Wahid F, Shehzad A, Khan T, Kim YY. MicroRNAs: synthesis, mechanism, function, and recent clinical trials. Biochim Biophys Acta. 2010. https://doi.org/10.1016/j.bbamcr.2010.06.013.
Article
Google Scholar
Chen R, Jiang N, Jiang Q, Sun X, Wang Y, Zhang H, Hu Z. Exploring microRNA-like small RNAs in the filamentous fungus Fusarium oxysporum. PLoS ONE. 2014. https://doi.org/10.1371/journal.pone.0104956.
Article
Google Scholar
Lee RC, Feinbaum RL, Ambros V. The C elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993. https://doi.org/10.1016/0092-8674(93)90529-Y.
Article
Google Scholar
Alvarez-Garcia I, Miska EA. MicroRNA functions in animal development and human disease. Development. 2005. https://doi.org/10.1242/dev.02073.
Article
Google Scholar
Kozomara A, Griffiths-Jones S. MiRBase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014. https://doi.org/10.1093/nar/gkt1181.
Article
Google Scholar
Kozomara A, Birgaoanu M, Griffiths-Jones S. MiRBase: From microRNA sequences to function. Nucleic Acids Res. 2019. https://doi.org/10.1093/nar/gky1141.
Article
Google Scholar
Cai Y, Yu X, Hu S, Yu J. A Brief Review on the Mechanisms of miRNA Regulation. Genomics Proteomics Bioinform. 2009. https://doi.org/10.1016/S1672-0229(08)60044-3.
Article
Google Scholar
Oyama Y, Bartman CM, Gile J, Eckle T. Circadian microRNAs in cardioprotection. Curr Pharm Design. 2017. https://doi.org/10.2174/1381612823666170707165319.
Article
Google Scholar
Chen W, Liu Z, Li T, Zhang R, Xue Y, Zhong Y, Bai W, Zhou D, Zhao Z. Regulation of Drosophila circadian rhythms by miRNA let-7 is mediated by a regulatory cycle. Nat Commun. 2014. https://doi.org/10.1038/ncomms6549.
Article
Google Scholar
Magri F, Vanoli F, Corti S. miRNA in spinal muscular atrophy pathogenesis and therapy. J Sel Mol Med. 2018. https://doi.org/10.1016/j.bbamcr.2010.06.013.
Article
Google Scholar
Ma Y. The Challenge of microRNA as a Biomarker of Epilepsy. Curr Neuropharmacol. 2017. https://doi.org/10.2174/1570159x15666170703102410.
Article
Google Scholar
Xue L, Wang Y, Yue S, Zhang J. The expression of miRNA-221 and miRNA-222 in gliomas patients and their prognosis. Neurol Sci. 2017. https://doi.org/10.1007/s10072-016-2710-y.
Article
Google Scholar
Wojciechowska A, Braniewska A, Kozar-Kamińska K. MicroRNA in cardiovascular biology and disease. Adv Clin Exp Med. 2017. https://doi.org/10.17219/acem/62915.
Article
Google Scholar
Biswas S, Haleyurgirisetty M, Lee S, Hewlett I, Devadas K. Development and validation of plasma miRNA biomarker signature panel for the detection of early HIV-1 infection. EBioMedicine. 2019. https://doi.org/10.1016/j.ebiom.2019.04.023.
Article
Google Scholar
Backes C, Meese E, Keller A. Specific miRNA disease biomarkers in blood, serum and plasma: challenges and prospects. Mol Diagn Therapy. 2016. https://doi.org/10.1007/s40291-016-0221-4.
Article
Google Scholar
Izzotti A, Carozzo S, Pulliero A, Zhabayeva D, Ravetti JL, Bersimbaev R. Extracellular MicroRNA in liquid biopsy: applicability in cancer diagnosis and prevention. Am J Cancer Res. 2016;6(7):1461–93.
Google Scholar
Shigeyasu K, Toden S, Zumwalt TJ, Okugawa Y, Goel A. Emerging role of microRNAs as liquid biopsy biomarkers in gastrointestinal cancers. Clin Cancer Res. 2017. https://doi.org/10.1158/1078-0432.CCR-16-1676.
Article
Google Scholar
Faruq O, Vecchione A. microRNA: Diagnostic perspective. Front Med. 2015. https://doi.org/10.3389/fmed.2015.00051.
Article
Google Scholar
Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, Galas DJ, Wang K. The microRNA spectrum in 12 body fluids. Clin Chem. 2010. https://doi.org/10.1373/clinchem.2010.147405.
Article
Google Scholar
Cheerla N, Gevaert O. MicroRNA based pan-cancer diagnosis and treatment recommendation. BMC Bioinform. 2017. https://doi.org/10.1186/s12859-016-1421-y.
Article
Google Scholar
World Health Organization. Cancer Today. 2018. https://doi.org/10.1371/journal.pone.0104956.
INCA: INCA. Estimativa 2020 : incidência de câncer no Brasil. Technical report, INCA; 2019.
Dong H, Luo L, Hong S, Siu H, **ao Y, ** L, Chen R, **ong M. Integrated analysis of mutations, miRNA and mRNA expression in glioblastoma. BMC Syst Biol. 2010. https://doi.org/10.1186/1752-0509-4-163.
Article
Google Scholar
Bayraktar R, Van Roosbroeck K, Calin GA. Cell-to-cell communication: microRNAs as hormones. Mol Oncol. 2017. https://doi.org/10.1002/1878-0261.12144.
Article
Google Scholar
Toms D, Pan B, Li J. Endocrine regulation in the ovary by MicroRNA during the estrous cycle. Front Electrol. 2018. https://doi.org/10.3389/fendo.2017.00378.
Article
Google Scholar
Olejniczak M, Kotowska-Zimmer A, Krzyzosiak W. Stress-induced changes in miRNA biogenesis and functioning. Cell Mol Life Sci. 2018. https://doi.org/10.1007/s00018-017-2591-0.
Article
Google Scholar
Iorio MV, Croce CM. MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol Med. 2012. https://doi.org/10.1002/emmm.201100209.
Article
Google Scholar
Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011. https://doi.org/10.1093/nar/gkr254.
Article
Google Scholar
Chen X, Liang H, Zhang J, Zen K, Zhang CY. Secreted microRNAs: a new form of intercellular communication. Trens Cell Biol. 2012. https://doi.org/10.1016/j.tcb.2011.12.001.
Article
Google Scholar
O’Brien J, Hayder H, Zayed Y, Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne). 2018. https://doi.org/10.3389/fendo.2018.00402.
Article
Google Scholar
Ludwig N, Leidinger P, Becker K, Backes C, Fehlmann T, Pallasch C, Rheinheimer S, Meder B, Stähler C, Meese E, Keller A. Distribution of miRNA expression across human tissues. Nucleic Acids Res. 2016. https://doi.org/10.1093/nar/gkw116.
Article
Google Scholar
Ying W, Riopel M, Bandyopadhyay G, Dong Y, Birmingham A, Seo JB, Ofrecio JM, Wollam J, Hernandez-Carretero A, Fu W, Li P, Olefsky JM. Adipose Tissue Macrophage-Derived Exosomal miRNAs Can Modulate in Vivo and in Vitro Insulin Sensitivity. Cell. 2017. https://doi.org/10.1016/j.cell.2017.08.035.
Article
Google Scholar
Vrtačnik P, Ostanek B, Mencej-Bedrač S, Marc J. The many faces of estrogen signaling. Biochem Med. 2014. https://doi.org/10.11613/BM.2014.035.
Article
Google Scholar
Shah MY, Ferrajoli A, Sood AK, Lopez-Berestein G, Calin GA. microRNA Therapeutics in Cancer - An Emerging Concept. EBioMedicine. 2016. https://doi.org/10.1016/j.ebiom.2016.09.017.
Article
Google Scholar
Chakraborty C, Sharma AR, Sharma G, Doss CGP, Lee SS. Therapeutic miRNA and siRNA: Moving from Bench to Clinic as Next Generation Medicine. Mol Ther Nucleic Acids. 2017. https://doi.org/10.1016/j.omtn.2017.06.005.
Article
Google Scholar
Baumann V, Winkler J. MiRNA-based therapies: strategies and delivery platforms for oligonucleotide and non-oligonucleotide agents. Future Med Chem. 2014. https://doi.org/10.4155/fmc.14.116.
Article
Google Scholar
Yang N, Zhu S, Lv X, Qiao Y, Liu YJ, Chen J. MicroRNAs: Pleiotropic regulators in the tumor microenvironment. Front Immunol. 2018. https://doi.org/10.3389/fimmu.2018.02491.
Article
Google Scholar
Curtale G. MiRNAs at the Crossroads between Innate Immunity and Cancer: Focus on Macrophages. Cells. 2018. https://doi.org/10.3390/cells7020012.
Article
Google Scholar
Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008–2030): A population-based study. Lancet Oncol. 2012. https://doi.org/10.1016/S1470-2045(12)70211-5.
Article
Google Scholar
Yang HY, Barbi J, Wu CY, Zheng Y, Vignali PDA, Wu X, Tao JH, Park BV, Bandara S, Novack L, Ni X, Yang X, Chang KY, Wu RC, Zhang J, Yang CW, Pardoll DM, Li H, Pan F. MicroRNA-17 Modulates Regulatory T Cell Function by Targeting Co-regulators of the Foxp3 Transcription Factor. Immunity. 2016. https://doi.org/10.1016/0092-8674(93)90529-Y.
Article
Google Scholar
Hippen KL, Loschi M, Nicholls J, MacDonald KPA, Blazar BR. Effects of MicroRNA on regulatory T Cells and implications for adoptive cellular therapy to ameliorate graft-versus-host disease. Front Immunol. 2018. https://doi.org/10.3389/fimmu.2017.02014.
Article
Google Scholar
Squadrito ML, Etzrodt M, De Palma M, Pittet MJ. MicroRNA-mediated control of macrophages and its implications for cancer. Trends Immunol. 2013. https://doi.org/10.1016/j.it.2013.02.003.
Article
Google Scholar
Kong YW, Ferland-McCollough D, Jackson TJ, Bushell M. MicroRNAs in cancer management. Lancet Oncol. 2012. https://doi.org/10.1016/S1470-2045(12)70073-6.
Article
Google Scholar
Yang Y, Chaerkady R, Beer MA, Mendell JT, Pandey A. Identification of miR-21 targets in breast cancer cells using a quantitative proteomic approach. Proteomics. 2009. https://doi.org/10.1002/pmic.200800551.
Article
Google Scholar
Feng YH, Tsao CJ. Emerging role of microRNA-21 in cancer. Review. 2016. https://doi.org/10.3892/br.2016.747.
Article
Google Scholar
Wang H, Tan Z, Hu H, Liu H, Wu T, Zheng C, Wang X, Luo Z, Wang J, Liu S, Lu Z, Tu J. MicroRNA-21 promotes breast cancer proliferation and metastasis by targeting LZTFL1. BMC Cancer. 2019. https://doi.org/10.1242/dev.02073.
Article
Google Scholar
Xu LF, Wu ZP, Chen Y, Zhu QS, Hamidi S, Navab R. MicroRNA-21 (miR-21) regulates cellular proliferation, invasion, migration, and apoptosis by targeting PTEN, RECK and Bcl-2 in lung squamous carcinoma, Gejiu City, China. PLoS ONE. 2014. https://doi.org/10.1242/dev.02073.
Article
Google Scholar
Li P, Xu T, Zhou X, Liao L, Pang G, Luo W, Han L, Zhang J, Luo X, **e X, Zhu K. Downregulation of miRNA-141 in breast cancer cells is associated with cell migration and invasion: involvement of ANP32E targeting. Cancer Med. 2017. https://doi.org/10.1242/dev.02073.
Article
Google Scholar
Long ZH, Bai ZG, Song JN, Zheng Z, Li J, Zhang J, Cai J, Yao HW, Wang J, Yang YC, Yin J, Zhang ZT. MiR-141 inhibits proliferation and migration of colorectal cancer sw480 cells. Anticancer Res. 2017. https://doi.org/10.1242/dev.02073.
Article
Google Scholar
Huang S, Wa Q, Pan J, Peng X, Ren D, Huang Y, Chen X, Tang Y. Downregulation of miR-141-3p promotes bone metastasis via activating NF-\(\kappa \)B signaling in prostate cancer. J Exp Clin Cancer Res. 2017. https://doi.org/10.1242/dev.02073.
Article
Google Scholar
Liu J, Zhang C, Zhao Y, Feng Z. MicroRNA Control of p53. J Cell Biochem. 2017. https://doi.org/10.1002/jcb.25609.
Article
Google Scholar
Goeman F, Strano S, Blandino G. MicroRNAs as Key Effectors in the p53 Network. Int Rev Cell Mol Biol. 2017. https://doi.org/10.1093/nar/gkt1181.
Article
Google Scholar
Luo Z, Cui R, Tili E, Croce C. Friend or Foe: MicroRNAs in the p53 network. Cancer Lett. 2018. https://doi.org/10.1093/nar/gkt1181.
Article
Google Scholar
Niazi S, Purohit M, Niazi JH. Role of p53 circuitry in tumorigenesis: A brief review. Eur Med Chem. 2018. https://doi.org/10.1093/nar/gkt1181.
Article
Google Scholar
Shenouda SK, Alahari SK. MicroRNA function in cancer: Oncogene or a tumor suppressor? Cancer Metastasis Rev. 2009. https://doi.org/10.1093/nar/gkt1181.
Article
Google Scholar
Suzuki HI, Yamagata K, Sugimoto K, Iwamoto T, Kato S, Miyazono K. Modulation of microRNA processing by p53. Nature. 2009. https://doi.org/10.1093/nar/gkt1181.
Article
Google Scholar
Feng Z, Zhang C, Wu R, Hu W. Tumor suppressor p53 meets microRNAs. J Mol Cell Biol. 2011. https://doi.org/10.1093/nar/gkt1181.
Article
Google Scholar
Hamam R, Hamam D, Alsaleh KA, Kassem M, Zaher W, Alfayez M, Aldahmash A, Alajez NM. Circulating microRNAs in breast cancer: novel diagnostic and prognostic biomarkers. Cell Death Dis. 2017. https://doi.org/10.1093/nar/gkt1181.
Article
Google Scholar
Mognato M, Celotti L. MicroRNAs Used in Combination with Anti-Cancer Treatments Can Enhance Therapy Efficacy. Mini-Rev Med Chem. 2015. https://doi.org/10.1093/nar/gkt1181.
Article
Google Scholar
Petrovic N, Ergun S. miRNAs as Potential Treatment Targets and Treatment Options in Cancer. Mol Diagn Ther. 2018. https://doi.org/10.1093/nar/gkt1181.
Article
Google Scholar
Si W, Shen J, Zheng H, Fan W. The role and mechanisms of action of microRNAs in cancer drug resistance. Review. 2019. https://doi.org/10.1186/s13148-018-0587-8.
Article
Google Scholar
Chuang JC, Jones PA. Epigenetics and microRNAs. Pediatr Res. 2007. https://doi.org/10.1093/nar/gky1141.
Article
Google Scholar
Wang H, Peng R, Wang J, Qin Z, Xue L. Circulating microRNAs as potential cancer biomarkers: the advantage and disadvantage. Clin Epigenet. 2018. https://doi.org/10.1093/nar/gky1141.
Article
Google Scholar
Farzanehpour M, Mozhgani SH, Jalilvand S, Faghihloo E, Akhavan S, Salimi V, Azad TM. Serum and tissue miRNAs: Potential biomarkers for the diagnosis of cervical cancer. Virol J. 2019. https://doi.org/10.1093/nar/gky1141.
Article
Google Scholar
Heneghan HM, Miller N, Lowery AJ, Sweeney KJ, Kerin MJ. MicroRNAs as Novel Biomarkers for Breast Cancer. J Oncol. 2010. https://doi.org/10.1093/nar/gky1141.
Article
Google Scholar
Qadir MI, Faheem A. miRNA: a diagnostic and therapeutic tool for pancreatic cancer. Crit Rev Eukaryotic Gene Express. 2017. https://doi.org/10.1093/nar/gky1141.
Article
Google Scholar
Li X, Kleeman S, Coburn SB, Fumagalli C, Perner J, Jammula S, Pfeiffer RM, Orzolek L, Hao H, Taylor PR, Miremadi A, Galeano-Dalmau N, Lao-Sirieix P, Tennyson M, MacRae S, Cook MB, Fitzgerald RC. Selection and Application of Tissue microRNAs for Nonendoscopic Diagnosis of Barrett’s Esophagus. Gastroenterology. 2018. https://doi.org/10.1093/nar/gky1141.
Article
Google Scholar
Hanna J, Hossain GS, Kocerha J. The potential for microRNA therapeutics and clinical research. Front Genet. 2019. https://doi.org/10.1093/nar/gky1141.
Article
Google Scholar
Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005. https://doi.org/10.1093/nar/gky1141.
Article
Google Scholar
Rosenfeld N, Aharonov R, Meiri E, Rosenwald S, Spector Y, Zepeniuk M, Benjamin H, Shabes N, Tabak S, Levy A, Lebanony D, Goren Y, Silberschein E, Targan N, Ben-Ari A, Gilad S, Sion-Vardy N, Tobar A, Feinmesser M, Kharenko O, Nativ O, Nass D, Perelman M, Yosepovich A, Shalmon B, Polak-Charcon S, Fridman E, Avniel A, Bentwich I, Bentwich Z, Cohen D, Chajut A, Barshack I. MicroRNAs accurately identify cancer tissue origin. Nat Biotechnol. 2008. https://doi.org/10.1093/nar/gky1141.
Article
Google Scholar
Paranjape T, Slack FJ, Weidhaas JB. MicroRNAs: Tools for cancer diagnostics. Gut. 2009. https://doi.org/10.1136/gut.2009.179531.
Article
Google Scholar
Sun Y, Guo W, Wang F, Peng F, Yang Y, Dai X, Liu X, Bai Z. Transcriptome and multivariable data analysis of Corynebacterium glutamicum under different dissolved oxygen conditions in bioreactors. PLoS ONE. 2016. https://doi.org/10.1016/S1672-0229(08)60044-3.
Article
Google Scholar
Frères P, Wenric S, Boukerroucha M, Fasquelle C, Thiry J, Bovy N, Struman I, Geurts P, Collignon J, Schroeder H, Kridelka F, Lifrange E, Jossa V, Bours V, Josse C, Jerusalem G. Circulating microRNA-based screening tool for breast cancer. Oncotarget. 2016. https://doi.org/10.1016/S1672-0229(08)60044-3.
Article
Google Scholar
Calin GA, Ferracin M, Cimmino A, Di Leva G, Shimizu M, Wojcik SE, Iorio MV, Visone R, Sever NI, Fabbri M, Iuliano R, Palumbo T, Pichiorri F, Roldo C, Garzon R, Sevignani C, Rassenti L, Alder H, Volinia S, Liu CG, Kipps TJ, Negrini M, Croce CM. A microRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med. 2005. https://doi.org/10.1016/S1672-0229(08)60044-3.
Article
Google Scholar
Hironaka-Mitsuhashi A, Matsuzaki J, Takahashi RU, Yoshida M, Nezu Y, Yamamoto Y, Shiino S, Kinoshita T, Ushijima T, Hiraoka N, Shimizu C, Tamura K, Ochiya T. A tissue microRNA signature that predicts the prognosis of breast cancer in young women. PLoS ONE. 2017. https://doi.org/10.1016/S1672-0229(08)60044-3.
Article
Google Scholar
Peskoe SB, Barber JR, Zheng Q, Meeker AK, De Marzo AM, Platz EA, Lupold SE. Differential long-term stability of microRNAs and RNU6B snRNA in 12–20 year old archived formalin-fixed paraffin-embedded specimens. BMC Cancer. 2017. https://doi.org/10.1016/S1672-0229(08)60044-3.
Article
Google Scholar
Du L, Pertsemlidis A. Cancer and neurodegenerative disorders: Pathogenic convergence through microRNA regulation. J Mol Cell Biol. 2011. https://doi.org/10.1016/S1672-0229(08)60044-3.
Article
Google Scholar
Saito Y, Saito H. MicroRNAs in cancers and neurodegenerative disorders. Front Genet. 2012. https://doi.org/10.1016/S1672-0229(08)60044-3.
Article
Google Scholar
Godlewski J, Lenart J, Salinska E. MicroRNA in brain pathology: neurodegeneration the other side of the brain cancer. Noncoding RNA. 2019. https://doi.org/10.1016/S1672-0229(08)60044-3.
Article
Google Scholar
Asano N, Matsuzaki J, Ichikawa M, Kawauchi J, Takizawa S, Aoki Y, Sakamoto H, Yoshida A, Kobayashi E, Tanzawa Y, Nakayama R, Morioka H, Matsumoto M, Nakamura M, Kondo T, Kato K, Tsuchiya N, Kawai A, Ochiya T. A serum microRNA classifier for the diagnosis of sarcomas of various histological subtypes. Nat Commun. 2019. https://doi.org/10.1016/S1672-0229(08)60044-3.
Article
Google Scholar
Søkilde R, Persson H, Ehinger A, Pirona AC, Fernö M, Hegardt C, Larsson C, Loman N, Malmberg M, Rydén L, Saal L, Borg Å, Vallon-Christerson J, Rovira C. Refinement of breast cancer molecular classification by miRNA expression profiles. BMC Genomics. 2019. https://doi.org/10.1186/s12864-019-5887-7.
Article
Google Scholar
Lopez-Rincon A, Martinez-Archundia M, Martinez-Ruiz GU, Schoenhuth A, Tonda A. Automatic discovery of 100-miRNA signature for cancer classification using ensemble feature selection. BMC Bioinform. 2019. https://doi.org/10.2174/1381612823666170707165319.
Article
Google Scholar
Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M, Ménard S, Palazzo JP, Rosenberg A, Musiani P, Volinia S, Nenci I, Calin GA, Querzoli P, Negrini M, Croce CM. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005. https://doi.org/10.2174/1381612823666170707165319.
Article
Google Scholar
Li P, Guo Y, Bledsoe G, Yang Z, Chao L, Chao J. Kallistatin induces breast cancer cell apoptosis and autophagy by modulating Wnt signaling and microRNA synthesis. Exp Cell Res. 2016. https://doi.org/10.2174/1381612823666170707165319.
Article
Google Scholar
Liu J, Li X, Wang M, **ao G, Yang G, Wang H, Li Y, Sun X, Qin S, Du N, Ren H, Pang Y. A miR-26a/E2F7 feedback loop contributes to tamoxifen resistance in ER-positive breast cancer. Int J Oncol. 2018. https://doi.org/10.2174/1381612823666170707165319.
Article
Google Scholar
Tormo E, Adam-Artigues A, Ballester S, Pineda B, Zazo S, González-Alonso P, Albanell J, Rovira A, Rojo F, Lluch A, Eroles P. The role of miR-26a and miR-30b in HER2+ breast cancer trastuzumab resistance and regulation of the CCNE2 gene. Scientific Rep. 2017. https://doi.org/10.2174/1381612823666170707165319.
Article
Google Scholar
Dinami R, Ercolani C, Petti E, Piazza S, Ciani Y, Sestito R, Sacconi A, Biagioni F, Le Sage C, Agami R, Benetti R, Mottolese M, Schneider C, Blandino G, Schoeftner S. miR-155 drives telomere fragility in human breast cancer by targeting TRF1. Cancer Res. 2014. https://doi.org/10.2174/1381612823666170707165319.
Article
Google Scholar
Rao X, Di Leva G, Li M, Fang F, Devlin C, Hartman-Frey C, Burow ME, Ivan M, Croce CM, Nephew KP. MicroRNA-221/222 confers breast cancer fulvestrant resistance by regulating multiple signaling pathways. Oncogene. 2011. https://doi.org/10.2174/1381612823666170707165319.
Article
Google Scholar
Miller TE, Ghoshal K, Ramaswamy B, Roy S, Datta J, Shapiro CL, Jacob S, Majumder S. MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27Kip1. J Biol Chem. 2008. https://doi.org/10.2174/1381612823666170707165319.
Article
Google Scholar
Zhao JJ, Lin J, Yang H, Kong W, He L, Ma X, Coppola D, Cheng JQ. MicroRNA-221/222 negatively regulates estrogen receptor \(\alpha \) and is associated with tamoxifen resistance in breast cancer. J Biol Chem. 2008. https://doi.org/10.2174/1381612823666170707165319.
Article
Google Scholar
Mishra S, Srivastava AK, Suman S, Kumar V, Shukla Y. Circulating miRNAs revealed as surrogate molecular signatures for the early detection of breast cancer. Cancer Lett. 2015. https://doi.org/10.1016/j.canlet.2015.07.045.
Article
Google Scholar
Moody L, He H, Pan YX, Chen H. Methods and novel technology for microRNA quantification in colorectal cancer screening. Clin Epigent. 2017. https://doi.org/10.1038/ncomms6549.
Article
Google Scholar
Garcia-Elias A, Alloza L, Puigdecanet E, Nonell L, Tajes M, Curado J, Enjuanes C, Díaz O, Bruguera J, Martí-Almor J, Comín-Colet J, Benito B. Defining quantification methods and optimizing protocols for microarray hybridization of circulating microRNAs. Scientific Rep. 2017. https://doi.org/10.1038/ncomms6549.
Article
Google Scholar
Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O’Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. 2008. https://doi.org/10.1038/ncomms6549.
Article
Google Scholar
Gelband H, Sankaranarayanan R, Gauvreau CL, Horton S, Anderson BO, Bray F, Cleary J, Dare AJ, Denny L, Gospodarowicz MK, Gupta S, Howard SC, Jaffray DA, Knaul F, Levin C, Rabeneck L, Rajaraman P, Sullivan T, Trimble EL, Jha P. Costs, affordability, and feasibility of an essential package of cancer control interventions in low-income and middle-income countries: Key messages from Disease Control Priorities, 3rd edition. 2016. https://doi.org/10.1016/S0140-6736(15)00755-2
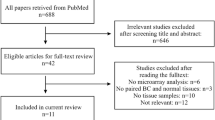
**ao Y, Watson M. Guidance on conducting a systematic literature review. J Plan Educ Res. 2019;39(1):93–112.
Article
Google Scholar
Liu C, Zhang S, Wang Q, Zhang X. Tumor suppressor miR-1 inhibits tumor growth and metastasis by simultaneously targeting multiple genes. Oncotarget. 2017. https://doi.org/10.1038/ncomms6549.
Article
Google Scholar
Gong Y, He T, Yang L, Yang G, Chen Y, Zhang X. The role of miR-100 in regulating apoptosis of breast cancer cells. Scientific Rep. 2015. https://doi.org/10.1038/ncomms6549.
Article
Google Scholar
Pakravan K, Babashah S, Sadeghizadeh M, Mowla SJ, Mossahebi-Mohammadi M, Ataei F, Dana N, Javan M. MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1\(\alpha \)/VEGF signaling axis in breast cancer cells. Cell Oncol. 2017. https://doi.org/10.1038/ncomms6549.
Article
Google Scholar
Akhavantabasi S, Sapmaz A, Tuna S, Erson-Bensan AE. MiR-125b targets ARID3B in breast cancer cells. Cell Struct Funct. 2012. https://doi.org/10.1038/ncomms6549.
Article
Google Scholar
Chen Y, Gelfond JA, McManus LM, Shireman PK. Reproducibility of quantitative rt-pcr array in mirna expression profiling and comparison with microarray analysis. BMC Genomics. 2009;10(1):1–10.
Article
Google Scholar
Chen J, Tian W, Cai H, He H, Deng Y. Down-regulation of microRNA-200c is associated with drug resistance in human breast cancer. Med Oncol. 2012. https://doi.org/10.1038/ncomms6549.
Article
Google Scholar
Wang CZ, Yuan P, Li Y. miR-126 regulated breast cancer cell invasion by targeting ADAM9. Int J Clin Exp Pathol. 2015;8(6):6547–53.
Google Scholar
Alhasan L. MiR-126 modulates angiogenesis in breast cancer by targeting VEGF-A -mRNA. Asian Pac J Cancer Prev. 2019. https://doi.org/10.1038/ncomms6549.
Article
Google Scholar
Zou C, Xu Q, Mao F, Li D, Bian C, Liu LZ, Jiang Y, Chen X, Qi Y, Zhang X, Wang X, Sun Q, Kung HF, Lin MC, Dress A, Wardle F, Jiang BH, Lai L. MiR-145 inhibits tumor angiogenesis and growth by N-RAS and VEGF. Cell Cycle. 2012. https://doi.org/10.4161/cc.20598.
Article
Google Scholar
Zheng M, Sun X, Li Y, Zuo W. MicroRNA-145 inhibits growth and migration of breast cancer cells through targeting oncoprotein ROCK1. Tumor Biol. 2016. https://doi.org/10.1016/j.bbamcr.2010.06.013.
Article
Google Scholar
Costales MG, Haga CL, Velagapudi SP, Childs-Disney JL, Phinney DG, Disney MD. Small Molecule Inhibition of microRNA-210 Reprograms an Oncogenic Hypoxic Circuit. J Am Chem Soc. 2017. https://doi.org/10.1016/j.bbamcr.2010.06.013.
Article
Google Scholar
Bao L, Hazari S, Mehra S, Kaushal D, Moroz K, Dash S. Increased expression of P-glycoprotein and doxorubicin chemoresistance of metastatic breast cancer is regulated by miR-298. Am J Pathol. 2012. https://doi.org/10.1016/j.bbamcr.2010.06.013.
Article
Google Scholar
Meng Y, Zou Q, Liu T, Cai X, Huang Y, Pan J. MicroRNA-335 inhibits proliferation, cell-cycle progression, colony formation, and invasion via targeting PAX6 in breast cancer cells. Mol Med Rep. 2015. https://doi.org/10.1016/j.bbamcr.2010.06.013.
Article
Google Scholar
Gao Y, Zeng F, Wu JY, Li HY, Fan JJ, Mai L, Zhang J, Ma DM, Li Y, Song F. MiR-335 inhibits migration of breast cancer cells through targeting oncoprotein c-Met. Tumor Biol. 2015. https://doi.org/10.1016/j.bbamcr.2010.06.013.
Article
Google Scholar
McAnena P, Tanriverdi K, Curran C, Gilligan K, Freedman JE, Brown JAL, Kerin MJ. Circulating microRNAs miR-331 and miR-195 differentiate local luminal a from metastatic breast cancer. BMC Cancer. 2019. https://doi.org/10.1016/j.bbamcr.2010.06.013.
Article
Google Scholar
Yerukala Sathipati S, Ho SY. Identifying a miRNA signature for predicting the stage of breast cancer. Scientific Rep. 2018. https://doi.org/10.1016/j.bbamcr.2010.06.013.
Article
Google Scholar
Nassar FJ, Chamandi G, Tfaily MA, Zgheib NK, Nasr R. Peripheral Blood-Based Biopsy for Breast Cancer Risk Prediction and Early Detection. Blood. 2020. https://doi.org/10.1016/j.bbamcr.2010.06.013.
Article
Google Scholar
Loh HY, Norman BP, Lai KS, Rahman NM, Alitheen NBM, Osman MA. The regulatory role of microRNAs in breast cancer. Int J Mol Sci. 2019. https://doi.org/10.1016/j.bbamcr.2010.06.013.
Article
Google Scholar
Singh R, Mo YY. Role of microRNAs in breast cancer. Cancer. 2013. https://doi.org/10.1016/j.bbamcr.2010.06.013.
Article
Google Scholar
Zou D, Zhou Q, Wang D, Guan L, Yuan L, Li S. The downregulation of MicroRNA-10b and its Role in cervical cancer. Oncol Res. 2016. https://doi.org/10.1016/j.bbamcr.2010.06.013.
Article
Google Scholar
Hou R, Wang D, Lu J. MicroRNA-10b inhibits proliferation, migration and invasion in cervical cancer cells via direct targeting of insulin-like growth factor-1 receptor. Oncol Lett. 2017. https://doi.org/10.1016/j.bbamcr.2010.06.013.
Article
Google Scholar
Shukla V, Varghese VK, Kabekkodu SP, Mallya S, Chakrabarty S, Jayaram P, Pandey D, Banerjee S, Sharan K, Satyamoorthy K. Enumeration of deregulated miRNAs in liquid and tissue biopsies of cervical cancer. Gynecol Oncol. 2019. https://doi.org/10.1016/j.bbamcr.2010.06.013.
Article
Google Scholar
Jiménez-Wences H, Martínez-Carrillo DN, Peralta-Zaragoza O, Campos-Viguri GE, Hernández-Sotelo D, Jiménez-López MA, Muñoz-Camacho JG, Garzón-Barrientos VH, Illades-Aguiar B, Fernández-Tilapa G. Methylation and expression of miRNAs in precancerous lesions and cervical cancer with HPV16 infection. Oncol Rep. 2016. https://doi.org/10.1016/j.bbamcr.2010.06.01313.
Article
Google Scholar
Zhao Y, Liu X, Lu Y. Microrna-143 regulates the proliferation and apoptosis of cervical cancer cells by targeting hif-1\(\alpha \). Eur Rev Med Pharmacol Sci. 2017;21(24):5580–6.
Google Scholar
Ma L, Hong Y, Lu C, Chen Y, Ma C. The occurrence of cervical cancer in Uygur women in **njiang Uygur Autonomous Region is correlated to microRNA-146a and ethnic factor. Int J Clin Exp Pathol. 2015;13(3):634.
Google Scholar
Yang Z, Chen S, Luan X, Li Y, Liu M, Li X, Liu T, Tang H. MicroRNA-214 is aberrantly expressed in cervical cancers and inhibits the growth of hela cells. IUBMB Life. 2009. https://doi.org/10.1016/j.bbamcr.2010.06.01314.
Article
Google Scholar
Wang X, **a Y. MicroRNA-328 inhibits cervical cancer cell proliferation and tumorigenesis by targeting TCF7L2. Biochem Biophys Res Commun. 2016. https://doi.org/10.1016/j.bbamcr.2010.06.01315.
Article
Google Scholar
Wang Y, Dong X, Hu B, **g Wang X, Wang Q, Wang W. The effects of Micro-429 on inhibition of cervical cancer cells through targeting ZEB1 and CRKL. Biomed Pharmacother. 2016. https://doi.org/10.1016/j.bbamcr.2010.06.01316.
Article
Google Scholar
Sun P, Shen Y, Gong JM, Zhou LL, Sheng JH, Duan FJ. A New MicroRNA Expression Signature for Cervical Cancer. Int J Gynecol Cancer. 2017. https://doi.org/10.1016/j.bbamcr.2010.06.01317.
Article
Google Scholar
Wen F, Xu JZ, Wang XR. Increased expression of miR-15b is associated with clinicopathological features and poor prognosis in cervical carcinoma. Arch Gynecol Obstetrics. 2017. https://doi.org/10.1016/j.bbamcr.2010.06.01318.
Article
Google Scholar
Xu J, Zhang W, Lv Q, Zhu D. Overexpression of miR-21 promotes the proliferation and migration of cervical cancer cells via the inhibition of PTEN. Oncol Rep. 2015. https://doi.org/10.1016/j.bbamcr.2010.06.01319.
Article
Google Scholar
Feng Y, Zhou S, Li G, Hu C, Zou W, Zhang H, Sun L. Nuclear factor-\(\kappa \)B-dependent microRNA-130a upregulation promotes cervical cancer cell growth by targeting phosphatase and tensin homolog. Arch Biochem Biophys. 2016. https://doi.org/10.1016/j.bbamcr.2010.06.01320.
Article
Google Scholar
Zhang J, Wu H, Li P, Zhao Y, Liu M, Tang H. Nf-\(\kappa \)b-modulated mir-130a targets tnf-\(\alpha \) in cervical cancer cells. J Transl Med. 2014;12(1):155.
Article
Google Scholar
Wang S, Gao B, Yang H, Liu X, Wu X, Wang W. MicroRNA-432 is downregulated in cervical cancer and directly targets FN1 to inhibit cell proliferation and invasion. Oncol Lett. 2019. https://doi.org/10.1016/j.bbamcr.2010.06.01321.
Article
Google Scholar
Nagy ZB, Wichmann B, Kalmár A, Galamb O, Barták BK, Spisák S, Tulassay Z, Molnár B. Colorectal adenoma and carcinoma specific miRNA profiles in biopsy and their expression in plasma specimens. Clin Epigenet. 2017. https://doi.org/10.1016/j.bbamcr.2010.06.01322.
Article
Google Scholar
Lo UG, Yang D, Hsieh JT. The role of microRNAs in prostate cancer progression. Cancer. 2013. https://doi.org/10.1016/j.bbamcr.2010.06.01323.
Article
Google Scholar
Vanacore D, Boccellino M, Rossetti S, Cavaliere C, D’Aniello C, Di Franco R, Romano FJ, Montanari M, La Mantia E, Piscitelli R, Nocerino F, Cappuccio F, Grimaldi G, Izzo A, Castaldo L, Pepe MF, Malzone MG, Iovane G, Ametrano G, Stiuso P, Quagliuolo L, Barberio D, Perdonà S, Muto P, Montella M, Maiolino P, Veneziani BM, Botti G, Caraglia M, Facchini G. Micrornas in prostate cancer: an overview. Oncotarget. 2017. https://doi.org/10.1016/j.bbamcr.2010.06.01324.
Article
Google Scholar
Hoey C, Ahmed M, Fotouhi Ghiam A, Vesprini D, Huang X, Commisso K, Commisso A, Ray J, Fokas E, Loblaw DA, He HH, Liu SK. Circulating miRNAs as non-invasive biomarkers to predict aggressive prostate cancer after radical prostatectomy. J Transl Med. 2019. https://doi.org/10.1016/j.bbamcr.2010.06.01325.
Article
Google Scholar
Murata T, Takayama K, Katayama S, Urano T, Horie-Inoue K, Ikeda K, Takahashi S, Kawazu C, Hasegawa A, Ouchi Y, Homma Y, Hayashizaki Y, Inoue S. miR-148a is an androgen-responsive microRNA that promotes LNCaP prostate cell growth by repressing its target CAND1 expression. Prostate Cancer Prostatic Dis. 2010. https://doi.org/10.1016/j.bbamcr.2010.06.01326.
Article
Google Scholar
Nip H, Dar AA, Saini S, Colden M, Varahram S, Chowdhary H, Yamamura S, Mitsui Y, Tanaka Y, Kato T, Hashimoto Y, Shiina M, Kulkarni P, Dasgupta P, Imai-Sumida M, Laura Tabatabai Z, Greene K, Deng G, Dahiya R, Majid S. Oncogenic microRNA-4534 regulates PTEN pathway in prostate cancer. Oncotarget. 2016. https://doi.org/10.1016/j.bbamcr.2010.06.01327.
Article
Google Scholar
Zuo ZH, Yu YP, Ding Y, Liu S, Martin A, Tseng G, Luo JH. Oncogenic Activity of miR-650 in Prostate Cancer Is Mediated by Suppression of CSR1 Expression. Am J Pathol. 2015. https://doi.org/10.1016/j.bbamcr.2010.06.01328.
Article
Google Scholar
Qingchuan D, ** M, Tao W, Weiwei Q, Weijun Q, Fuli W, Jianlin Y, Zhinan C, Angang Y, He W. MicroRNA let-7a inhibits proliferation of human prostate cancer cells In Vitro and In Vivo by targeting E2F2 and CCND2. PLoS ONE. 2010. https://doi.org/10.1016/j.bbamcr.2010.06.01329.
Article
Google Scholar
Nadiminty N, Tummala R, Lou W, Zhu Y, Shi XB, Zou JX, Chen H, Zhang J, Chen X, Luo J, deVere White RW, Kung HJ, Evans CP, Gao AC. MicroRNA let-7c is downregulated in prostate cancer and suppresses prostate cancer growth. PLoS ONE. 2012. https://doi.org/10.1016/j.bbamcr.2010.06.01330.
Article
Google Scholar
Sung SY, Liao CH, Wu HP, Hsiao WC, Wu IH, **pu Yu, Lin SH, Hsieh CL. Loss of let-7 microRNA upregulates IL-6 in bone marrow-derived mesenchymal stem cells triggering a reactive stromal response to prostate cancer. PloS one. 2013. https://doi.org/10.1371/journal.pone.0071637
Wang M, Ren D, Guo W, Wang Z, Huang S, Du H, Song L, Peng X. Loss of miR-100 enhances migration, invasion, epithelial-mesenchymal transition and stemness properties in prostate cancer cells through targeting Argonaute 2. Int J Oncol. 2014. https://doi.org/10.1016/j.bbamcr.2010.06.01331.
Article
Google Scholar
Zhang W, Mao YQ, Wang H, Yin WJ, Zhu SX, Wang WC. MiR-124 suppresses cell motility and adhesion by targeting talin 1 in prostate cancer cells. Cancer Cell Int. 2015. https://doi.org/10.1016/j.bbamcr.2010.06.01332.
Article
Google Scholar
Qin W, Pan Y, Zheng X, Li D, Bu J, Xu C, Tang J, Cui R, Lin P, Yu X. MicroRNA-124 regulates TGF-\(\alpha \)-induced epithelial-mesenchymal transition in human prostate cancer cells. Int J Oncol. 2014. https://doi.org/10.1016/j.bbamcr.2010.06.01333.
Article
Google Scholar
Shi XB, Xue L, Yang J, Ma AH, Zhao J, Xu M, Tepper CG, Evans CP, Kung HJ, White RWDV. An androgen-regulated miRNA suppresses Bak1 expression and induces androgen-independent growth of prostate cancer cells. Proc Natl Acad Sci USA. 2007. https://doi.org/10.1016/j.bbamcr.2010.06.01334.
Article
Google Scholar
Shi XB, Xue L, Ma AH, Tepper CG, Kung HJ, White RWD. MiR-125b promotes growth of prostate cancer xenograft tumor through targeting pro-apoptotic genes. Prostate. 2011. https://doi.org/10.1016/j.bbamcr.2010.06.01335.
Article
Google Scholar
Fu W, Tao T, Qi M, Wang L, Hu J, Li X, **ng N, Du R, Han B. MicroRNA-132/212 Upregulation Inhibits TGF-\(\beta \)-Mediated Epithelial-Mesenchymal Transition of Prostate Cancer Cells by Targeting SOX4. Prostate. 2016. https://doi.org/10.1016/j.bbamcr.2010.06.01336.
Article
Google Scholar
Zhang HL, Qin XJ, Cao DL, Zhu Y, Yao XD, Zhang SL, Dai B, Ye DW. An elevated serum miR-141 level in patients with bone-metastatic prostate cancer is correlated with more bone lesions. Asian J Androl. 2013. https://doi.org/10.1016/j.bbamcr.2010.06.01337.
Article
Google Scholar
Zhou P, Chen WG, Li XW. MicroRNA-143 acts as a tumor suppressor by targeting hexokinase 2 in human prostate cancer. Am J Cancer Res. 2015;9:12.
Google Scholar
Xu B, Niu X, Zhang X, Tao J, Wu D, Wang Z, Li P, Zhang W, Wu H, Feng N, Wang Z, Hua L, Wang X. MiR-143 decreases prostate cancer cells proliferation and migration and enhances their sensitivity to docetaxel through suppression of KRAS. Mol Cell Biochem. 2011. https://doi.org/10.1016/j.bbamcr.2010.06.01338.
Article
Google Scholar
Guo W, Ren D, Chen X, Tu X, Huang S, Wang M, Song L, Zou X, Peng X. HEF1 promotes epithelial mesenchymal transition and bone invasion in prostate cancer under the regulation of microRNA-145. J Cell Biochem. 2013. https://doi.org/10.1016/j.bbamcr.2010.06.01339.
Article
Google Scholar
Chen X, Gong J, Zeng H, Chen N, Huang R, Huang Y, Nie L, Xu M, **a J, Zhao F, Meng W, Zhou Q. MicroRNA145 targets BNIP3 and suppresses prostate cancer progression. Cancer Res. 2010. https://doi.org/10.1016/j.bbamcr.2010.06.01340.
Article
Google Scholar
Zhang X, Tao T, Liu C, Guan H, Huang Y, Xu B, Chen M. Downregulation of miR-195 promotes prostate cancer progression by targeting HMGA1. Oncol Rep. 2016. https://doi.org/10.1016/j.bbamcr.2010.06.01341.
Article
Google Scholar
Wu J, Ji A, Wang X, Zhu Y, Yu Y, Lin Y, Liu Y, Li S, Liang Z, Xu X, Zheng X, **e L. MicroRNA-195-5p, a new regulator of Fra-1, suppresses the migration and invasion of prostate cancer cells. J Transl Med. 2015. https://doi.org/10.1016/j.bbamcr.2010.06.01342.
Article
Google Scholar
Liu X, Chen Q, Yan J, Wang Y, Zhu C, Chen C, Zhao X, Xu M, Sun Q, Deng R, Zhang H, Qu Y, Huang J, Jiang B, Yu J. MiRNA-296-3p-ICAM-1 axis promotes metastasis of prostate cancer by possible enhancing survival of natural killer cell-resistant circulating tumour cells. Cell Death Dis. 2013. https://doi.org/10.1016/j.bbamcr.2010.06.01343.
Article
Google Scholar
Rasheed SAK, Teo CR, Beillard EJ, Voorhoeve PM, Casey PJ. MicroRNA-182 and MicroRNA-200a control G-protein subunit \(\alpha \)-13 (GNA13) expression and cell invasion synergistically in prostate cancer cells. J Biol Chem. 2013. https://doi.org/10.1016/j.bbamcr.2010.06.01344.
Article
Google Scholar
Galardi S, Mercatelli N, Giorda E, Massalini S, Frajese GV, Ciafrè SA, Farace MG. miR-221 and miR-222 expression affects the proliferation potential of human prostate carcinoma cell lines by targeting p27Kip1. J Biol Chem. 2007. https://doi.org/10.1016/j.bbamcr.2010.06.01345.
Article
Google Scholar
Zheng C, Yinghao S, Li J. MiR-221 expression affects invasion potential of human prostate carcinoma cell lines by targeting DVL2. Med Oncol. 2012;29(2):815–22. https://doi.org/10.1016/j.bbamcr.2010.06.01346.
Article
Google Scholar
Yang X, Yang Y, Gan R, Zhao L, Li W, Zhou H, Wang X, Lu J, Meng QH. Down-regulation of miR-221 and miR-222 restrain prostate cancer cell proliferation and migration that is partly mediated by activation of SIRT1. PLoS ONE. 2014. https://doi.org/10.1016/j.bbamcr.2010.06.01347.
Article
Google Scholar
Wei JJ, Wu X, Peng Y, Shi G, Olca B, Yang X, Daniels G, Osman I, Ouyang J, Hernando E, Pellicer A, Rhim JS, Melamed J, Lee P. Regulation of HMGA1 expression by MicroRNA-296 affects prostate cancer growth and invasion. Clinical Cancer Res. 2011. https://doi.org/10.1016/j.bbamcr.2010.06.01348.
Article
Google Scholar
Lee KH, Lin FC, Hsu TI, Lin JT, Guo JH, Tsai CH, Lee YC, Lee YC, Chen CL, Hsiao M, Lu PJ. MicroRNA-296-5p (miR-296-5p) functions as a tumor suppressor in prostate cancer by directly targeting Pin1. Biochimica et Biophysica Acta. 2014. https://doi.org/10.1016/j.bbamcr.2010.06.01349.
Article
Google Scholar
Wen J, Li R, Wen X, Chou G, Lu J, Wang X, ** Y. Dysregulation of cell cycle related genes and microRNAs distinguish the low- from high-risk of prostate cancer. Diagnostic Pathol. 2014. https://doi.org/10.1186/s13000-014-0156-1.
Article
Google Scholar
Bryant RJ, Pawlowski T, Catto JWF, Marsden G, Vessella RL, Rhees B, Kuslich C, Visakorpi T, Hamdy FC. Changes in circulating microRNA levels associated with prostate cancer. Br J Cancer. 2012. https://doi.org/10.1038/bjc.2011.595.
Article
Google Scholar
Zhang W, Liu J, Qiu J, Fu X, Tang Q, Yang F, Zhao Z, Wang H. MicroRNA-382 inhibits prostate cancer cell proliferation and metastasis through targeting COUP-TFII. Oncol Rep. 2016. https://doi.org/10.3892/or.2016.5141.
Article
Google Scholar
Noonan EJ, Place RF, Pookot D, Basak S, Whitson JM, Hirata H, Giardina C, Dahiya R. MiR-449a targets HDAC-1 and induces growth arrest in prostate cancer. Oncogene. 2009. https://doi.org/10.1016/j.bbamcr.2010.06.01353.
Article
Google Scholar
Noonan EJ, Place RF, Basak S, Pookot D, Li LC. miR-449a causes Rb-dependent cell cycle arrest and senescence in prostate cancer cells. Oncotarget. 2010. https://doi.org/10.1016/j.bbamcr.2010.06.01354.
Article
Google Scholar
Mariotto AB, Robin Yabroff K, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011. https://doi.org/10.1093/jnci/djq495.
Article
Google Scholar
Dusetzina SB. Drug pricing trends for orally administered anticancer medications reimbursed by commercial health plans, 2000–2014. JAMA Oncol. 2016. https://doi.org/10.1001/jamaoncol.2016.0648.
Article
Google Scholar
Dolgin E. Bringing down the cost of cancer treatment. Nature. 2018. https://doi.org/10.1016/j.bbamcr.2010.06.01357.
Article
Google Scholar
Atun R, Cavalli F. The global fight against cancer: challenges and opportunities. Lancet. 2018. https://doi.org/10.1016/S0140-6736(18)30156-9.
Article
Google Scholar
Massuda A, Hone T, Leles FAG, De Castro MC, Atun R. The Brazilian health system at crossroads: Progress, crisis and resilience. BMJ Global Health. 2018. https://doi.org/10.1016/j.bbamcr.2010.06.01359.
Article
Google Scholar
Liu R, Li J, Lai Y, Liao Y, Liu R, Qiu W. Hsa-miR-1 suppresses breast cancer development by down-regulating K-ras and long non-coding RNA MALAT1. Int J Biol Macromol. 2015. https://doi.org/10.1016/j.ijbiomac.2015.08.016.
Article
Google Scholar
Yan L, Yu MC, Gao GL, Liang HW, Zhou XY, Zhu ZT, Zhang CY, Wang YB, Chen X. MiR-125a-5p functions as a tumour suppressor in breast cancer by downregulating BAP1. J Cell Biochem. 2018. https://doi.org/10.1002/jcb.27124.
Article
Google Scholar
Ru P, Steele R, Hsueh EC, Ray RB. Anti-mir-203 upregulates socs3 expression in breast cancer cells and enhances cisplatin chemosensitivity. Genes Cancer. 2011;2(7):720–7.
Article
Google Scholar
Camps C, Saini HK, Mole DR, Choudhry H, Reczko M, Guerra-Assunção JA, Tian YM, Buffa FM, Harris AL, Hatzigeorgiou AG, Enright AJ, Ragoussis J. Integrated analysis of microRNA and mRNA expression and association with HIF binding reveals the complexity of microRNA expression regulation under hypoxia. Mol Cancer. 2014. https://doi.org/10.1186/1476-4598-13-28.
Article
Google Scholar
Zhang Y, Yan J, Wang L, Dai H, Li N, Hu W, Cai H. HIF-1\(\alpha \) Promotes Breast Cancer Cell MCF-7 Proliferation and Invasion Through Regulating miR-210. Cancer Biother Radiopharm. 2017. https://doi.org/10.1089/cbr.2017.2270.
Article
Google Scholar
Mohammadi-Yeganeh S, Paryan M, Arefian E, Vasei M, Ghanbarian H, Mahdian R, Karimipoor M, Soleimani M. MicroRNA-340 inhibits the migration, invasion, and metastasis of breast cancer cells by targeting Wnt pathway. Tumor Biol. 2016. https://doi.org/10.1007/s13277-015-4513-9.
Article
Google Scholar
Yao J, Deng B, Zheng L, Dou L, Guo Y, Guo K. MiR-27b is upregulated in cervical carcinogenesis and promotes cell growth and invasion by regulating CDH11 and epithelial-mesenchymal transition. Oncol Rep. 2016. https://doi.org/10.3892/or.2015.4500.
Article
Google Scholar
Miska EA, Alvarez-Saavedra E, Townsend M, Yoshii A, Sestan N, Rakic P, Constantine-Paton M, Horvitz HR. Microarray analysis of microRNA expression in the develo** mammalian brain. Genome Biol. 2004. https://doi.org/10.1186/gb-2004-5-9-r68.
Article
Google Scholar
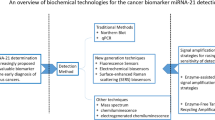
Li W, Ruan K. MicroRNA detection by microarray. Anal Bioanal Chem. 2009. https://doi.org/10.1007/s00216-008-2570-2.
Article
Google Scholar
Wu Q, Lu Z, Li H, Lu J, Guo L, Ge Q. Next-generation sequencing of microRNAs for breast cancer detection. J Biomed Biotechnol. 2011. https://doi.org/10.1155/2011/597145.
Article
Google Scholar
Leshkowitz D, Horn-Saban S, Parmet Y, Feldmesser E. Differences in microRNA detection levels are technology and sequence dependent. RNA. 2013. https://doi.org/10.1016/j.bbamcr.2010.06.01369.
Article
Google Scholar
Várallyay É, Burgyán J, Havelda Z. MicroRNA detection by northern blotting using locked nucleic acid probes. Nature Protocols. 2008. https://doi.org/10.1016/j.bbamcr.2010.06.01370.
Article
Google Scholar
Koscianska E, Starega-Roslan J, Sznajder LJ, Olejniczak M, Galka-Marciniak P, Krzyzosiak WJ. Northern blotting analysis of microRNAs, their precursors and RNA interference triggers. BMC Mol Biol. 2011. https://doi.org/10.1186/1471-2199-12-14.
Article
Google Scholar
Jia H, Li Z, Liu C, Cheng Y. Ultrasensitive detection of microRNAs by exponential isothermal amplification. Angewandte Chemie. 2010. https://doi.org/10.1002/anie.201001375.
Article
Google Scholar
Duan R, Zuo X, Wang S, Quan X, Chen D, Chen Z, Jiang L, Fan C, **a F. Lab in a tube: Ultrasensitive detection of MicroRNAs at the single-cell level and in breast cancer patients using quadratic isothermal amplification. J Am Chem Soc. 2013. https://doi.org/10.1021/ja311313b.
Article
Google Scholar
Deng R, Zhang K, Li J. Isothermal Amplification for MicroRNA Detection: From the Test Tube to the Cell. Acc Chem Res. 2017. https://doi.org/10.1021/acs.accounts.7b00040.
Article
Google Scholar