ebihara T, Funayama Y, et al. Characteristics of bacteremia caused by extended-spectrum beta-lactamase-producing Proteus mirabilis. J Infect Chemotherapy: Official J Japan Soc Chemother. 2013;19(5):799–805.
Mohamudha Parveen R, Harish BN, Parija SC. Ampc Beta lactamases among gram negative clinical isolates from a tertiary hospital, South India. Brazilian J Microbiology: [publication Brazilian Soc Microbiology]. 2010;41(3):596–602.
Helmy MM, Wasfi R. Phenotypic and molecular characterization of plasmid mediated AmpC β-lactamases among Escherichia coli, Klebsiella spp, and Proteus mirabilis isolated from urinary tract infections in Egyptian hospitals. BioMed research international. 2014;2014.
Joji RM, Al-Mahameed AE, Jishi TA, Fatani DI, Saeed NK, Jaradat A, et al. Molecular detection of plasmid-derived AmpC β-lactamase among clinical strains of Enterobacteriaceae in Bahrain. Annals Thorac Med. 2021;16(3):287–93.
Tan TY, Ng LS, He J, Koh TH, Hsu LY. Evaluation of screening methods to detect plasmid-mediated AmpC in Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis. Antimicrob Agents Chemother. 2009;53(1):146–9.
Mendes RE, Castanheira M, Woosley LN, Stone GG, Bradford PA, Flamm RK. Characterization of β-Lactamase content of Ceftazidime-resistant pathogens recovered during the Pathogen-Directed phase 3 REPRISE trial for Ceftazidime-Avibactam: correlation of efficacy against β-Lactamase producers. Antimicrob Agents Chemother. 2019;63(6).
Halat DH, Moubareck CA. The current Burden of carbapenemases: review of significant properties and dissemination among Gram-negative Bacteria. Antibiotics. 2020;9(4).
Ohno Y, Nakamura A, Hashimoto E, Matsutani H, Abe N, Fukuda S, et al. Molecular epidemiology of carbapenemase-producing Enterobacteriaceae in a primary care hospital in Japan, 2010–2013. J Infect Chemotherapy: Official J Japan Soc Chemother. 2017;23(4):224–9.
Naas T, Cuzon G, Villegas MV, Lartigue MF, Quinn JP, Nordmann P. Genetic structures at the origin of acquisition of the beta-lactamase blaKPC gene. Antimicrob Agents Chemother. 2008;52(4):1257–63.
Guillard T, Grillon A, de Champs C, Cartier C, Madoux J, Berçot B et al. Mobile insertion cassette elements found in small non-transmissible plasmids in Proteeae may explain qnrD mobilization. PLoS ONE. 2014;9(2).
Karageorgopoulos DE, Wang R, Yu XH, Falagas ME. Fosfomycin: evaluation of the published evidence on the emergence of antimicrobial resistance in Gram-negative pathogens. J Antimicrob Chemother. 2012;67(2):255–68.
Sokhn ES, Salami A, El Roz A, Salloum L, Bahmad HF, Ghssein G. Antimicrobial susceptibilities and Laboratory profiles of Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis isolates as agents of urinary tract infection in Lebanon: paving the way for Better Diagnostics. Med Sci 2020;8(3).
Gomaa S, Serry F, Abdellatif H, Abbas H. Elimination of multidrug-resistant Proteus mirabilis biofilms using bacteriophages. Arch Virol. 2019;164(9):2265–75.
Shilpakar A, Ansari M, Rai KR, Rai G, Rai SK. Prevalence of multidrug-resistant and extended-spectrum beta-lactamase producing Gram-negative isolates from clinical samples in a tertiary care hospital of Nepal. Trop Med Health. 2021;49(1).
Fursova NK, Astashkin EI, Knyazeva AI, Kartsev NN, Leonova ES, Ershova ON, et al. The spread of Bla OXA-48 and bla OXA-244 carbapenemase genes among Klebsiella pneumoniae, Proteus mirabilis and Enterobacter spp. isolated in Moscow, Russia. Ann Clin Microbiol Antimicrob. 2015;14:46–55.
Wei Q, Hu Q, Li S, Lu H, Chen G, Shen B, et al. A novel functional class 2 integron in clinical Proteus mirabilis isolates. J Antimicrob Chemother. 2014;69(4):973–6.
Mokracka J, Gruszczyńska B, Kaznowski A. Integrons, β-lactamase and qnr genes in multidrug resistant clinical isolates of Proteus mirabilis and P. Vulgaris. APMIS: Acta Pathologica Microbiol et Immunol Scand. 2012;120(12):950–8.
Yekani M, Memar MY, Baghi HB, Sefidan FY, Alizadeh N, Ghotaslou R. Association of integrons with multidrug-resistant isolates among phylogenic groups of uropathogenic Escherichia coli. J Microbiol Res. 2018;9(1).
Funding
This study was self-funded.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
The study was conceived and designed by RB and HSS. Experimental work was performed by ME. HSS and ME performed data preparation, interpretation, and bioinformatics analyses. HSS conducted statistical analyses, data graphing and visualization. ME and HSS wrote the manuscript. RB and HSS has revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by Research Ethics Committee of Faculty of Pharmacy, Mansoura University, Egypt (Code number: 2021 − 367).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.



Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
ElTaweel, M., Said, H.S. & Barwa, R. Emergence of extensive drug resistance and high prevalence of multidrug resistance among clinical Proteus mirabilis isolates in Egypt. Ann Clin Microbiol Antimicrob 23, 46 (2024). https://doi.org/10.1186/s12941-024-00705-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12941-024-00705-3