Abstract
Background
While previous studies primarily focused on the structure of the normal whole mouse lung, the whole bronchus and cytoarchitectural details of the mouse intact lung lobe have been discovered at single-cell resolution. Revealing the sophisticated lung adenocarcinoma structure at three-dimensional (3D) and single-cell level remains a fundamental and critical challenge for the pathological mechanism research of lung adenocarcinoma (LA).
Methods
Fluorescence micro-optical Sectioning Tomography (fMOST) combined with PI staining were used to obtain the 3D imaging of the human LA tissue at single-cell resolution.
Results
With a spatial resolution of 0.32 × 0.32 × 1.0 μm3, the dataset of human LA with single-cell precision consists of two channels, each of which contains information about the bronchi and the cytoarchitecture. The bronchial wall is thicker and the lumen is smaller in the cancer tissue, in which its original normal structure is vanished. More solid components, more clustered cancer cells with larger nucleoli, and more significant atypia are found in cancer tissue. In paracancerous tissue, the bronchial wall cells have a monolayer or bilayer structure, cluster along the wall, and are relatively dispersed. Few fibrous structures and occasional dissemination of spread through air spaces (STAS) are observed.
Conclusions
Based on the human LA tissue dataset obtained by fMOST and PI staining, the bronchi and cells were reconstructed and visualized. This work provides a technical roadmap for studying the bronchus and cytoarchitectural structure and their spatial relationship in LA tissue, which may help with the understanding of the main histological structure of LA among pathologists.
Similar content being viewed by others
Introduction
Lung cancer is one of the serious public health problems worldwide [1]. About 85% of all cases of lung cancer are non-small cell lung cancer (NSCLC). Due to the absence of specific symptoms in patients with early NSCLC, approximately 70% of NSCLC patients were already in advanced stage once diagnosis, accompanied by local or distant metastases (stage III and IV), which led to a 5-year overall survival (OS) rate of only 10–15% [2]. However, the 5-year OS rate of NSCLC patients at early-stage after surgery is 60–90%. In addition, patients with NSCLC also have a high likelihood of disease impoverishing and the incidence of catastrophic health expenditures is as high as 82.3% [3]. Lung adenocarcinoma (LA) accounts for 68.6% of NSCLC. In recent 20 years, the incidence of LA in China is the highest among people under the age of 49, posing an immeasurable loss of human resources to the society [4].
According to the 2015 World Health Organization (WHO) classification of lung adenocarcinoma, lung adenocarcinoma is divided into five subtypes: micropapillary, lepidic, acinar, solid and papillary. Classification and stratification according to the primary structure of lung adenocarcinoma can predict the efficacy of adjuvant chemotherapy [5]. These five subtypes are classified into three prognostic groups based on the pathology: low grade (mostly lepidic), intermediate grade (primarily acinar or papillary) and high grade (primarily solid or micropapillary) [6]. LA is histologically heterogeneous, displaying an aggregate of multiple structures and proportions.
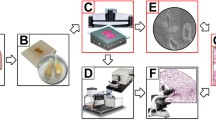
The acinar type is the most prevalent (40–50%) and has the greatest prognostic spectrum when categorized solely by main structures [7]. However, some pathologists may classify these structures as high grade (solid) or intermediate grade (acinar) due to a lack of knowledge about these histological features, leading to uncertainty in tumor classification. Currently, the reproducibility of histological structure assessment of lung adenocarcinoma by multiple pathologists is a challenge [8]. In the study by Moreira AL et al. [Processing of images Two detection channels performed all image preprocessing of the obtained data. Based on precise spatial orientation and nearby overlap, the stripes of each coronal portion were stitched to generate a full section. Section by section, lateral illumination adjustment was carried out. Calculating the mean intensity along each direction and fitting the relevant polynomial curves led to the determination of the correction coefficient along each direction. By calculating the images’ average grey-scale values, it was possible to adjust for axial lighting by balancing the brightness of the various coronal portions. Finally, using LZW compression, we created a single image at the imaging plane for image storage in a 16-bit depth TIFF format. Matlab and C + + were implemented to preprocess obtained images. On a computer server with 32 cores operating at 2.9 GHz per core, image preprocesses for the LA tissue data set at the voxel resolution of 0.32 × 0.32 × 1.0 µm3 were carried out. To create the charts and movies, we used the Amira software (version 2020.1, FEI, Merignac Cedex, France) and the Imaris software (version 9.7.2, bitplane, Switzerland) to visualize the dataset. Amira software was used to import the preprocessed dataset using a desktop graphical workstation (7920 with one Intel Xeon Gold 6226R CPU, 512 GB memory and an Nvidia GeForce RTX 3090 graphics card, Dell Inc., Round Rock, Texas, USA). We used the OTSU thresholding technique in conjunction with manually corrected parameters on the red channel cytoarchitecture pictures to reconstruct the bronchus. Amira was used to convert the TIFF data format to the native LDA type so that the TB-sized data could be processed on a single workstation. The extraction of the data in the range of interest, sampling or interpolation, reslicing of the images, determination of the maximum intensity projection, volume and surface rendering, and creation of movies using Amira's main module were all steps in the visualization process [30, 31]. The quality of samples directly determines the success of fMOST imaging, so the sample should be selected strictly. A small piece of tissue was scraped off the surface of the tissue and made into HE sections for pathological examination. The sections were examined back-to-back by two professional and technical personnel of the department of pathology, and the typical stage II lung adenocarcinoma were confirmed as qualified samples.Preprocessing of images
Reconstruction and visualization
Quality control
Availability of data and materials
The datasets supporting this study's findings are included in the publication and its supplemental materials. On reasonable request, the corresponding author will provide raw data.
Abbreviations
- 3D:
-
Three dimensional
- LA:
-
Lung adenocarcinoma
- fMOST:
-
Fluorescence micro-optical sectioning tomography
- STAS:
-
Spread through air spaces
- PI:
-
Propidium iodide
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- WHO:
-
World Health Organization
- 2D:
-
Two dimensional
- WSI:
-
Whole slide imaging
- HE:
-
Hematoxylin–eosin
- VP:
-
Ventral pallidum
References
He J, Zhang JX, Chen CT, Ma Y, De Guzman R, Meng J, et al. The relative importance of clinical and socio-demographic variables in prognostic prediction in Non-small cell lung cancer: a variable importance approach. Med Care. 2020;58(5):461–7.
Lemjabbar-Alaoui H, Hassan OU, Yang YW, Buchanan P. Lung cancer: biology and treatment options. Biochim Biophys Acta. 2015;1856(2):189–210.
Lortet-Tieulent J, Soerjomataram I, Ferlay J, Rutherford M, Weiderpass E, Bray F. International trends in lung cancer incidence by histological subtype: adenocarcinoma stabilizing in men but still increasing in women. Lung Cancer. 2014;84(1):13–22.
Chen GB, Du W, Zhou JP, Lu XZ, Huang XA, Hu WC, et al. Analysis on the trend of lung cancer case distribution and pathological features. Chin J Lung Dis (Electronic version). 2021;14(05):590–2.
Kim TH, Buonocore D, Petre EN, Durack JC, Maybody M, Johnston RP, et al. Utility of core biopsy specimen to identify histologic subtype and predict outcome for lung adenocarcinoma. Ann Thorac Surg. 2019;108(2):392–8.
Travis WD, World Health Organization. International agency for research on cancer, international association for the study of lung cancer., International academy of pathology: WHO classification of tumours of the lung, pleura, thymus and heart. Lyon Oxford: IARC Press, Oxford University Press; 2015.
Warth A, Muley T, Kossakowski C, Stenzinger A, Schirmacher P, Dienemann H, et al. Prognostic impact and clinicopathological correlations of the cribriform pattern in pulmonary adenocarcinoma. J Thorac Oncol. 2015;10(4):638–44.
Thunnissen E, Beasley MB, Borczuk AC, Brambilla E, Chirieac LR, Dacic S, et al. Reproducibility of histopathological subtypes and invasion in pulmonary adenocarcinoma. An international interobserver study. Mod Pathol. 2012;25(12):1574–83.
Moreira AL, Ocampo PSS, **a Y, Zhong H, Russell PA, Minami Y, et al. A grading system for invasive pulmonary adenocarcinoma: a proposal from the international association for the study of lung cancer pathology committee. J Thorac Oncol. 2020;15(10):1599–610.
Yagi Y, Aly RG, Tabata K, Barlas A, Rekhtman N, Eguchi T, et al. Three-dimensional histologic, immunohistochemical, and multiplex immunofluorescence analyses of dynamic vessel co-option of spread through air spaces in lung adenocarcinoma. J Thorac Oncol. 2020;15(4):589–600.
Gong H, Zeng S, Yan C, Lv X, Yang Z, Xu T, et al. Continuously tracing brain-wide long-distance axonal projections in mice at a one-micron voxel resolution. Neuroimage. 2013;74:87–98.
Dupret-Bories A, Vergez S, Meresse T, Brouillet F, Bertrand G. Contribution of 3D printing to mandibular reconstruction after cancer. Eur Ann Otorhinolaryngol Head Neck Dis. 2018;135(2):133–6.
Hadeed K, Acar P, Dulac Y, Cuttone F, Alacoque X, Karsenty C. Cardiac 3D printing for better understanding of congenital heart disease. Arch Cardiovasc Dis. 2018;111(1):1–4.
Kuroda S, Kobayashi T, Ohdan H. 3D printing model of the intrahepatic vessels for navigation during anatomic resection of hepatocellular carcinoma. Int J Surg Case Rep. 2017;41:219–22.
Shimizu K, Nagashima T, Ohtaki Y, Obayashi K, Nakazawa S, Kamiyoshihara M, et al. Analysis of the variation pattern in right upper pulmonary veins and establishment of simplified vein models for anatomical segmentectomy. Gen Thorac Cardiovasc Surg. 2016;64(10):604–11.
Akiba T. Utility of three-dimensional computed tomography in general thoracic surgery. Gen Thorac Cardiovasc Surg. 2013;61(12):676–84.
Akiba T, Marushima H, Harada J, Kobayashi S, Morikawa T. Importance of preoperative imaging with 64-row three-dimensional multidetector computed tomography for safer video-assisted thoracic surgery in lung cancer. Surg Today. 2009;39(10):844–7.
Kato H, Oizumi H, Suzuki J, Hamada A, Watarai H, Sadahiro M. Thoracoscopic anatomical lung segmentectomy using 3D computed tomography simulation without tumour markings for nonpalpable and non-visualized small lung nodules. Interact Cardiovasc Thorac Surg. 2017;25(3):434–41.
Ji Y, Zhang T, Yang L, Wang X, Qi L, Tan F, et al. The effectiveness of three-dimensional reconstruction in the localization of multiple nodules in lung specimens: a prospective cohort study. Transl Lung Cancer Res. 2021;10(3):1474–83.
Tsai PC, Yeh YC, Hsu PK, Chen CK, Chou TY, Wu YC. CT-guided core biopsy for peripheral sub-solid pulmonary nodules to predict predominant histological and aggressive subtypes of lung adenocarcinoma. Ann Surg Oncol. 2020;27(11):4405–12.
Choi SH, Jeong JY, Lee SY, Shin KM, Jeong SY, Park TI, et al. Clinical implication of minimal presence of solid or micropapillary subtype in early-stage lung adenocarcinoma. Thorac Cancer. 2021;12(2):235–44.
Emoto K, Eguchi T, Tan KS, Takahashi Y, Aly RG, Rekhtman N, et al. Expansion of the concept of micropapillary adenocarcinoma to include a newly recognized filigree pattern as well as the classical pattern based on 1468 stage I lung adenocarcinomas. J Thorac Oncol. 2019;14(11):1948–61.
Mäkinen JM, Laitakari K, Johnson S, Mäkitaro R, Bloigu R, Pääkkö P, et al. Histological features of malignancy correlate with growth patterns and patient outcome in lung adenocarcinoma. Histopathology. 2017;71(3):425–36.
Kadota K, Kushida Y, Kagawa S, Ishikawa R, Ibuki E, Inoue K, et al. Cribriform subtype is an independent predictor of recurrence and survival after adjustment for the eighth Edition of TNM staging system in patients with resected lung adenocarcinoma. J Thorac Oncol. 2019;14(2):245–54.
Zhang Q, Li A, Chen S, Yuan J, Jiang T, Li X, et al. Multiscale reconstruction of various vessels in the intact murine liver lobe. Commun Biol. 2022;5(1):260.
Wang X, **ong H, Liu Y, Yang T, Li A, Huang F, et al. Chemical sectioning fluorescence tomography: high-throughput, high-contrast, multicolor, whole-brain imaging at subcellular resolution. Cell Rep. 2021;34(5):108709.
Gong H, Xu D, Yuan J, Li X, Guo C, Peng J, et al. High-throughput dual-colour precision imaging for brain-wide connectome with cytoarchitectonic landmarks at the cellular level. Nat Commun. 2016;7:12142.
Feng Q, An S, Wang R, Lin R, Li A, Gong H, et al. Whole-brain reconstruction of neurons in the ventral pallidum reveals diverse projection patterns. Front Neuroanat. 2021;15:801354.
Wu Z, Huang Z, Qin Y, Jiao W. Progress in three-dimensional computed tomography reconstruction in anatomic pulmonary segmentectomy. Thorac Cancer. 2022;13(13):1881–7.
Guo J, Meng S, Su H, Zhang B, Li T. Non-invasive optical monitoring of human lungs: Monte Carlo modeling of photon migration in Visible Chinese Human and an experimental test on a human. Biomed Opt Express. 2023;13(12):6389–403.
Meng S, Su H, Guo J, Wang L, Li T. Noninvasive optical monitoring of pulmonary embolism: a Monte Carlo study on visible Chinese human thoracic tissues. J Biomed Opt. 2023;28(1):015001.
Acknowledgements
None declared.
Funding
The research was funded by National Natural Science Foundation of Tian** (No.21JCYBJC00260), Tian** Key Medical Discipline (Thoracic Surgery) Construction Project (No. TJYXZDXK-018A), National Natural Science Foundation of China (No.81971660), Medical & Health Innovation Project (2021-I2M-1-042, 2021-I2M-1-058), Sichuan Science and Technology Program (No. 2021YFH0004), Tian** Outstanding Youth Fund Project (No. 20JCJQIC00230), Program of Chinese Institute for Brain Research in Bei**g (2020-NKX-XM-14) and CAMS Innovation Fund for Medical Sciences (2022-I2M-C&T-B-001).
Author information
Authors and Affiliations
Contributions
XL and BZ prepared the manuscript; LM prepared the figures and movies; TL participated in the results interpretation. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by Institutional Review Board of Tian** Chest Hospital and informed consent was taken from the patient.
Consent for publication
All the authors give consent for the publication.
Competing interests
The authors have no competing interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Imaging of bronchi channel of human lung adenocarcinoma tissue.
Additional file 2.
Imaging of cytoarchitecture channel of human lung adenocarcinoma tissue.
Additional file 3. 3D bird’s-eye view of human lung adenocarcinoma tissue.
Additional file 4. A bronchus was tracked and located in paracancerous (left) and cancer (right) tissue respectively.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, X., Zhang, B., Liang, Y. et al. Multiscale reconstruction of bronchus and cancer cells in human lung adenocarcinoma. BioMed Eng OnLine 22, 11 (2023). https://doi.org/10.1186/s12938-023-01072-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12938-023-01072-4