Abstract
Background
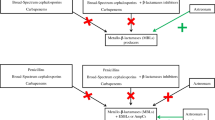
Antibiotics exert an outstanding selective pressure on bacteria, forcing their chromosomal gene mutations and drug resistance genes to spread. The objective of this study is to evaluate the expression of the New Delhi Metallo-β-Lactamase-1 gene (blaNDM−1) in the clinical isolate (Klebsiella pneumoniae TH-P12158), transformant strains Escherichia coli BL21 (DE3)-blaNDM−1, and Escherichia coli DH5α- blaNDM−1 when exposed to imipenem.
Methods
β-Lactamase genes (blaSHV, blaTEM−1, blaCTX−M−9, blaIMP, blaNDM−1, blaKPC, blaOXA, blaGES, and blaDHA) from randomly selected carbapenems-sensitive K.pneumoniae (n = 20) and E.coli (n = 20) strains were amplified by PCR. The recombinant plasmid of pET-28a harboring blaNDM−1 was transformed into E.coli BL21 (DE3) and E.coli DH5α by electroporation. The resistance phenotype and higher blaNDM−1 expression in K.pneumoniae TH-P12158, transformant E.coli BL21 (DE3)-blaNDM−1, and E.coli DH5α-blaNDM−1 were observed when exposed to imipenem with grade increasing, decreasing, and canceling doses, respectively.
Results
After being exposed to different doses of imipenem, the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) of antimicrobial drugs and blaNDM−1 expression of strains increased, which was positively correlated with doses of imipenem. On the contrary, with the decrease or cancellation of imipenem doses, the blaNDM−1 expression was deteriorated, while the MIC and MBC values remained relatively stable. These results demonstrated that low doses of imipenem (˂MIC) could press blaNDM−1 positive strains producing stable drug resistance memory and altered blaNDM−1 expression.
Conclusions
Low doses of imipenem could press blaNDM−1 positive strains producing sustained resistance memory and altered blaNDM−1 expression. In particular, the positive correlation between the resistance genes expression and antibiotics exposure shows promising guiding significance for clinical medication.
Similar content being viewed by others
Background
The gram-negative bacteria family Enterobacteriaceae has become the main source of both community and hospital-acquired infections that range from abscesses to blood infections, intra-abdominal infections, meningitis, pneumonia, and urinary tract infections [1]. Furthermore, the continuous emergence of drug-resistant Enterobacteriaceae (DRE) leads to increased morbidity, mortality, and healthcare costs [2]. The typical effect of DRE is the inactivation of β-lactams by β-lactamases such as extended-spectrum β-lactamases (ESBLs), AmpC cephalosporinases, and carbapenemases [3]. According to Ambler classification (similarity of amino acid sequences), β-lactamases are divided into four categories of class A, B, C, and D. Key enzyme families includes TEM, SHV, CTX-M, and KPC (class A); IMP, VIM, SPM, GIM, SIM, and NDM (class B); CMY, DHA, and ADC (class C); Class D enzymes are all termed OXA [4,5,6,7,8,9,10]. Class A, C, and D enzymes contain a serine residue at the active site of β-lactamase, while class B enzymes (Metallo-β-Lactamases, MBLs) contain one or two zinc ions at the active site. The genes encoding MBLs are located in both chromosome and plasmids, the primary structure of the enzyme is highly variable, and the homology of the amino acid sequence is less than 23% [11, 12]. In 2009, Timothy R. Walsh discovered a new type of MBLs, New Delhi Metallo-β-Lactamases-1 (NDM-1), hydrolyzes β-lactams (including carbapenems), which greatly damage antibacterial chemotherapy based β-lactam [13]. Nowadays, the blaNDM−1 encoding NDM has already disseminated worldwide and has been detected in Enterobacteriaceae, including Klebsiella pneumoniae, Escherichia coli, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterobacter cloacae, and Raoultella ornithinolytica [13,14,15].
Enterobacteriaceae is able to capture, accumulate and transmit resistance genes through the migration of gene elements (plasmids, insertion sequences, transposons, and integrons) within and between species [16, 17]. blaTEM,blaSHV, and blaCTX−M have become the main epidemic trend, the subtypes and variants of these gene families are develo** and spreading geographically and in a variety of bacterial species [8], E.coli and K.pneumoniae are important bacteria for accumulating these resistance genes. The plasmids carrying blaNDM−1 isolated Enterobacteriaceae also coexist with other drug resistance genes (blaNDM−5, blaOXA23, blaOXA52, armA, blaTEM−1, and blaCTX−M−9), which confer resistance to different classes of antibiotics [14, 18, 19]. Moreover, these plasmids carrying multiple drug-resistance genes can be exchanged by conjugation within different enterobacterial species [14].
Carbapenems are the mainstay antimicrobial agents for treating severe infection for their strong antibacterial activity and comprehensive antibacterial spectrum against gram-positive and gram-negative bacteria [20]. β-lactamase inhibitors cannot prevent the hydrolysis of carbapenems induced by NDM-1. Facing the reality that blaNDM−1 bacteria spread worldwide and greatly weaken the application effect of antibiotics, researchers have paid too much attention to the mechanism of resistance (mutation and gene transfer) and co** strategies [21, 22], although little attention is paid to the evolution of drug resistance of carbapenemase producing strains. In this study, we conducted the laboratory evolution of K.pneumoniae and E.coli carrying blaNDM−1 gene exposed to imipenem in vitro, to provide theoretical support for clinical control of the transmission of blaNDM−1 and the infection of the strain carrying it.
Methods
Bacterial strains
A total of 102 strains of K.pneumoniae and 91 strains of E.coli were isolated from sputum, blood, urine, cerebrospinal fluid, and secretions of patients admitted at the University Affiliated Hospital in China from July to August 2020. The VITEK-2 compact automatic microbiological analysis system (bioMerieux, Marcy-l′Etoile, France) is used to detect the minimum inhibitory concentration (MIC) of clinically recommended antibiotics. At the same time, the sensitivity of carbapenems was verified by the disk diffusion method (K-B method) [23].
E.coli BL21 (DE3) and E.coli DH5α strains (Novagen, Darmstadt, Germany) were used as an expression cell of pET-28a (+)-blaNDM−1 plasmid in the laboratory.
Molecular identification of β-lactamase genes
All randomly selected carbapenems-sensitive K.pneumoniae (n = 20) and E.coli(n = 20) strains were identified by 16SrDNA sequence analysis. The ESBLs (blaSHV, blaTEM−1, and blaCTX−M−9), AmpC (blaDHA), and carbapenemase (blaIMP, blaNDM−1, blaKPC, blaGES, and blaOXA) resistance genes carried by these strains were amplified by polymerase chain reaction (PCR) as previously described [14]. Primers for PCR were shown in Table 1. Amplification system: 2×NovoStar Green PCR Mix 25µL, Upstream, downstream primer 1µL (10µmol/L), DNA template 1µL, ddH2O 22µL, total volume 50µL. Amplification conditions: pre-denaturation at 95 °C for 5 min, denaturation at 95 °C for 30s, annealing at 55 °C (blaSHV) or 49 °C (blaTEM−1) or 50 °C (blaCTX−M−9) or 56 °C (blaIMP) or 55 °C (blaNDM−1) or 52 °C (blaKPC) or 57 °C (blaOXA) or 56 °C (blaGES) or 50 °C (blaDHA) for 30s (Table 1), extension at 72 °C for 1 min, a total of 34 cycles, extension at 72 °C for 5 min. Amplified products were separated by 1% agarose gel electrophoresis (110 V, 30 min) and sequenced. The gene sequence was compared with the GenBank database to determine the genotype.
Bla NDM−1-pET28a (+) plasmid
According to the literature, the blaNDM−1 gene was amplified by PCR and cloned into a pET-28a vector containing a 6×His tag.
Primers blaNDM−1 (Fwd-5′-GGATCCATGGAATTGCCCAATATTATGCA-3′ and Rev-5′-GTCG ACTCAGCGCAGCTTGTCGGCCAT-3′) were designed with BamH Ӏ & Sal Ӏ enzyme digestion sites added at both ends. The pCYNDM01 plasmid DNA (accession no. MK510953) was used as a template for PCR amplification of the blaNDM−1 gene (837 bp). Amplification reactions were performed in a total volume of 20µL (2×Taq PCR Green Mix 10µL, NDM-1-Fwd 0.5µL, NDM-1-Rev 0.5µL, DNA 1µL, and ddH2O 8µL). The mixture was heat denatured at 95 °C for 30s, annealed at 55 °C for 30s, extended at 68 °C for 2 min, and the reaction was carried out for 35 cycles. The PCR products were detected by 1% agarose gel electrophoresis (110 V, 27 min) and purified by PCR purification Kit (Qiagen).
5µL of PCR products (blaNDM−1) and pET28a (+) vector containing a 6×His tag used in a enzyme digestion reaction with BamH Ӏ 0.5µL, Sal Ӏ 0.5µL,10×Buffer 2µL, and ddH2O 8µL in a total volume of 20µL was incubated for 4 h at 37 °C. Then, the reaction system of 20µL (blaNDM−1 6µL, pET28a (+) 2µL, T4 DNA ligase 1µL, and 10× Ligase Buffer 1µL) was connected overnight at 4 °C.
The recombinant plasmid was sequenced and transformed into E.coli BL21 (DE3) and E.coliDH5α as described previously [24]. After overnight incubation at 37 °C (200 rpm) in LB liquid medium, the positive expression strains E.coli BL21 (DE3)-blaNDM−1 and E.coli DH5α-blaNDM−1 were screened on LB solid medium containing 100 µg/mL kanamycin and 0.5 µg/mL imipenem.
Imipenem exposure
According to the clinical guidance, a MIC of ≥ 4 µg/mL for imipenem is considered as carbapenems-resistant Enterobacteriaceae [25]. Different doses of imipenem were assessed for their effects on the drug resistance phenotype and genotype of clinical isolate K.pneumoniae TH-P12158 (carbapenem-sensitive strain carrying blaNDM−1 gene), E.coli BL21 (DE3)-blaNDM−1, and E.coli DH5α-blaNDM−1.
Subculture growth
Taking OD600 of 1.5-2.0 (1.5 × 108 CFU/mL) as the subculture growth standard of strains, K.pneumoniae TH-P12158, E.coli BL21 (DE3)-blaNDM−1, and E.coli DH5α-blaNDM−1 were incubated in Mueller-Hinton (MH) liquid medium containing 0.5×MIC imipenem at 37 °C for 200 rpm until the OD600 was 1.5-2.0. The same process was repeated until the OD600 value of the strain reached 1.5-2.0 within 11–12 h at the same imipenem concentration, and then the next higher imipenem concentration was exposed for subculture growth.
MIC, MBC, and K-B method
Regardless of whether imipenem increased (4 µg/mL, 8 µg/mL and 12 µg/mL), decreased (12 µg/mL to 8 µg/mL, 8 µg/mL to 4 µg/mL, and 4 µg/mL to 0 µg/mL) or canceled (12 µg/mL to 0 µg/mL, 8 µg/mL to 0 µg/mL, and 4 µg/mL to 0 µg/mL), the MIC and the minimum bactericidal concentration (MBC) of seven antimicrobial drugs (imipenem, meropenem, cefuroxime, ceftazidime, cefoperazone sodium/sulbactam sodium, piperacillin sodium/tazobactam sodium, levofloxacin) against three strains exposed to imipenem were detected, respectively. Meanwhile, the K-B method was used to verify the sensitivity of three strains to imipenem.
The MIC was interpreted using CLSI M100 (in 2019). Antimicrobial drugs were diluted into Mueller-Hinton liquid medium at a final ratio of 1280 µg/mL, 640 µg/mL, 320 µg/mL, 160 µg/mL, 80 µg/mL, 40 µg/mL, 20 µg/mL, 10 µg/mL, 5 µg/mL, and 2.5 µg/mL. A volume of 90µL strain (1.5 × 108 cfu/mL) was seeded in each well of 96-well plates containing 10µL antibacterial drugs and incubated for 16-24 h at 37 °C. The MIC of antibacterial drugs was evaluated by absorbance value at 600 nm. All experiments were repeated for 3 times.
100µL of bacterial solution from 96-well plates exhibiting complete bacteriostatic activity were incubated on MH solid medium without antibiotics at 37 °C for 48 h, and the minimum drug concentration without bacterial growth was denoted as the minimum bactericidal concentration (MBC). All experiments were repeated for three times.
A volume of 100µL three strains (1.5 × 108 cfu/mL) were inoculated on MH solid medium, respectively. The disk containing imipenem of 10 µg was attached to the plate and incubated at 37 °C for 18-20 h. The diameter of the inhibition zone was considered to be the degree of drug resistance of strains exposed to imipenem. The experiment was repeated three times to calculate the mean and standard error.
Quantitative real-time-PCR (qRT-PCR)
The qRT-PCR was used to detect the expression of blaNDM−1 of the last generation cells (OD600 reached 1.5-2.0 within 11–12 h) exposed to imipenem with grade increasing (4 µg/mL, 8 µg/mL, and 12 µg/mL), decreasing (12 µg/mL to 8 µg/mL, 8 µg/mL to 4 µg/mL, and 4 µg/mL to 0 µg/mL), and canceling doses (12 µg/mL to 0 µg/mL, 8 µg/mL to 0 µg/mL, and 4 µg/mL to 0 µg/mL).
The CDS sequence of blaNDM− 1 was obtained from the NCBI database to design its fluorescent quantitative primers (Fwd-5′-ACTGGATCAAGCAGGAGATCAACC-3′ and Rev-5′-CCATTGGC GGCGAAAGTCA-3′) with oligo 7.0. The mRNA was isolated from the strain exposed to imipenem by using TIANGEN RNAprep pure Cell/Bacteria Kit (China) and cDNA was synthesized by reverse transcription by using TOYOBO ReverTra Ace® qPCR RT Kit (Japan). The cDNA was used as the template to detect the expression levels of blaNDM− 1; the 16SrRNA gene was used as the reference gene. Results are presented as ratios of gene expression between the blaNDM− 1 (target) and the reference gene.
Statistical analysis
GraphPadPrism 7 and SPSS 25.0 software were used for drawing and statistical analysis. All the experiments were repeated not less than 3 times, and the results were expressed by mean ± standard deviation. T-test was used to compare the data among groups, P-values of < 0.05 were considered statistically significant.
Results
Drug sensitivity analysis
The drug sensitivity results of clinical isolates showed that the carbapenem resistance rates of K.pneumoniae and E.coli were 18.6% (n = 19) and 1% (n = 1), respectively.
All randomly selected carbapenems-sensitive K.pneumoniae and E.coli strains showed resistance against selected antibiotics (Table 2), especially to cephalosporins, such as 35% (n = 7) and 75% (n = 15) of cefazolin (first generation), 40% (n = 8) and 70% (n = 14) of cefuroxime (second generation), 30% (n = 6) and 70% (n = 14) of ceftriaxone (third generation), 15% (n = 3) and 60% (n = 12) of cefepime (fourth generation), respectively. In addition, they also showed certain resistance to β-lactamase inhibitors, such as ampicillin/sulbactam of 30% (n = 6) and 35% (n = 7), and ticarcillin/clavulanic acid of 20% (n = 4) and 20% (n = 4), respectively. The results also showed that drug-resistant K.pneumoniae mostly came from sputum, while E. coli came from urine (Table S1). However, all strains of K.pneumoniae and E.coli were highly susceptible to carbapenems (MIC ˂1 µg/mL) (Table 2).
Molecular identification of β-lactamase genes
The β-lactamases genes carried by K.pneumoniae and E.coli strains. Based on the neighbor-joining method, the phylogenetic tree was constructed by comparing the 16SrDNA sequences of K.pneumoniae (A) and E.coli strains (B). The β-Lactamases genes of blaSHV (red), blaTEM−1 (yellow), blaCTX−M−9 (green), blaIMP (blue), and blaNDM−1 (purple) amplified by PCR were placed on the right side of the strain, but this did not indicate the location of these genes in the genome of the strain
The detected β-lactamase genes are shown in Fig. 1. The detection rates of blaSHV, blaTEM−1, blaCTX−M−9, and blaIMP in K.pneumoniae were 100% (n = 20), 20% (n = 4), 70% (n = 14), and 5% (n = 1), respectively, while those in E.coli were 10% (n = 2), 35% (n = 7), 50% (n = 10), and 20% (n = 4) respectively. K.pneumoniae generally carried blaSHV and blaCTX−M−9, while E.coli mostly carried blaCTX−M−9. Surprisingly, blaNDM−1 and blaIMP (carbapenemase gene) were detected in K.pneumoniae (TH-P12158 and TH-P12498) (Fig. 1A) and E.coli (TH-U3224, TH-U3268, TH-U3226, and TH-U3265) (Fig. 1A, B).
Imipenem exposure
The blaNDM−1 gene was inserted into BamH I and Sal I sites of the pET-28a (+) vector (Fig. S1, S2). The structure of blaNDM−1-pET28a (+) plasmid transferred to the competent cells of E.coli BL21 (DE3) and E.coli DH5α was displayed in Fig. 2.
Subculture growth
Effect of imipenem on the growth of all three strains. Firstly, the same strain was exposed to imipenem of 0.125 µg/mL (MIC ˂1 µg/mL), and then exposed to imipenem at grade increasing (0.25 µg/mL, 0.5 µg/mL, 1 µg/mL, 2 µg/mL, 4 µg/mL, 8 µg/mL, and 12 µg/mL), decreasing (12 µg/mL to 8 µg/mL, 8 µg/mL to 4 µg/mL, and 4 µg/mL to 0 µg/mL), and canceling (12 µg/mL to 0 µg/mL, 8 µg/mL to 0 µg/mL, and 4 µg/mL to 0 µg/mL) doses, respectively. Bacterial growth curve was drawn with GraphPad 7.0 software
Effects of imipenem on bacterial growth are displayed in Fig. 3. The MICs of imipenem against K.pneumoniae TH-P12158, E.coli BL21 (DE3)-blaNDM−1, and E.coli DH5α-blaNDM−1 were 0.5 µg/mL, 0.25 µg/mL, and 0.25 µg/mL, respectively. With the increased doses of imipenem, the subculture times of K.pneumoniaeTH-P12158, E.coli BL21 (DE3)-blaNDM−1, and E.coli DH5α-blaNDM−1 were also increased. However, E.coli BL21 (DE3)-blaNDM−1 and E.coliDH5α-blaNDM−1 need more subculture times to make them grow normally (11-12 h, OD600 = 1.5-2.0) than K.pneumoniae TH-P12158 (Fig. 3A, B, C).
From Fig. 3, it can be seen that the subculture times of three strains were significantly shortened at the decreased doses of imipenem (12 µg/mL to 8 µg/mL, 8 µg/mL to 4 µg/mL, 4 µg/mL to 0 µg/mL) (Fig. 3D, E, and F). When the imipenem was canceled, the cells of the same strain exposed to imipenem of 12 µg/mL, 8 µg/mL, or 4 µg/mL could proliferate to OD600 of 1.5-2.0 in 8-12 h with the same subculture times (Fig. 3G, H, and I).
MIC, MBC, and K-B method
The MIC values of seven antimicrobial agents against three strains exposed to imipenem are displayed in Table 3. Under the exposure of imipenem of 0 µg/mL, 4 µg/mL, 8 µg/mL, and 12 µg/mL, the MIC values of Imipenem against K.pneumoniae TH-12,158, E.coli BL21(DE3)-blaNDM−1, and E.coli DH5α-blaNDM−1 were increased from 1×MIC (0.5 µg/mL), 1×MIC (0.25 µg/mL), and 1×MIC (0.25 µg/mL) at 0 µg/mL to 16×MIC, 8×MIC,and 8×MIC at 12 µg/mL, respectively. Meanwhile, the MIC values of meropenem and other drugs except norfloxacin were increased significantly. When the doses of imipenem were decreased or canceled from high to low, the MIC values of imipenem against the three strains remained unchanged for 20 generations (Table S2) (subsequent experiments were not done).
The same antibacterial effect also showed that the MBC values of seven antimicrobial agents were 2–4 times that of MIC, whether the exposure doses increased or decreased, or canceled on the strains (Table 3).
The resistance changes of three strains exposed to imipenem at series doses were shown in Table 4. Under the exposure of imipenem of 0 µg/mL, 4 µg/mL, 8 µg/mL, and 12 µg/mL, the inhibition zone of imipenem against K.pneumoniae TH-P12158 was significantly reduced from 26.00 ± 2.45 mm to 16.33 ± 1.25 mm (p < 0.05), 13.67 ± 0.47 mm (p < 0.05), and 11.00 ± 0.00 mm (p < 0.05), respectively. Meanwhile, E.coli DH5α-blaNDM−1 and E.coli BL21 (DE3)-blaNDM−1 showed also gradually increasing resistance to imipenem from 30.00 ± 2.16 mm, and 26.00 ± 1.63 mm of 0 µg/mL to 12.67 ± 0.47 mm (p < 0.05) and 13.00 ± 0.82 mm (p < 0.05) of 12 µg/mL, respectively.
Table 4 also showed that the inhibition zone of all strains exposed to imipenem of 12 µg/ml did not change significantly, regardless of whether the imipenem was decreased or canceled.
qRT-PCR
The expression levels of blaNDM−1 of all three strains exposed to imipenem were shown in Fig. 4. Under the exposure of imipenem of 0 µg/mL (control), 4 µg/mL, 8 µg/mL, and 12 µg/mL, the expression values of blaNDM−1 of K.pneumoniaeTH-P12158 were approximately threefold (relative quantification [RQ] = 2.8, p < 0.001 ), fivefold (RQ = 4.79, p < 0.001), and sevenfold (RQ = 6.22, p < 0.001) compared to the control without imipenem, respectively (Fig. 4A). Based on the same method, the expression levels of blaNDM−1 of E.coli DH5α-blaNDM−1 and E.coli BL21 (DE3)-blaNDM−1 were found to be lower than K.pneumoniae TH-P12158. The expression values of blaNDM−1 of E.coli DH5α-blaNDM−1 were approximately twofold (RQ = 1.43, p < 0.05), twofold (RQ = 2.10, p < 0.01), and twofold (RQ = 2.31, p < 0.001) from the control (RQ = 1), respectively (Fig. 4B). Meanwhile, the RQ expression values of blaNDM−1 of E.coli BL21 (DE3)-blaNDM−1 also were up-regulated 1.61(p > 0.05), 2.11(p < 0.01), and 2.54(p < 0.01) (Fig. 4C). With the gradually decreased or canceled exposure to imipenem, the expression levels of blaNDM−1 of all three strains were gradually decreased, and the RQ value was even less than 0.4 (Fig. 4F, H, I). However, the expression values of strains exposed to imipenem at 12 µg/mL to 8 µg/mL or 12 µg/mL to 0 µg/mL were higher than the control, suggesting that the strain exposed to imipenem of 12 µg/mL produced relatively stable blaNDM−1 expression (Fig. 4G, H, I), and the MIC values also supported this speculation.
Effect of imipenem on the blaNDM−1 expression of all three strains. The qRT-PCR was applied to detect the expression of blaNDM−1 of the last generation cells (OD600 reached 1.5-2.0 within 11-13 h) exposed to imipenem with grade increasing (control, 4 µg/mL, 8 µg/mL, and 12 µg/mL), decreasing (12 µg/mL to 8 µg/mL, 8 µg/mL to 4 µg/mL, and 4 µg/mL to 0 µg/mL), and canceling (12 µg/mL to 0 µg/mL, 8 µg/mL to 0 µg/mL, and 4 µg/mL to 0 µg/mL) doses, respectively
Discussion
K.pneumoniae and E.coli are important pathogens of community- and hospital-acquired infections, and they are also multi-drug-resistant bacteria posing a serious threat to the clinic. K.pneumoniae and E.coli generally carry many types of β-lactamase genes, such as early common blaTEM, blaSHV, and blaCTX−M, blaKPC, and blaNDM−1, which have become mainstream in recent years [13, 26–29]. The coexistence of blaNDM−1 and other β-lactamase genes, and their drug resistance phenotype have attracted much attention; however, not enough attention was drawn to the sensitive bacteria carrying blaNDM−1. In this study, the gene analysis of 40 isolates showed that the higher cephalosporin resistance rates of K.pneumoniae and E.coli were probably related to their drug resistance genes. K.pneumoniae generally carried blaSHV (100%, n = 20) and blaCTX−M−9 (70%, n = 14). K.pneumoniae TH-P12498 and K.pneumoniae TH-P12158 carried blaIMP and blaNDM−1 of carbapenemase genes respectively and coexisted with blaSHV and blaCTX−M−9. Compared with K.pneumoniae, E.coli carried higher blaTEM−1 (35%, n = 7) and blaCTX−M−9 (50%, n = 10). Among them, all four strains of E.coli (TH-U3224, TH-U3226, TH-U3265, and TH-U3268) isolated from patients’ urine samples carried carbapenem gene blaIMP and coexisted with blaTEM−1 and blaCTX−M−9. The phenotypic and genotypic characteristics of 40 isolates showed that blaSHV, blaTEM−1, blaCTX−M−9, and blaIMP might be transmitted horizontally within or between K.pneumoniae and E.coli. The high carrier rate of blaIMP in E.coli should be highlighted, although strains did not show carbapenem resistance phenotype.
The rapid expansion of acquired carbapenem resistance is increasingly propagated by mobile genetic elements such as epidemic plasmids that transfer carbapenemase genes within and between Enterobacteriaceae [27]. Plasmids harboring blaNDM are frequently larger than 100 kb and belong to the incompatibility groups IncA/C, IncX3 or IncF with a broad-host range. The higher fitness cost of blaNDM−1 plasmid in E.coli may determine that it mostly exists in K.pneumoniae, but rarely in E.coli [26]. Although our study also found that most of the blaNDM−1 positive drug-resistant bacteria are K.pneumoniae, the wild plasmid (123KB) coexisting with other drug-resistant genes (blaCTX−M−9, blaTEM−1), or the artificially constructed blaNDM−1-pET28a (+) plasmid can be transferred to E. coli[14].
Wild type or artificially constructed blaNDM−1 plasmid do not show carbapenem resistance phenotype (MIC ˂1 µg/mL), which may be related to a variety of factors, such as host compatibility, antibiotic stress, and so on. Antibiotics are considered to be the most important driving force for accelerating the transformation of drug-resistant genes. They exert selective pressure on bacteria to make the mutation of chromosome genes and the spread of existing or rising genes, thus enhancing the resistance and virulence of bacteria [11, 12, 27, 30]. Antibiotic-induced mutagenesis is typically studied at antibiotic concentrations close to, but below the MIC value [28]. Under the exposure of imipenem, meropenem, and ertapenem stress, an upregulated expression of blaNDM−1 was observed by quantitative real-time polymerase chain reaction [18]. K.pneumoniae TH-P12158, E.coli DH5α-blaNDM−1, and E.coli BL21 (DE3)-blaNDM−1 showed resistance phenotype and genotype changes positively correlated with increased imipenem exposure, this suggests that the blaNDM−1 experienced the same up-regulation. However, with the decrease or cancellation of imipenem exposure, the expression of blaNDM−1 was down-regulated, although the resistant phenotype remained unchanged. Based on the MIC and MBC values of imipenem, we can confirm that the resistant phenotypes of strains carrying blaNDM−1 have produced stable memory.
Cross-resistance is a phenomenon where the acquisition of resistance to a specific drug causes resistance to another drug simultaneously [29]. K.pneumoniae TH-P12158, E.coli DH5α-blaNDM−1, and E.coli BL21 (DE3)-blaNDM−1 showed enhanced resistance to exposed and unexposed antibiotics (MEM, CXM, CAZ, CSS, and PSTS), indicating that a specific antibiotic exposure will inevitably lead to an expanded antimicrobial spectrum. We do not know whether imipenem can specifically press blaNDM−1 or only is an antibiotic stress factor. But so far, it is certain that the drug resistance phenotype of the exposed strains can be stably sub-cultured with chromosome mutation and horizontal transmission to the 20th passage (Table S3). Special attention should be paid to drug resistance caused by the mutation of sensitive bacteria or the horizontal transfer of drug-resistant genes caused by antibiotic exposure. The transmission or expression of drug-resistant genes after the decrease or cancellation of exposure factors is more significant for the use of clinical drugs.
Data Availability
The datasets used or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- bla NDM−1 :
-
New Delhi Metallo-β-Lactamase-1 gene
- MIC:
-
Minimum inhibitory concentration
- MBC:
-
Minimum bactericidal concentration
- DRE:
-
Drug-resistant Enterobacteriaceae
- ESBLs:
-
Extended-spectrum β-lactamases
- MBLs:
-
Metallo-β-Lactamases
- NDM-1:
-
New Delhi Metallo-β-Lactamases-1
- qRT-PCR:
-
Quantitative real-time-PCR
- SAM:
-
Ampicillin/sulbactam
- CAP:
-
Chloramphenicol
- CIP:
-
Ciprofloxacin
- CRO:
-
Ceftriaxone
- CFZ:
-
Cefazolin
- CXM:
-
Cefuroxime
- FOX:
-
Cefoxitin
- CAZ:
-
Ceftazidime
- FEP:
-
Cefepime
- CN:
-
Gentamicin
- TIM:
-
Ticarcillin/Clavulanic acid
- PTZ:
-
Piperacillin/tazobactam
- MI:
-
Minocycline
- SXT:
-
Sulfamethoxazole
- LEV:
-
Levofloxacin
- IPM:
-
Imipenem
- MEM:
-
Meropenem
- CSSS:
-
Cefoperazone sodium/sulbactam sodium
- PSTS:
-
Piperacillin sodium /tazobactam sodium.
References
Iredell J, Brown J, Tagg K. Antibiotic resistance in Enterobacteriaceae: mechanisms and clinical implications. BMJ. 2016;352:h6420.
Friedman ND, Temkin E, Carmeli Y. The negative impact of antibiotic resistance. Clin Microbiol Infect. 2016;22:416–22.
Bush K, Bradford PA. Epidemiology of β-Lactamase-producing pathogens. Clin Microbiol Rev. 2020;33:e00047–19.
Tooke CL, Hinchliffe P, Bragginton EC, Colenso CK, Hirvonen VHA, Takebayashi Y, et al. β-Lactamases and β-Lactamase inhibitors in the 21st century. J Mol Biol. 2019;431:3472–500.
Yigit H, Queenan AM, Anderson GJ, Domenech-Sanchez A, Biddle JW, Steward CD, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother. 2001;45:1151–61.
Majiduddin FK, Palzkill T. Amino acid sequence requirements at residues 69 and 238 for the SME-1 β-lactamase to confer resistance to β-lactam antibiotics. Antimicrob Agents Chemother. 2003;47:1062–7.
Smith CA, Nossoni Z, Toth M, Stewart NK, Frase H, Vakulenko SB. Role of the conserved disulfide bridge in class A carbapenemases. J Biol Chem. 2016;291:22196–206.
Fonseca F, Chudyk EI, van der Kamp MW, Correia A, Mulholland AJ, Spencer J. The basis for carbapenem hydrolysis by class A β-lactamases: a combined investigation using crystallography and simulations. J Am Chem Soc. 2012;134:18275–85.
Rasmussen BA, Bush K, Keeney D, Yang YJ, Hare R, O’gare C, et al. Characterization of IMI-1 β-lactamase, a class A carbapenem-hydrolyzing enzyme from Enterobacter cloacae. Antimicrob Agents Chemother. 1996;40:2080–6.
Rodríguez-Martínez JM, Poirel L, Nordmann P. Extended-spectrum cephalosporinases in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2009;53:1766–71.
Prudhomme M, Attaiech L, Sanchez G, Martin B, Claverys JP. Antibiotic stress induces genetic transformability in the human pathogen Streptococcus pneumoniae. Science. 2006;313:89–92.
Charpentier X, Polard P, Claverys JP. Induction of competence for genetic transformation by antibiotics: convergent evolution of stress responses in distant bacterial species lacking SOS? Curr Opin Microbiol. 2012;15:570–6.
Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, Lee K, et al. Characterization of a new metallo-β-lactamase gene, blaNDM–1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 2009;53:5046–54.
Yu CF, Wei XL, Wang ZH, Liu L, Liu ZX, Liu J, et al. Occurrence of two NDM-1-producing Raoultella ornithinolytica and Enterobacter cloacae in a single patient in China: probable a novel antimicrobial resistance plasmid transfer in vivo by conjugation. J Glob Antimicrob Resist. 2020;22:835–41.
Du N, Liu SM, Niu M, Duan Y, Zhang SM, Yao J, et al. Transmission and characterization of blaNDM–1 in Enterobacter cloacae at a teaching hospital in Yunnan, China. Ann Clin Microbiol Antimicrob. 2017;16:58.
Zhao WH, Hu ZQ. Acquired Metallo-β-lactamases and their genetic association with class 1 integrons and ISCR elements in gram-negative bacteria. Future Microbiol. 2015;10:873–87.
Schwaber MJ, Carmeli Y. Carbapenem-resistant Enterobacteriaceae: a potential threat. JAMA. 2008;300:2911–13.
Paul D, Garg A, Bhattacharjee A. Occurrence of blaNDM–1 and blaNDM–5 in a tertiary referral hospital of North India. Microb Drug Resist. 2017;23:815–21.
Karthikeyan K, Thirunarayan MA, Krishnan P. Coexistence of blaOXA–23 with blaNDM–1 and armA in clinical isolates of Acinetobacter baumannii from India. J Antimicrob Chemother. 2010;65:2253–4.
Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. Carbapenems: past, present, and future. Antimicrob Agents Chemother. 2011;55:4943–60.
Chen C, Sun LY, Gao H, Kang PW, Li JQ, Zhen JB, et al. Identification of cisplatin and palladium(II) complexes as potent Metallo-β-lactamase inhibitors for targeting carbapenem-resistant Enterobacteriaceae. ACS Infect Dis. 2020;6:975–85.
King DT, Worrall LJ, Gruninger R, Strynadka NC. New Delhi Metallo-β-lactamase: structural insights into β-lactam recognition and inhibition. J Am Chem Soc. 2012;134:11362–5.
Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial susceptibility tests for Bacteria that grow aerobically. 7th ed. Wayne, PA: CLSI; 2006.
Chan WT, Verma CS, Lane DP, Gan SK. A comparison and optimization of methods and factors affecting the transformation of Escherichia coli. Biosci Rep. 2013;33:e00086.
Centers for Disease Control and Prevention. Facility guidance for control of carbapenem-resistant Enterobacteriaceae (CRE). Available from http://www.cdc.gov/hai/pdfs/cre/CRE-guidance-508.pdf. Accessed November. 2015.
Göttig S, Riedel-Christ S, Saleh A, Kempf VA, Hamprecht A. Impact of blaNDM–1 on fitness and pathogenicity of Escherichia coli and Klebsiella pneumoniae. Int J Antimicrob Agents. 2016;47:430–5.
Brink AJ. Epidemiology of carbapenem-resistant Gram-negative infections globally. Curr Opin Infect Dis. 2019;32:609–16.
Revitt-Mills SA, Robinson A. Antibiotic-induced mutagenesis: under the microscope. Front Microbiol. 2020;11:585175.
Suzuki S, Horinouchi T, Furusawa C. Prediction of antibiotic resistance by gene expression profiles. Nat Commun. 2014;5:5792.
Neu HC. The crisis in antibiotic resistance. Science. 1992;257:1064–73.
Acknowledgements
Not applicable.
Funding
This work was supported by special funds from the Foundation of Health Commission of Hubei Province (WJ2021F049), the Advantages Discipline Group (Biology and Medicine) Project in Higher Education of Hubei Province (2021–2025) (2022BMXKQY1), and the Open Project of Hubei Key Laboratory of Wudang Local Chinese Medicine Research (Hubei University of Medicine) (Grant No.WDCM2019016).
Author information
Authors and Affiliations
Contributions
ZJ.LM.and HZ.designed and drafted the study and QZ.LS.JY.and ZW.FY.collected the isolates and performed the antimicrobial susceptibility test and qRT-PCR experiment and LW.and CY.carried out the molecular biology experiments. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
Not required.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12941_2023_598_MOESM1_ESM.doc
Additional files 1: Table S1. The resistance of 20 clinical isolates to 17 antibiotics.
Additional files 2: Table S2. Transmission of drug resistance phenotype of imipenem exposed strains in subculture cells.
Additional files 3: Fig. S1. The electropherogram map of blaNDM−1-pET28a (+) plasmid.
Additional files 4: Fig. S2. The electropherogram map of blaNDM−1 gene amplified from blaNDM−1-pET28a (+) plasmid.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhao, Q., Sha, L., Wu, Z. et al. Evolution of carbapenem resistance in klebsiella pneumoniae and escherichia coli carrying blaNDM−1 gene: imipenem exposure results in sustained resistance memory of strains in vitro. Ann Clin Microbiol Antimicrob 22, 46 (2023). https://doi.org/10.1186/s12941-023-00598-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12941-023-00598-8