Abstract
V-domain immunoglobulin suppressor of T cell activation (VISTA) is a novel negative checkpoint receptor (NCR) primarily involved in maintaining immune tolerance. It has a role in the pathogenesis of autoimmune disorders and cancer and has shown promising results as a therapeutic target. However, there is still some ambiguity regarding the ligands of VISTA and their interactions with each other. While V-Set and Immunoglobulin domain containing 3 (VSIG-3) and P-selectin glycoprotein ligand-1(PSGL-1) have been extensively studied as ligands for VISTA, the others have received less attention. It seems that investigating VISTA ligands, reviewing their functions and roles, as well as outcomes related to their interactions, may allow an understanding of their full functionality and effects within the cell or the microenvironment. It could also help discover alternative approaches to target the VISTA pathway without causing related side effects. In this regard, we summarize current evidence about VISTA, its related ligands, their interactions and effects, as well as their preclinical and clinical targeting agents.
Similar content being viewed by others
Introduction
With the advent of immunotherapy, cancer treatment modalities have undergone a revolution heralding a new era of specialized treatments that are supposed to improve the chances of successful therapies. Indeed, immunotherapy aims to activate or suppress the immune system to boost anti-tumor responses or attenuate the specific adaptive immune response against self-antigens. Since lymphocytes are the most critical players in immune responses, activatory and inhibitory receptors within them are the center of attention during immunotherapy [1]. Immune checkpoint receptors (ICRs) regulate the balance between the immune system’s stimulatory and inhibitory pathways and help maintain immunosurveillance. With the introduction of the inhibitory ICRs, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), and programmed cell death protein 1 (PD-1) by Alison et al., more attention was paid to this field in cancer patients, and the initial immune checkpoint inhibitors (ICIs) showed remarkable therapeutic effects. In addition to CTLA-4 and PD-1, several ICRs have been identified over the years, and they are in various stages of clinical trials. Moreover, the number of cases receiving United States Food and Drug Administration (FDA) approval is on the rise. Their brilliant results in creating dramatic and long-term clinical outcomes, especially in refractory cancers such as non-small cell lung cancer (NSCLC) and melanoma, have raised hopes for cancer therapy [2,3,4].
Among the novel ICRs, VISTA, also known as B7-H5, PDCD-1 homolog (PD-1H), stress-induced secreted protein 1 (SISP1), death domain1alpha (DD1a), Gi24, and differentiation of embryonic stem cells 1 (Dies1) plays a central role in immune system functions, and its association with several human disorders, including autoimmune disease, inflammatory diseases, infection, and cancer was confirmed [5]. Although there has been considerable research regarding the function and role of VISTA and its potential therapeutic target, little information is available regarding the ligands of VISTA and their interactions. This article will provide a comprehensive review of VISTA, its related ligands' roles and functions, the impact of thier interactions, and the newest targeting agents for them to evaluate their potential as therapeutic targets in the future.
VISTA structure
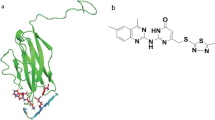
VISTA, as a type I transmembrane protein (55–65 kDa), is encoded by a gene called VSIR and is located on 10q22.1 within an intron of the CDH23 gene. VISTA protein structure (without signal peptide: 32 amino acids) contains 279-aa; 130-aa in the hyperglycosylated extracellular Ig-V domain, 33-aa in the stalk region, 20-aa in the transmembrane domain, and 96-aa in the cytoplasmic domain [6].
The extracellular domain (ECD) of VISTA adopts a canonical β-sandwich formation with the front face representing H-, A-, G-, F-, C-, and C′ β-strands, while the back face represents A′-, B-, E-, D-, and C′′ β-strands. Moreover, three disulfide bonds exist between the strands of B-F (Cys22–Cys114), A′-H (Cys12–Cys146), and CC′ loop-F (Cys51–Cys113) as a result of the presence of six cysteines. The ECD of VISTA, mainly the C–C′ loop, contains a considerable number of histidine (H) residues with a positive charge. These residues are responsible for forming pH-dependent binding sites, which enhance the binding of VISTA to its ligands in acidic environments such as tumor microenvironment (TME) [5, 7]. The cytoplasmic region of VISTA does not contain tyrosine-based signaling motifs, including immunoreceptor tyrosine-based activation motif (ITAM), immunoreceptor tyrosine-based inhibitory motif (ITIM), and immunoreceptor tyrosine-based switch motif (ITSM). Additionally, the presence of proline-rich motifs, including three C-terminal Src homology domain 3 (SH3) binding motifs (PxxP) and a Src homology domain 2 (SH2) binding motif (YxxQ), as well as specific sequences for phosphokinase C (PKC) and casein kinase 2 (CK2) binding sites enable VISTA to alter some cellular functions within the cell and act both as a receptor and a ligand. The cytoplasmic region of VISTA, which is very important in terms of intracellular functions, is similar to members of the CD28 family, such as PD-1 and CTLA-4. Then, VISTA seems to share common functional properties with the CD28 family members [6, 7].
Phylogenetic analysis showed that VISTA has a highly conserved sequence, especially between humans and mice with 76% similarity. In light of the sequence similarity between VISTA and the other members of the B7 family, especially in the ECD, it has been classified as a member of this group. Analysis of the Ig-V domain within ECD showed that the highest homology rate between VISTA and B7 family members belongs to programmed death-ligand 1 (PD-L1), where 23% of the sequences are identical. Nevertheless, VISTA still exhibits some unique characteristics that make it stand unique from other members of the B7 family: (1) chromosomal locus in which VSIR gene is located apart from others, (2) conformational differences in the ECD domain; containing ten β-strands compared to nine in the B7 family fold, having an extra helix sequence (FQDL) and unique C–C′ unstructured loop of 21 residues, 3) Two extra disulfide bonds in IgV-like domain with unique cysteine residues (Cys44, Cys83, Cys144, and Cys177) that are absent in others, 4) While B7 family members have both IgC and IgV-like domains, VISTA lacks an IgC-like domain and exhibits extremely large IgV-like, and 5) lacking any ITAM, ITIM, ITSM motifs, which are existed in other B7 family members [5, 7].
VISTA expression
At a steady state, VISTA is expressed in a wide range of human tissues, particularly hematopoietic compartments and tissues containing infiltrating leukocytes, e.g., bone marrow (BM), thymus, spleen, and lymph node [8, 9]. Evidence also indicates the presence of VISTA in secretory form [10, 11].
In terms of hematopoietic tissues, peripheral blood mononuclear cells (PBMCs), mainly myeloid lineages, including monocytes, myeloid dendritic cells (DCs), macrophages, neutrophils, and basophils have the highest expression level of VISTA [8, 9]. Concerning lymphoid cells with lower expression of VISTA than myeloid cells, while subsets of CD4 + T cells, exceedingly naïve CD4 + T cells, and forkhead box P3 (FoxP3 +) regulatory T cells (Tregs) exhibit higher levels of VISTA, CD8 + T cells, plasma cells, lymphoid DCs, CD56low natural killer (NK) cells, and thymocytes express it at lower expression levels. However, its expression level on CD19 + B cells and CD56high NK cells has not yet been observed. Regarding the expression of VISTA in mice, it has almost the same expression pattern as humans, mainly confined to hematopoietic tissues [8, 9].
Activation of immune cells could increase the expression level of VISTA depending on each cell type [6]. Nevertheless, this upregulation in CD4 + and CD8 + T cells decreases over time [5]. Hu et al. showed that activated CD4 + T cells showed higher expression levels of VISTA than activated CD8 + T cells; however, the intensity of increased expression was higher in activated CD8 + T cells than in activated CD4 + T cells [65]. Gao et al. [66] showed that following ipilimumab therapy in prostate cancer patients, the expression level of VISTA and PD-L1 and infiltration of immune cells, especially CD68 + macrophages (VISTA + and PD-L1 +) that have M2 phenotype (suppressive phenotype) within the TME increased. It was shown that blocking VISTA in combination with CTLA-4 and PD-1 showed a synergistic effect and prevented the development of resistance to therapy in cancer patients [67]. Accordingly, it is suggested that inhibiting VISTA simultaneously with other ICs may not only result in more effective therapeutic outcomes but also prevent drug resistance.
The important side effect to consider in using ICIs in cancer treatment is the possibility of immune-related adverse events (irAEs), resulting in complications similar to those of autoimmune diseases caused by a disruption of self-tolerance. As a matter of fact, blocking a non-redundant NCR can result in a less suppressed immune system and a higher likelihood of over-reacted immune responses. Depending on the organ involved, these side effects include colitis, dermatitis, pneumonitis, myalgias, arthralgias, etc. [68,69,70]. According to a large meta-analysis, the incidence of irAE is 83% for CTLA-4 inhibitors, 72% for PD-1 inhibitors, and 60% for PD-L1 inhibitors, which are high rates [71]. In terms of the number of studies conducted on irAEs associated with VISTA, there are only a few. There was evidence that mice lacking the VISTA gene produced more IFN-γ-secreting T cells and showed chronic inflammation. Nevertheless, no organ-specific autoimmune disease developed [27]. Consequently, identifying alternative targets for ICIs, such as ligands of ICRs that suppress the immune checkpoint pathways, may increase the possibility of treating cancer patients while reducing the possibility of develo** subsequent irAEs.
Ligands of VISTA
As mentioned before, VISTA acts as both a ligand and a receptor. VISTA expressed within cells other than T cells (e.g., APCs and tumor cells) acts as a ligand via binding to unknown receptors on T cells. VISTA expressed on T cells acts as a receptor that interacts with ligands and inhibits T cells' activity via transducing downstream inhibitory pathways related to TCR [9, 13, 30]. On the other hand, VISTA shows homophilic interactions that facilitate its correlation with other VISTA proteins expressed in other cells. A confirmatory study of this issue showed that VISTA homophilic interactions intermediate macrophages’ efferocytosis and inhibition of T cell activation [14].
Although VISTA co-inhibitory ligands have not been fully elucidated, recent studies have suggested VSIG-3 and PSGL-1 as prominent and Galectin-9 (Gal-9), VSIG-8, matrix metalloproteinase-13 (MMP-13), syndecan-2 (Sdc2), and leucine-rich repeats and immunoglobulin-like domains 1 (LRIG1) as less well-confirmed receptors (Fig. 2).
VISTA and its related ligands. VISTA interacts with its associated ligands through the ECD region, where histidine and cysteine residues play important roles. ECD (extracellular domain), VISTA (V-domain immunoglobulin suppressor of T cell activation), PSGL-1 (P-selectin glycoprotein ligand-1), VSIG-3 (V-Set and Immunoglobulin domain containing 3), Gal-9 (Galectin-9), VSIG-8 (V-Set and Immunoglobulin domain containing 8), MMP-13 (matrix metalloproteinase-13), Sdc2 (syndecan-2), LRIG1 (leucine-rich repeats and immunoglobulin-like domains 1), NH2 (N-terminus), COOH (C-terminus), PKC (phosphokinase C), CK2 (casein kinase 2), SH2 (Src homology domain 2), SH3 (Src homology domain 3). Created with BioRender.com
V-set and immunoglobulin domain containing 3 (VSIG-3)
VSIG-3, also mainly known as BT-IgSF and IGSF11, is a single-pass (type I) transmembrane protein belonging to the Ig superfamily and consists of several domains, including N-terminal (Ig-like V type and Ig-like C2 type domains), transmembrane, and C-terminal (PDZ domain). VSIG-3 gene is located on chromosome 3q13.32 and contains 431 amino acids in humans [72, 134]. Mehta et al. [7] compared binding affinities of VSIG-3 and PSGL-1 for VISTA at different pH. They showed that at pH 7.4 (physiological pH), VISTA has a binding affinity of 20 nM for VSIG-3, while no binding was detected for PSGL-1. Conversely, at pH 6.0, while VSIG-3 showed an apparent binding affinity of 80 nM (fourfold decrease), PSGL-1 showed 4 nM (significant increase). Considering that limited information is available regarding VISTA interaction details with other ligands, we are not able to discuss more about them and the signaling pathways involved. Furthermore, it should be noted that most studies on the effects of VISTA binding to its ligands have been conducted in the context of cancer; hence, our knowledge about the impact of VISTA interaction with ligands has mostly been derived from research related to cancer, which mostly dampens anti-cancer responses and inflammation within the TME. Little is known about the effect of their interactions on the pathogenesis of autoimmune or inflammatory diseases (Fig. 3).
The effect of VISTA interaction with ligands and their binding effects. a The interaction between VSIG-3 and VSIG-8 expressed within tumor cells with VISTA on T cells causes inhibition in T cell activation and proliferation, reduction in IFN-γ, IL-2, IL-17, CCL3, CCL5, CXCL11 production, and suppression of immune cell infiltration to the TME. b The binding of Gal-9 secreted from the AML cells to VISTA expressed on T cells induces apoptosis in activated T cells and inhibits immune responses in the TME. c The interaction between PSGL-1 expressed on T cells with VISTA expressed on tumor cells, TILs, and TAMs/MDSCs not only suppresses T cell activation (blocking NF-κB pathway and reduction in IFN-γ production) and proliferation but also decreases the production of anti-inflammatory mediators in TME. d The MMP-13 produced by MM tumor cells binds to the VISTA expressed on the osteoclasts and T cells, causing bone resorption and T cell suppression, respectively. e The binding of VISTA to its unidentified ligand on monocyte surfaces is associated with Sdc-2 interactions, which have an impact on monocyte biological functions. VISTA (V-domain immunoglobulin suppressor of T cell activation), VSIG-3 (V-Set and Immunoglobulin domain containing 3, VSIG-8 (V-Set and Immunoglobulin domain containing 8), PSGL-1 (P-selectin glycoprotein ligand-1), Gal-9 (Galectin-9), MMP-13 (matrix metalloproteinase-13), Sdc2 (syndecan-2), IFN-γ (interferon gamma), TNF (tumor necrosis factor), NF-κB (nuclear factor kappa B), IL (interleukin), CCL3 (chemokine (C–C motif) ligand 3), CCL5 (chemokine (C–C motif) ligand 5), CXCL11 (C-X-C motif chemokine 11), Cas-3 (caspase 3), TAMs (tumor-associated macrophages), MDSCs (myeloid-derived suppressor cells), TME (tumor microenvironment), AML (acute myeloid leukemia), MM (multiple myeloma). Created with BioRender.com
VISTA and its ligands in clinical trials
As mentioned before, VISTA occupies unique features that make it stand out among others: distinct signaling pathway from CTLA-4 and PD-1/PD-L1, synergistic effects with anti-CTLA-4 and –PD-1, and involvement in resistance to anti-CTLA-4/PD-1 therapies. Therefore, targeting VISTA in TME suppresses the tumor-promoting effects and induces anti-tumoral responses. In terms of combination therapy, no information has been available regarding the simultaneous inhibition of VISTA and the use of other standard treatments, such as chemotherapy or radiotherapy. VISTA also plays a crucial role in maintaining self-tolerance, and its agonists have also been shown to be valuable in treating autoimmune and inflammatory diseases. According to the most recent multiple preclinical and clinical studies, VISTA appears to have tremendous therapeutic potential, either as an agonist or antagonist (Table 1).
Agonists
VISTA agonistic mAbs promote the activity of VISTA; induction of activation-induced cell death (AICD) and enhancement of peripheral T cell tolerance, inhibit myeloid chemotaxis, and reprogramming macrophages towards an anti-inflammatory profile [16, 135]. Hence, they can be a suitable treatment option for inflammatory and autoimmune disorders. Recent animal studies showed that using VISTA agonist antibodies results in immunomodulatory effects, such as suppressing NF-κB signaling pathway and production of pro-inflammatory cytokines [16]. In GVHD, targeting VISTA in donor CD4 + T cells with agonistic antibody before the transfer led to the deletion of donor alloreactive T cells via the T cell-intrinsic pathway and prevented disease [30]. As mentioned before, the VISTA agonist antibody (4C11) suppressed lung inflammation and reduced the severity of the disease [41]. Five agonistic anti-VISTA mAbs are under investigation in autoimmune disorders, 8G8, INX803, 7G1, 7G5, and 7E12, which target VISTA in mice, humans, or both. Studies indicate that these antibodies induce VISTA signaling and suppress immunity, although little information about their effects is available [136, 137]. It should be noted that only VISTA is currently being developed as a checkpoint agonist in clinical studies.
Antagonists
Small molecule inhibitors
CA-170
A small molecule inhibitor named CA-170, taken orally, targets VISTA (H strand) and PD-L1/L2 pathways without interrupting PD-1/PD-L1 interaction. Since 2015, Curis has licensed the technology from Aurigene, and in vitro studies showed that CA-170 promotes cell proliferation and IFN-γ production in T cells suppressed by VISTA or PD-1/PD-L1 [138, 139]. In syngeneic mouse models of melanoma and colon cancer (B16, CT26, and MC38), CA-170 suppressed tumor growth, promoted the activation of peripheral T cells, and activation of TILs [140]. Phase I trial (NCT02812875) showed its safety and effectiveness in solid tumors and lymphoma. Patients involved presented increased activated CD4 + and CD8 + T cells in the periphery [141]. Phase II studies in lung cancer, Hodgkin lymphoma, head and neck/oral cavity, and MSI-high cancers are currently underway by Aurigene in India [142]. Despite all these findings, the binding of human VISTA to CA-170 was not confirmed [143].
AUPM-493
Small molecule developed by Aurigene and acts as PD-L1 and VISTA antagonist. In a preclinical study, AUPM-493 suppressed the interaction between VISTA and VSIG-8, which led to the activation of T cells and IFN-γ production. It also showed anti-tumoral effects in syngeneic models of melanoma and colon cancer [117].
Blocking antibodies
VSTB112
Regarding mAbs blocking VISTA, JNJ-61610588 or VSTB112 is the first humanized IgG1κ antibody developed by ImmuNext/Janssen, targeting human VISTA through C–C′ loops (H121 and H122 residues) and adjacent Helix. There is no pH dependence on the interaction between VSTB and VISTA [144]. It was found that VSTB112 suppressed VISTA signaling in vitro and also tumor regression in a mouse model of bladder cancer (human VISTA knock-in mice) as a result [145]. In 2016, Janssen Biotech started a phase I trial (NCT02671955) to assess its safety, tolerability, and pharmacokinetics in advanced tumors, including lung, pancreatic, head and neck, colorectal, and cervical cancers [146]. The study was prematurely terminated after 2 years for unknown reasons in a situation where one of 12 patients experienced cytokine release syndrome.
CI-8993
Curis is currently pursuing VSTB112 (JNJ-61610588) as CI-8993 (Onvatilimab) in the phase I trial (NCT04475523) in relapsed/refractory solid tumors [147]. It is important to note that even at subtherapeutic doses, CI-8993 triggers a significant release of cytokines that can cause neurotoxicity, maybe due to its cell-depleting IgG1 backbone.
W018 (K01401-020)
The newest anti-VISTA mAb with IgG1 subtype, developed by Pierre Fabre, inhibits PSGL-1 binding to VISTA at pH 6–7.4 [148]. W018 phase I trial (NCT04564417) has recently commenced (149).
BMS767 (P1-068767)
Anti-VISTA human mAb investigated by Bristol-Myers Squibb. It is the only VISTA pH-sensitive antibody interacting with VISTA (H121, H122, and some C–C' loop residues) at pH 6.0 (not physiological pH) [144].
SG7
Developed by yeast surface display technology and has inhibitory effects against VISTA. It has overlap** epitopes with VSTB112 and BMS767, binding only to human VISTA. However, it has unique regions for binding to VISTA expressed in murine and cynomolgus monkey, which makes SG7 species cross-reactive antibody with high affinity. Jurkat T cell activation assay showed that the activation level of T cells was restored by SG7, and blocking VISTA via SG7 reduced tumor growth in syngeneic tumor models. SG7 was also found to reduce the number of polymorphonuclear MDSCs (PMN-MDSCs) in a TME from 4T1-bearing mice and increased the number of CD4 + and CD8 + T cells. PMN-MDSCs are cells with a high expression of VISTA and suppress anti-tumoral responses in TME. Nevertheless, no effect was observed on other examined myeloid cells such as CD11c + DCs, CD11b + macrophages, and monocytic myeloid-derived suppressor cells (M-MDSCs). SG7 inhibited VISTA's interaction with VSIG-3 at pH 7.4 and PSGL-1 at pH 6.0, mainly via H122 and E125 residues [144].
HMBD-002
An IgG4 anti-VISTA mAb developed by Hummingbird Biosciences, which primarily interacts with the C–C' loop of VISTA, where VISTA interacts with VSIG-3 and LRIG1. It neutralizes VISTA functions without depleting VISTA + cells by acting via an Fc-independent mechanism. Regarding VISTA/VSIG-3 interactions, HMBD-002 resolved the suppressory effects of VSIG-3, and anti-CD3-activated T cells produced IFN-γ. Additionally, it has been shown that HMBD-002 reverses the inhibitory effects of MDSCs on T cells, suppresses tumor cell invasion, and, most importantly, enables T cells to shift toward Th1/Th17 [150]. In several humanized and syngeneic murine models of breast, colorectal, and lung cancer, HMBD-002 showed therapeutical effects and suppressed tumor growth without apparent toxicity. A preclinical study showed that in combination with pembrolizumab (anti-PD-L1), HMBD-002 demonstrated superior efficacy, particularly in tumors with high infiltration of MDSCs [151,152,153]. It is now under investigation in the phase 1/2 trial (NCT05082610) as a single agent and combined with pembrolizumab in advanced solid tumors expressing VISTA [154].
Both CI-8993 and W018 induce anti-tumor effects through Fc-dependent activities of their IgG1, which frequently result in ADCC or complement-dependent cytotoxicity (CDC)-mediated cell death [155]. It is important to note that VISTA is expressed in a wide range of healthy cells, which means that this activity can cause the death of a large number of cells that are not targeted. HMBD-002 epitope is distinct from CI-8993 and W018 and exhibits high binding specificity for VISTA in various species (human, rat, monkey, and murine orthologs). VSTB112, SG7, and BMS767 all interact with the H122 residue within VISTA to effectively inhibit the binding of both VSIG-3 and PSGL-1. SG7 showed more affinity binding around 25 to 50 fold compared with VSTB112 or BMS767. While the interaction between BMS767 and VISTA is pH-dependent, the binding of SG7 and VSTB112 is not, which makes BMS767 a potential anti-VISTA targeting mAb homing TME. Furthermore, VSTB112 and BMS767 show depletion in VISTA-expressing cells (active Fc), but SG7 does not (dead Fc) [144, 156]. Currently, some mAbs are in the preclinical stage of development, such as KVA 12.1 [157], PMC-309 [158], APX-201, VTX-0811, SNS-101 [159], IMT-18, and IGN-381 for which only a few publications have been published so far, and some are still in the development process [160, 161].
Conclusions and future perspectives
There is no doubt that VISTA has attracted attention in immunotherapy thanks to its distinguishing features compared with other NCRs, where it shows more profound immunoregulatory effects as a result of these options. The impressive results that have been published from treatments based on VISTA targeting highlight the importance and highest value of examining VISTA in more detail [67, 135]. Regarding therapy efficacy, VISTA agonists appear to be more effective in autoimmune and inflammatory disorders than antagonists in cancer because of VISTA-associated irAEs and bi-directional role. However, it should be kept in mind that in MS and lupus cases, various elements such as genetics and inflammatory factors could affect the expression of VISTA. In this regard, investigating the expression pattern of VISTA in autoimmune disorders may be helpful before starting any therapy based on this gene.
Based on the information discussed above, it is clear that all VISTA ligands, apart from VISTA, play an important role in tumor development and growth. In some cases, their importance is so great that they have even been proposed as targets for immunotherapy. Among the VISTA ligands, PSGL-1 and VSIG-3 are valuable options to be considered due to their unique expression and functions, which may reduce side effects related to VISTA targeting. Nevertheless, newly identified ligands, such as Gal-9 and MMP-13, can also be investigated as potential targets blocking the VISTA pathway in the future. Because blocking their interaction with VISTA showed that their inhibition has the potential not only to activate immune responses but also suppress tumor growth. However, in proposing VISTA ligands as alternative targets for blocking the VISTA inhibitory pathway, consideration should be given to their functions in other parts of the body as well as the immune system. Because even if its inhibition suppresses tumor cell growth, it can result in serious secondary complications. Therefore, in determining which of the identified ligands for VISTA should be targeted, choosing the most effective option in suppressing VISTA signaling with fewer irAEs is advisable.
Regarding designing mAbs, it would be advantageous to choose specific residues shared between multiple VISTA ligands to inhibit all relevant VISTA pathways and/or the choice of non-overlap** regions to block a particular path. This case requires identifying the details of the regions through which the ligands are attached to VISTA. Moreover, the Fc activity of the antibodies is another critical factor to consider. Fc-independent antibodies have a high priority in the effort to eliminate ADCC and CDC. It is also possible for bispecific antibodies to be used as a combination therapy in order to inhibit VISTA and its related pathways, as well as other ICs.
On the other hand, environmental factors are also important for improving therapy efficacy. As discussed before, the environment’s pH is a critical factor in VISTA’s performance and binding to its ligands [162]. Hence, some strategies could be used to optimize this factor. For example, PSGL-1 interacts with VISTA in an acidic pH environment and, therefore, has the highest priority to inhibit the VISTA pathway in TME and would be a suitable therapeutic option for cancer therapy. BMS767 is the only VISTA-targeting antibody developed based on this point.
Altogether, it is clear that VISTA's ligands are just as important as VISTA itself, and their interactions play a significant role in many diseases related to the immune system, especially cancer. Therefore, VISTA and its ligands can be quite promising candidates when it comes to considering new immunotherapeutic targets. Nevertheless, VISTA's interaction with ligands, especially other than PSGL-1 and VSIG-3, their related effects, and intracellular pathways remain a work in progress. As a result, it is imperative that more studies be conducted in vitro, particularly in vivo, to fill these knowledge gaps and to maximize the potential of targeting VISTA through new therapeutic approaches.
Availability of data and materials
Not applicable.
Abbreviations
- VISTA:
-
V-domain immunoglobulin suppressor of T cell activation
- ICs:
-
Immune checkpoints
- ICRs:
-
Immune checkpoint receptors
- CTLA-4:
-
Cytotoxic T-lymphocyte-associated protein 4
- PD-1:
-
Programmed cell death protein 1/PD-L1 (Programmed death-ligand 1)
- FDA:
-
Food and drug administration
- NSCLC:
-
Non-small cell lung cancer
- PD-1H:
-
PDCD-1 homolog
- SISP1:
-
Stress-induced secreted protein 1
- DD1a:
-
Death domain1alpha
- Dies1:
-
Differentiation of embryonic stem cells 1
- ITAM:
-
Immunoreceptor tyrosine-based activation motif
- ITIM:
-
Immunoreceptor tyrosine-based inhibitory motif
- ITSM:
-
Immunoreceptor tyrosine-based switch motif
- SH3:
-
Src homology domain 3
- SH2:
-
Src homology domain 2
- PKC:
-
Phosphokinase C
- CK2:
-
Casein kinase 2
- ECD:
-
Extracellular domain
- TME:
-
Tumor microenvironment
- EAE:
-
Experimental autoimmune encephalomyelitis
- MS:
-
Multiple sclerosis
- Th2:
-
T helper 2
- MDSC:
-
Myeloid-derived suppressor cells
- MAIDS:
-
Murine model of AIDS
- mAb:
-
Monoclonal antibody
- TNF:
-
Tumor necrosis factor
- IL:
-
Interleukin
- CXCL2:
-
C-X-C motif chemokine ligand 2
- DCs:
-
Dendritic cells
- TLRs:
-
Toll-like receptors
- MyD88:
-
Myeloid differentiation primary response 88
- APCs:
-
Antigen-presenting cells
- CNS:
-
Central nervous system
- CKO:
-
Conditional knockout
- BM:
-
Bone marrow
- PBMCs:
-
Peripheral blood mononuclear cells
- FoxP3 + :
-
Forkhead box P3
- Tregs:
-
Regulatory T cells
- NK:
-
Natural killer
- TFs:
-
Transcription factors
- NF-κB:
-
Nuclear factor kappa B
- HIF-1α:
-
Hypoxia-inducible factor 1-alpha
- FOXD3:
-
Forkhead box D3
- miRNA:
-
MicroRNA
- LPS:
-
Lipopolysaccharide
- IFN-γ:
-
Interferon-γ
- Tfh:
-
T follicular helper
- DLE:
-
Discoid lupus erythematosus
- SLE:
-
Systemic lupus erythematosus
- RRMS:
-
Relapsing–remitting multiple sclerosis
- RA:
-
Rheumatoid arthritis
- GVHD:
-
Graft-versus-host disease
- MCP-1:
-
Monocyte chemoattractant protein-1
- WT:
-
Wild-type
- TILs:
-
Tumor-infiltrating lymphocytes
- GC:
-
Gastric cancer
- AML:
-
Acute myeloid leukemia
- OSCC:
-
Oral squamous cell carcinoma
- IrAEs:
-
Immune-related adverse events
- TGF-β:
-
Transforming growth factor beta
- VSIG-3:
-
V-Set and Immunoglobulin domain containing 3
- PSGL-1:
-
P-selectin glycoprotein ligand-1
- Gal-9:
-
Galectin-9
- VSIG-8:
-
V-Set and Immunoglobulin domain containing 8
- MMP-13:
-
Matrix metalloproteinase-13
- Sdc2:
-
Syndecan-2
- LRIG1:
-
Leucine-rich repeats and immunoglobulin-like domains 1
- CTLs:
-
Cytotoxic T lymphocytes
- BC:
-
Breast cancer
- IDO:
-
Indoleamine 2,3-dioxygenase
- HSCs:
-
Hematopoietic stem cells
- TIM-3:
-
T-cell immunoglobulin and mucin-domain containing-3
- LAG3:
-
Lymphocyte-activation gene 3
- CRDs:
-
Carbohydrate-recognition domains
- MM:
-
Multiple myeloma
- PDI:
-
Protein disulfide isomerase
- LRP1:
-
Lipoprotein receptor-related protein 1
- TIMPs:
-
Inhibitors of metalloproteinases
- HSPG:
-
Heparan sulfate proteoglycan
- EGFR:
-
Epidermal growth factor receptor
- AICD:
-
Activation-induced cell death
- CDC:
-
Complement-dependent cytotoxicity
- PMN-MDSCs:
-
Polymorphonuclear MDSCs
- M-MDSCs:
-
Monocytic myeloid-derived suppressor cells
References
Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20(11):651–68.
Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–5.
Hosseini A, Gharibi T, Marofi F, Babaloo Z, Baradaran B. CTLA-4: from mechanism to autoimmune therapy. Int Immunopharmacol. 2020;80:106221.
Singh S, Numan A, Agrawal N, Tambuwala MM, Singh V, Kesharwani P. Role of immune checkpoint inhibitors in the revolutionization of advanced melanoma care. Int Immunopharmacol. 2020;83:106417.
Xu W, Hiếu T, Malarkannan S, Wang L. The structure, expression, and multifaceted role of immune-checkpoint protein VISTA as a critical regulator of anti-tumor immunity, autoimmunity, and inflammation. Cell Mol Immunol. 2018;15(5):438–46.
Flies DB, Wang S, Xu H, Chen L. Cutting edge: a monoclonal antibody specific for the programmed death-1 homolog prevents graft-versus-host disease in mouse models. J Immunol. 2011;187(4):1537–41.
Mehta N, Maddineni S, Mathews II, Andres Parra Sperberg R, Huang PS, Cochran JR. Structure and functional binding epitope of V-domain Ig suppressor of T cell activation. Cell Rep. 2019;28(10):2509–16.
Bharaj P, Chahar HS, Alozie OK, Rodarte L, Bansal A, Goepfert PA, et al. Characterization of programmed death-1 homologue-1 (PD-1H) expression and function in normal and HIV infected individuals. PLoS ONE. 2014;9(10):e109103.
Lines JL, Pantazi E, Mak J, Sempere LF, Wang L, O’Connell S, et al. VISTA is an immune checkpoint molecule for human T cells. Can Res. 2014;74(7):1924–32.
Yasinska IM, Meyer NH, Schlichtner S, Hussain R, Siligardi G, Casely-Hayford M, et al. Ligand-receptor interactions of galectin-9 and VISTA suppress human T lymphocyte cytotoxic activity. Front Immunol. 2020;11:580557.
Noubissi Nzeteu GA, Schlichtner S, David S, Ruppenstein A, Fasler-Kan E, Raap U, et al. Macrophage differentiation and polarization regulate the release of the immune checkpoint protein V-domain Ig suppressor of T cell activation. Front Immunol. 2022;13:837097.
Hu L, Chen L, **ao Z, Zheng X, Chen Y, **an N, et al. Ablation of T cell-associated PD-1H enhances functionality and promotes adoptive immunotherapy. JCI Insight. 2022. https://doi.org/10.1172/jci.insight.148247.
Wang L, Rubinstein R, Lines JL, Wasiuk A, Ahonen C, Guo Y, et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med. 2011;208(3):577–92.
Yoon KW, Byun S, Kwon E, Hwang SY, Chu K, Hiraki M, et al. Control of signaling-mediated clearance of apoptotic cells by the tumor suppressor p53. Science. 2015;349(6247):1261669.
ElTanbouly MA, Schaafsma E, Noelle RJ, Lines JL. VISTA: Coming of age as a multi-lineage immune checkpoint. Clin Exp Immunol. 2020;200(2):120–30.
ElTanbouly MA, Zhao Y, Nowak E, Li J, Schaafsma E, Le Mercier I, et al. VISTA is a checkpoint regulator for naïve T cell quiescence and peripheral tolerance. Science. 2020. https://doi.org/10.1126/science.aay0524.
Deng J, Li J, Sarde A, Lines JL, Lee YC, Qian DC, et al. Hypoxia-induced VISTA promotes the suppressive function of myeloid-derived suppressor cells in the tumor microenvironment. Cancer Immunol Res. 2019;7(7):1079–90.
Rosenbaum SR, Knecht M, Mollaee M, Zhong Z, Erkes DA, McCue PA, et al. FOXD3 regulates VISTA expression in melanoma. Cell Rep. 2020;30(2):510–24.
Oliveira P, Carvalho J, Rocha S, Azevedo M, Reis I, Camilo V, et al. Dies1/VISTA expression loss is a recurrent event in gastric cancer due to epigenetic regulation. Sci Rep. 2016;6:34860.
Borggrewe M, Grit C, Den Dunnen WFA, Burm SM, Bajramovic JJ, Noelle RJ, et al. VISTA expression by microglia decreases during inflammation and is differentially regulated in CNS diseases. Glia. 2018;66(12):2645–58.
Wang G, Tai R, Wu Y, Yang S, Wang J, Yu X, et al. The expression and immunoregulation of immune checkpoint molecule VISTA in autoimmune diseases and cancers. Cytokine Growth Factor Rev. 2020;52:1–14.
Dolatkhah K, Alizadeh N, Mohajjel-Shoja H, Abdoli Shadbad M, Hajiasgharzadeh K, Aghebati-Maleki L, et al. B7 immune checkpoint family members as putative therapeutics in autoimmune disease: an updated overview. Int J Rheum Dis. 2022;25(3):259–71.
ElTanbouly MA, Schaafsma E, Smits NC, Shah P, Cheng C, Burns C, et al. VISTA re-programs macrophage biology through the combined regulation of tolerance and anti-inflammatory pathways. Front Immunol. 2020;11:580187.
Vivian Ma YH, Sparkes A, Saha S, Gariépy J. VISTA as a ligand downregulates LPS-mediated inflammation in macrophages and neutrophils. Cell Immunol. 2022;379:104581.
Broughton TWK, ElTanbouly MA, Schaafsma E, Deng J, Sarde A, Croteau W, et al. Defining the signature of VISTA on myeloid cell chemokine responsiveness. Front Immunol. 2019;10:2641.
Ceeraz S, Eszterhas SK, Sergent PA, Armstrong DA, Ashare A, Broughton T, et al. VISTA deficiency attenuates antibody-induced arthritis and alters macrophage gene expression in response to simulated immune complexes. Arthritis Res Ther. 2017;19(1):270.
Xu W, Dong J, Zheng Y, Zhou J, Yuan Y, Ta HM, et al. Immune-checkpoint protein VISTA regulates antitumor immunity by controlling myeloid cell-mediated inflammation and immunosuppression. Cancer Immunol Res. 2019;7(9):1497–510.
Borggrewe M, Kooistra SM, Wesseling EM, Gierschek FL, Brummer ML, Nowak EC, et al. VISTA regulates microglia homeostasis and myelin phagocytosis, and is associated with MS lesion pathology. Acta Neuropathol Commun. 2021;9(1):91.
Dübbel L, Koch KW, Bremer E. Characterization of the novel negative checkpoint regulator V-domain immunoglobulin-containing suppressor of T-cell activation (VISTA) on antigen presenting cells. Oldenburg: Carl von Ossietzky Universität Oldenburg; 2020.
Flies DB, Higuchi T, Chen L. Mechanistic assessment of PD-1H coinhibitory receptor-induced T Cell tolerance to allogeneic antigens. J Immunol. 2015;194(11):5294–304.
Aloia L, Parisi S, Fusco L, Pastore L, Russo T. Differentiation of embryonic stem cells 1 (Dies1) is a component of bone morphogenetic protein 4 (BMP4) signaling pathway required for proper differentiation of mouse embryonic stem cells. J Biol Chem. 2010;285(10):7776–83.
Parisi S, Battista M, Musto A, Navarra A, Tarantino C, Russo T. A regulatory loop involving Dies1 and miR-125a controls BMP4 signaling in mouse embryonic stem cells. FASEB J. 2012;26(10):3957–68.
Ren G, Beech C, Smas CM. The immunoglobulin superfamily protein differentiation of embryonic stem cells 1 (dies1) has a regulatory role in preadipocyte to adipocyte conversion. PLoS ONE. 2013;8(6):e65531.
Ceeraz S, Sergent PA, Plummer SF, Schned AR, Pechenick D, Burns CM, et al. VISTA deficiency accelerates the development of fatal murine lupus nephritis. Arthr Rheumatol. 2017;69(4):814–25.
Han X, Vesely MD, Yang W, Sanmamed MF, Badri T, Alawa J, et al. PD-1H (VISTA)-mediated suppression of autoimmunity in systemic and cutaneous lupus erythematosus. Sci Trans Med. 2019. https://doi.org/10.1126/scitranslmed.aax1159.
Sergent PA, Plummer SF, Pettus J, Mabaera R, DeLong JK, Pechenick DA, et al. Blocking the VISTA pathway enhances disease progression in (NZB × NZW) F1 female mice. Lupus. 2018;27(2):210–6.
Derakhshani A, Asadzadeh Z, Baradaran B, Safarpour H, Rahmani S, Leone P, et al. The expression pattern of VISTA in the PBMCs of relapsing-remitting multiple sclerosis patients: a single-cell RNA sequencing-based study. Biomed pharmacother. 2022;148:112725.
Rendon A, Schäkel K. Psoriasis pathogenesis and treatment. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20061475.
Li N, Xu W, Yuan Y, Ayithan N, Imai Y, Wu X, et al. Immune-checkpoint protein VISTA critically regulates the IL-23/IL-17 inflammatory axis. Sci Rep. 2017;7(1):1485.
Ohno T, Kondo Y, Zhang C, Kang S, Azuma M. Immune checkpoint molecule, VISTA regulates T-cell-mediated skin inflammatory responses. J Invest Dermatol. 2017;137(6):1384–6.
Liu H, Li X, Hu L, Zhu M, He B, Luo L, et al. A crucial role of the PD-1H coinhibitory receptor in suppressing experimental asthma. Cell Mol Immunol. 2018;15(9):838–45.
Venegas Garrido C, Mukherjee M, Bhalla A, Nair P. Airway autoimmunity, asthma exacerbations, and response to biologics. Clin Exp Allergy. 2022;52(12):1365–78.
Ohno T, Zhang C, Kondo Y, Kang S, Furusawa E, Tsuchiya K, et al. The immune checkpoint molecule VISTA regulates allergen-specific Th2-mediated immune responses. Int Immunol. 2018;30(1):3–11.
Flies DB, Han X, Higuchi T, Zheng L, Sun J, Ye JJ, et al. Coinhibitory receptor PD-1H preferentially suppresses CD4+ T cell-mediated immunity. J Clin Investig. 2014;124(5):1966–75.
Green KA, Wang L, Noelle RJ, Green WR. Selective involvement of the checkpoint regulator VISTA in suppression of B-cell, but not T-cell, responsiveness by monocytic myeloid-derived suppressor cells from mice infected with an immunodeficiency-causing retrovirus. J Virol. 2015;89(18):9693–8.
Zhao SJ, Muyayalo KP, Luo J, Huang D, Mor G, Liao AH. Next generation of immune checkpoint molecules in maternal-fetal immunity. Immunol Rev. 2022;308(1):40–54.
Jiang X, Wang J, Deng X, **ong F, Ge J, **ang B, et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer. 2019;18(1):10.
Zhou K, Guo S, Li F, Sun Q, Liang G. Exosomal PD-L1: new insights into tumor immune escape mechanisms and therapeutic strategies. Front Cell Dev Biol. 2020;8:569219.
Nakamura K, Smyth MJ. Myeloid immunosuppression and immune checkpoints in the tumor microenvironment. Cell Mol Immunol. 2020;17(1):1–12.
Le Mercier I, Chen W, Lines JL, Day M, Li J, Sergent P, et al. VISTA regulates the development of protective antitumor immunity. Can Res. 2014;74(7):1933–44.
Villarroel-Espindola F, Yu X, Datar I, Mani N, Sanmamed M, Velcheti V, et al. Spatially resolved and quantitative analysis of VISTA/PD-1H as a novel immunotherapy target in human non-small cell lung cancer. Clin Cancer Res. 2018;24(7):1562–73.
Zhang M, Pang HJ, Zhao W, Li YF, Yan LX, Dong ZY, et al. VISTA expression associated with CD8 confers a favorable immune microenvironment and better overall survival in hepatocellular carcinoma. BMC Cancer. 2018;18(1):511.
Liao H, Zhu H, Liu S, Wang H. Expression of V-domain immunoglobulin suppressor of T cell activation is associated with the advanced stage and presence of lymph node metastasis in ovarian cancer. Oncol Lett. 2018;16(3):3465–72.
Hong S, Yuan Q, **a H, Zhu G, Feng Y, Wang Q, et al. Analysis of VISTA expression and function in renal cell carcinoma highlights VISTA as a potential target for immunotherapy. Protein Cell. 2019;10(11):840–5.
Wu L, Deng WW, Huang CF, Bu LL, Yu GT, Mao L, et al. Expression of VISTA correlated with immunosuppression and synergized with CD8 to predict survival in human oral squamous cell carcinoma. Cancer Immunol Immunother CII. 2017;66(5):627–36.
Böger C, Behrens HM, Krüger S, Röcken C. The novel negative checkpoint regulator VISTA is expressed in gastric carcinoma and associated with PD-L1/PD-1: a future perspective for a combined gastric cancer therapy? Oncoimmunology. 2017;6(4):e1293215.
**e S, Huang J, Qiao Q, Zang W, Hong S, Tan H, et al. Expression of the inhibitory B7 family molecule VISTA in human colorectal carcinoma tumors. Cancer Immunol Immunother CII. 2018;67(11):1685–94.
Lei CJ, Wang B, Long ZX, Ren H, Pan QY, Li Y. Investigation of PD-1H in DEN-induced mouse liver cancer model. Eur Rev Med Pharmacol Sci. 2018;22(16):5194–9.
Kondo Y, Ohno T, Nishii N, Harada K, Yagita H, Azuma M. Differential contribution of three immune checkpoint (VISTA, CTLA-4, PD-1) pathways to antitumor responses against squamous cell carcinoma. Oral Oncol. 2016;57:54–60.
Zong L, Zhou Y, Zhang M, Chen J, **ang Y. VISTA expression is associated with a favorable prognosis in patients with high-grade serous ovarian cancer. Cancer Immunol Immunother CII. 2020;69(1):33–42.
Zhang M, Zhang J, Liu N, Wang B, Zhou Y, Yang J. VISTA is associated with immune infiltration and predicts favorable prognosis in TNBC. Front Oncol. 2022;12:961374.
Cao X, Ren X, Zhou Y, Mao F, Lin Y, Wu H, et al. VISTA expression on immune cells correlates with favorable prognosis in patients with triple-negative breast cancer. Front Oncol. 2020;10:583966.
Liu J, Yuan Y, Chen W, Putra J, Suriawinata AA, Schenk AD, et al. Immune-checkpoint proteins VISTA and PD-1 nonredundantly regulate murine T-cell responses. Proc Natl Acad Sci USA. 2015;112(21):6682–7.
Deng J, Le Mercier I, Kuta A, Noelle RJ. A New VISTA on combination therapy for negative checkpoint regulator blockade. J Immunother Cancer. 2016;4:86.
Kakavand H, Jackett LA, Menzies AM, Gide TN, Carlino MS, Saw RPM, et al. Negative immune checkpoint regulation by VISTA: a mechanism of acquired resistance to anti-PD-1 therapy in metastatic melanoma patients. Mod Pathol. 2017;30(12):1666–76.
Gao J, Ward JF, Pettaway CA, Shi LZ, Subudhi SK, Vence LM, et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat Med. 2017;23(5):551–5.
Yum JI, Hong YK. Terminating cancer by blocking VISTA as a novel immunotherapy: hasta la vista, baby. Front Oncol. 2021;11:658488.
Chennamadhavuni A, Abushahin L, ** N, Presley CJ, Manne A. Risk factors and biomarkers for immune-related adverse events: a practical guide to identifying high-risk patients and rechallenging immune checkpoint inhibitors. Front Immunol. 2022;13:779691.
Conroy M, Naidoo J. Immune-related adverse events and the balancing act of immunotherapy. Nat Commun. 2022;13(1):392.
Li N, Wang G, Hou X, Tai R, Huang S, He Z, et al. Adverse and unconventional reactions related to immune checkpoint inhibitor therapy for cancer. Int Immunopharmacol. 2022;108:108803.
Song P, Zhang D, Cui X, Zhang L. Meta-analysis of immune-related adverse events of immune checkpoint inhibitor therapy in cancer patients. Thoracic Cancer. 2020;11(9):2406–30.
Suzu S, Hayashi Y, Harumi T, Nomaguchi K, Yamada M, Hayasawa H, et al. Molecular cloning of a novel immunoglobulin superfamily gene preferentially expressed by brain and testis. Biochem Biophys Res Commun. 2002;296(5):1215–21.
**e X, Chen C, Chen W, Jiang J, Wang L, Li T, et al. Structural basis of VSIG3: the ligand for VISTA. Front Immunol. 2021;12:625808.
Watanabe T, Suda T, Tsunoda T, Uchida N, Ura K, Kato T, et al. Identification of immunoglobulin superfamily 11 (IGSF11) as a novel target for cancer immunotherapy of gastrointestinal and hepatocellular carcinomas. Cancer Sci. 2005;96(8):498–506.
Harada H, Suzu S, Hayashi Y, Okada S. BT-IgSF, a novel immunoglobulin superfamily protein, functions as a cell adhesion molecule. J Cell Physiol. 2005;204(3):919–26.
Kim H, Takegahara N, Walsh MC, Choi Y. CD44 can compensate for IgSF11 deficiency by associating with the scaffold protein PSD-95 during osteoclast differentiation. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21072646.
Jang S, Oh D, Lee Y, Hosy E, Shin H, van Riesen C, et al. Synaptic adhesion molecule IgSF11 regulates synaptic transmission and plasticity. Nat Neurosci. 2016;19(1):84–93.
Pelz L, Purfürst B, Rathjen FG. The cell adhesion molecule BT-IgSF is essential for a functional blood-testis barrier and male fertility in mice. J Biol Chem. 2017;292(52):21490–503.
Chen B, Zhu G, Yan A, He J, Liu Y, Li L, et al. IGSF11 is required for pericentric heterochromatin dissociation during meiotic diplotene. PLoS Genet. 2021;17(9):e1009778.
Grelet S, Fréreux C, Obellianne C, Noguchi K, Howley BV, Dalton AC, et al. TGFβ-induced expression of long noncoding lincRNA Platr18 controls breast cancer axonogenesis. Life Sci Alliance. 2022. https://doi.org/10.26508/lsa.202101261.
Wang J, Wu G, Manick B, Hernandez V, Renelt M, Erickson C, et al. VSIG-3 as a ligand of VISTA inhibits human T-cell function. Immunology. 2019;156(1):74–85.
Ghouzlani A, Rafii S, Karkouri M, Lakhdar A, Badou A. The promising IgSF11 immune checkpoint is highly expressed in advanced human gliomas and associates to poor prognosis. Front Oncol. 2020;10:608609.
Moore KL. Structure and function of P-selectin glycoprotein ligand-1. Leuk Lymphoma. 1998;29(1–2):1–15.
Baïsse B, Galisson F, Giraud S, Schapira M, Spertini O. Evolutionary conservation of P-selectin glycoprotein ligand-1 primary structure and function. BMC Evol Biol. 2007;7:166.
Ley K, Kansas GS. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat Rev Immunol. 2004;4(5):325–35.
Silván J, González-Tajuelo R, Vicente-Rabaneda E, Pérez-Frías A, Espartero-Santos M, Muñoz-Callejas A, et al. Deregulated PSGL-1 expression in B cells and dendritic cells may be implicated in human systemic sclerosis development. J Invest Dermatol. 2018;138(10):2123–32.
Vachino G, Chang XJ, Veldman GM, Kumar R, Sako D, Fouser LA, et al. P-selectin glycoprotein ligand-1 is the major counter-receptor for P-selectin on stimulated T cells and is widely distributed in non-functional form on many lymphocytic cells. J Biol Chem. 1995;270(37):21966–74.
Martinez M, Joffraud M, Giraud S, Baïsse B, Bernimoulin MP, Schapira M, et al. Regulation of PSGL-1 interactions with L-selectin, P-selectin, and E-selectin: role of human fucosyltransferase-IV and -VII. J Biol Chem. 2005;280(7):5378–90.
Pouyani T, Seed B. PSGL-1 recognition of P-selectin is controlled by a tyrosine sulfation consensus at the PSGL-1 amino terminus. Cell. 1995;83(2):333–43.
Veerman KM, Williams MJ, Uchimura K, Singer MS, Merzaban JS, Naus S, et al. Interaction of the selectin ligand PSGL-1 with chemokines CCL21 and CCL19 facilitates efficient homing of T cells to secondary lymphoid organs. Nat Immunol. 2007;8(5):532–9.
Yang J, Hirata T, Croce K, Merrill-Skoloff G, Tchernychev B, Williams E, et al. Targeted gene disruption demonstrates that P-selectin glycoprotein ligand 1 (PSGL-1) is required for P-selectin-mediated but not E-selectin-mediated neutrophil rolling and migration. J Exp Med. 1999;190(12):1769–82.
Hirata T, Furie BC, Furie B. P-, E-, and L-selectin mediate migration of activated CD8+ T lymphocytes into inflamed skin. J Immunol. 2002;169(8):4307–13.
Yang J, Furie BC, Furie B. The biology of P-selectin glycoprotein ligand-1: its role as a selectin counterreceptor in leukocyte-endothelial and leukocyte-platelet interaction. Thromb Haemost. 1999;81(1):1–7.
Urzainqui A, Martínez del Hoyo G, Lamana A, de la Fuente H, Barreiro O, Olazabal IM, et al. Functional role of P-selectin glycoprotein ligand 1/P-selectin interaction in the generation of tolerogenic dendritic cells. J Immunol. 2007;179(11):7457–65.
Lévesque JP, Zannettino AC, Pudney M, Niutta S, Haylock DN, Snapp KR, et al. PSGL-1-mediated adhesion of human hematopoietic progenitors to P-selectin results in suppression of hematopoiesis. Immunity. 1999;11(3):369–78.
Zaongo SD, Liu Y, Harypursat V, Song F, **a H, Ma P, et al. P-selectin glycoprotein ligand 1: a potential HIV-1 therapeutic target. Front Immunol. 2021;12:710121.
Tinoco R, Carrette F, Barraza ML, Otero DC, Magaña J, Bosenberg MW, et al. PSGL-1 is an immune checkpoint regulator that promotes T cell exhaustion. Immunity. 2016;44(5):1190–203.
Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–7.
Johnston RJ, Su LJ, Pinckney J, Critton D, Boyer E, Krishnakumar A, et al. VISTA is an acidic pH-selective ligand for PSGL-1. Nature. 2019;574(7779):565–70.
Bänfer S, Jacob R. Galectins in intra- and extracellular vesicles. Biomolecules. 2020. https://doi.org/10.3390/biom10091232.
Popa SJ, Stewart SE, Moreau K. Unconventional secretion of annexins and galectins. Semin Cell Dev Biol. 2018;83:42–50.
Moar P, Tandon R. Galectin-9 as a biomarker of disease severity. Cell Immunol. 2021;361:104287.
Liang T, Wang X, Wang F, Feng E, You G. Galectin-9: a predictive biomarker negatively regulating immune response in glioma patients. World Neurosurg. 2019;132:e455–62.
Tao L, ** L, Dechun L, Hongqiang Y, Changhua K, Guijun L. Galectin-3 expression in colorectal cancer and its correlation with clinical pathological characteristics and prognosis. Open Med. 2017;12:226–30.
Chang WA, Tsai MJ, Kuo PL, Hung JY. Role of galectins in lung cancer. Oncol Lett. 2017;14(5):5077–84.
Yang R, Sun L, Li CF, Wang YH, Yao J, Li H, et al. Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat Commun. 2021;12(1):832.
Yang R, Sun L, Li CF, Wang YH, **a W, Liu B, et al. Development and characterization of anti-galectin-9 antibodies that protect T cells from galectin-9-induced cell death. J Biol Chem. 2022;298(4):101821.
Bi S, Hong PW, Lee B, Baum LG. Galectin-9 binding to cell surface protein disulfide isomerase regulates the redox environment to enhance T-cell migration and HIV entry. Proc Natl Acad Sci USA. 2011;108(26):10650–5.
Niki T, Tsutsui S, Hirose S, Aradono S, Sugimoto Y, Takeshita K, et al. Galectin-9 is a high affinity IgE-binding lectin with anti-allergic effect by blocking IgE-antigen complex formation. J Biol Chem. 2009;284(47):32344–52.
Cao A, Alluqmani N, Buhari FHM, Wasim L, Smith LK, Quaile AT, et al. Galectin-9 binds IgM-BCR to regulate B cell signaling. Nat Commun. 2018;9(1):3288.
Wu C, Thalhamer T, Franca RF, **ao S, Wang C, Hotta C, et al. Galectin-9-CD44 interaction enhances stability and function of adaptive regulatory T cells. Immunity. 2014;41(2):270–82.
Sharma S, Sundararajan A, Suryawanshi A, Kumar N, Veiga-Parga T, Kuchroo VK, et al. T cell immunoglobulin and mucin protein-3 (Tim-3)/Galectin-9 interaction regulates influenza A virus-specific humoral and CD8 T-cell responses. Proc Natl Acad Sci USA. 2011;108(47):19001–6.
Yang R, Sun L, Li C-F, Wang Y-H, Yao J, Li H, et al. Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat Commun. 2021;12(1):832.
Rice RH, **a Y, Alvarado RJ, Phinney BS. Proteomic analysis of human nail plate. J Proteome Res. 2010;9(12):6752–8.
Lee YJ, Rice RH, Lee YM. Proteome analysis of human hair shaft: from protein identification to posttranslational modification. Mol Cell Proteomics MCP. 2006;5(5):789–800.
Yap EH, Rosche T, Almo S, Fiser A. Functional clustering of immunoglobulin superfamily proteins with protein-protein interaction information calibrated hidden Markov model sequence profiles. J Mol Biol. 2014;426(4):945–61.
Sasikumar PG, Naremaddepalli SS, Ramachandra RK, Gowda N, Yerramsetti MR, Bandireddy SR, et al. Abstract B006: functional antagonism of VSIG8-mediated immune suppression by oral VISTA agents. Mol Cancer Ther. 2018;17(1):006.
Wang J, Manick B, Renelt M, Hansen L, Wu G, Kalabokis V. VSIG-8 is a co-inhibitory ligand and an immune checkpoint molecule for human T cells. J Immunol. 2018. https://doi.org/10.4049/jimmunol.200.Supp.47.4.
Molloy M, Guo Y, Rothstein JAY, Rosenzweig M, inventors; Immunext INC molloy michael guo yalin rothstein jay, assignee. identification of vsig8 as the putative vista receptor and its use thereof to produce vista/vsig8 modulatorS. WO patent WO 2016/090347 A1. 2016 2015/12/05.
Chen W, Qie C, Hu X, Wang L, Jiang J, Liu W, et al. A small molecule inhibitor of VSIG-8 prevents its binding to VISTA. Invest New Drugs. 2022;40(4):690–9.
Fromm G, Silva Sd, Johannes K, Patel A, Hornblower J, Schreiber TH. Abstract 5564: agonist redirected checkpoint, VSIG8-Fc-OX40L, for cancer immunotherapy. Cancer Res. 2018;78(13):5564.
Leeman MF, Curran S, Murray GI. The structure, regulation, and function of human matrix metalloproteinase-13. Crit Rev Biochem Mol Biol. 2002;37(3):149–66.
Li S, Pritchard DM, Yu LG. Regulation and function of matrix metalloproteinase-13 in cancer progression and metastasis. Cancers. 2022. https://doi.org/10.3390/cancers14133263.
Yamamoto K, Okano H, Miyagawa W, Visse R, Shitomi Y, Santamaria S, et al. MMP-13 is constitutively produced in human chondrocytes and co-endocytosed with ADAMTS-5 and TIMP-3 by the endocytic receptor LRP1. Matrix Biol. 2016;56:57–73.
Cui N, Hu M, Khalil RA. Biochemical and biological attributes of matrix metalloproteinases. Prog Mol Biol Transl Sci. 2017;147:1–73.
Quillard T, Tesmenitsky Y, Croce K, Travers R, Shvartz E, Koskinas KC, et al. Selective inhibition of matrix metalloproteinase-13 increases collagen content of established mouse atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31(11):2464–72.
Li XD, Zhang XR, Li ZH, Yang Y, Zhang D, Zheng H, et al. Effect of matrix metallopeptidase 13 on the function of mouse bone marrow-derived dendritic cells. Chin Med J. 2017;130(6):717–21.
Amar S, Smith L, Fields GB. Matrix metalloproteinase collagenolysis in health and disease. Biochim et Biophys Acta Mol Cell Res. 2017;1864(11):1940–51.
Howes JM, Pugh N, Hamaia SW, Jung SM, Knäuper V, Malcor JD, et al. MMP-13 binds to platelet receptors αIIbβ3 and GPVI and impairs aggregation and thrombus formation. Res Pract Thromb Haemostasis. 2018;2(2):370–9.
Fu J, Li S, Yang C, Brown LM, Weiss SJ, Drake CG, et al. Checkpoint inhibitor PD-1H/VISTA functions as MMP-13 receptor on osteoclasts and mediates MMP-13 induced osteoclast activation in multiple myeloma. Blood. 2019;134(1):3072.
Fu J, Li S, Yang C, Brown LM, Weiss SJ, Drake CG, et al. Checkpoint inhibitor PD-1H/VISTA functions As MMP-13 receptor on osteoclasts and mediates MMP-13 induced osteoclast activation in multiple myeloma. Blood. 2019;134:3072.
Rogers BM, Smith L, Dezso Z, Shi X, DiGiammarino E, Nguyen D, et al. VISTA is an activating receptor in human monocytes. J Exp Med. 2021. https://doi.org/10.1084/jem.20201601.
Sun D, Wang YAN, Gordon Catherine A, Chai YI, Williams Samuel AF, inventors; Immutics Inc, assignee. Treating cancer by blocking the interaction of vista and its binding partner. WO patent WO 2019/165233 A1. 2019 2019/02/22.
Yuan L, Tatineni J, Mahoney KM, Freeman GJ. VISTA: a mediator of quiescence and a promising target in cancer immunotherapy. Trends Immunol. 2021;42(3):209–27.
ElTanbouly MA, Zhao Y, Schaafsma E, Burns CM, Mabaera R, Cheng C, et al. VISTA: a target to manage the innate cytokine storm. Front Immunol. 2020;11:595950.
Molloy M, Rothstein JAY, Pechenick DOV, Snyder L, Powers G, inventors; IMMUNEXT INC JANSSEN PHARMACEUTICALS INC, assignee. Anti-human VISTA antibodies and use thereof. US patent US 11525000 B2. 2022 2017/04/14.
Ma Y-HV, Sparkes A, Romão E, Saha S, Gariépy J. Agonistic nanobodies and antibodies to human VISTA. mAbs. 2021;13(1):2003281.
Li K, Tian H. Development of small-molecule immune checkpoint inhibitors of PD-1/PD-L1 as a new therapeutic strategy for tumour immunotherapy. J Drug Target. 2019;27(3):244–56.
Lazorchak AS, Patterson T, Ding Y, Sasikumar PG, Sudarshan NS, Gowda NM, et al. Abstract A36: CA-170, an oral small molecule PD-L1 and VISTA immune checkpoint antagonist, promotes T cell immune activation and inhibits tumor growth in pre-clinical models of cancer. Cancer Immunol Res. 2017;5(3):36.
Sasikumar PG, Sudarshan NS, Adurthi S, Ramachandra RK, Samiulla DS, Lakshminarasimhan A, et al. PD-1 derived CA-170 is an oral immune checkpoint inhibitor that exhibits preclinical anti-tumor efficacy. Commun Biol. 2021;4(1):699.
Curis I. A Study of CA-170 (Oral PD-L1, PD-L2 and VISTA Checkpoint Antagonist) in Patients With Advanced Tumors and Lymphomas. https://ClinicalTrials.gov/show/NCT02812875; 2016.
Radhakrishnan V, Bakhshi S, Prabhash K, Deshmukh C, Nag S, Lakshmaiah K. Phase 2 trial of CA-170, a novel oral small molecule dual inhibitor of immune checkpoints VISTA and PD-1, in patients (pts) with advanced solid tumor and Hodgkin lymphoma. J Immunother Cancer. 2018;6:P714.
Gabr MT, Gambhir SS. Discovery and optimization of small-molecule ligands for V-domain Ig suppressor of T-cell activation (VISTA). J Am Chem Soc. 2020;142(38):16194–8.
Mehta N, Maddineni S, Kelly RL, Lee RB, Hunter SA, Silberstein JL, et al. An engineered antibody binds a distinct epitope and is a potent inhibitor of murine and human VISTA. Sci Rep. 2020;10(1):15171.
Snyder L, Powers G, Sepulveda Manuel A, Alvarez John D, inventors; JANSSEN PHARMACEUTICA NV, assignee. ANTI-VISTA ANTIBODIES AND FRAGMENTS. WO patent WO 2016/207717 A8. 2017 2016/06/23.
Research J, Development L. A Study of Safety, Pharmacokinetics, Pharmacodynamics of JNJ-61610588 in Participants With Advanced Cancer. https://ClinicalTrials.gov/show/NCT02671955; 2016.
Curis I. Phase 1 Study of CI-8993 Anti-VISTA Antibody in Patients With Advanced Solid Tumor Malignancies. https://ClinicalTrials.gov/show/NCT04475523; 2020.
Cruzalegui F, Loukili N, Delfour O, Boute N, Thuilliez C, Champion T, et al. VISTA interaction with PSGL1, a likely VISTA receptor in tumors, is effectively disrupted by K0401–020 anti-VISTA antibody. Cancer Res. 2020;80(16):3372.
Medicament PF. First-In-Human (FIH) Study of W0180 as Single Agent and in Combination With Pembrolizumab in Adults With Locally Advanced or Metastatic Solid Tumors. https://ClinicalTrials.gov/show/NCT04564417; 2020.
Thakkar D, Paliwal S, Dharmadhikari B, Guan S, Liu L, Kar S, et al. Rationally targeted anti-VISTA antibody that blockades the C-C’ loop region can reverse VISTA immune suppression and remodel the immune microenvironment to potently inhibit tumor growth in an Fc independent manner. J Immunother Cancer. 2022. https://doi.org/10.1136/jitc-2021-003382.
Thakkar D, Paliwal S, Dharmadhikari B, Guan S, Liu L, Kar S, et al. Rationally targeted anti-VISTA antibody that blockades the C-C’ loop region can reverse VISTA immune suppression and remodel the immune microenvironment to potently inhibit tumor growth in an Fc independent manner. J Immunother Cancer. 2022;10(2):e003382.
Ingram PJ, Thakkar D, Boyd-Kirkup JD. HMBD002, a novel neutralizing antibody targeting a specific epitope on the co-inhibitory immune checkpoint receptor VISTA, displays potent anti-tumor effects in pre-clinical models. Cancer Res. 2017;77(13):587.
Boyd-Kirkup JD, Thakkar D, Paszkiewicz K, Ingram PJ. Integrative immune profiling of syngeneic tumor models provides predictive immune signatures for treatment response with HMBD002, a novel anti-VISTA neutralizing antibody. Cancer Res. 2018;78(13):1729.
Hummingbird Bioscience I. A Study of HMBD-002, a Monoclonal Antibody Targeting VISTA, as Monotherapy and Combined With Pembrolizumab. https://ClinicalTrials.gov/show/NCT05082610; 2022.
Martinez E, Faris J, Von Roemeling R, Angelides S, Johnson M. 392 Phase 1 study of CI-8993 anti-VISTA antibody in patients with advanced solid tumor malignancies. London: BMJ Specialist Journals; 2020.
Mostböck S, Wu HH, Fenn T, Riegler B, Strahlhofer S, Huang Y, et al. Distinct immune stimulatory effects of anti-human VISTA antibodies are determined by Fc-receptor interaction. Front Immunol. 2022;13:862757.
Guillaudeux T, Iadonato S, Tarcha E, Philips C. 268 KVA 121 a novel fully human anti-VISTA antibody: clinical trial design in monotherapy and in combination with an anti-PD1 antibody. J Immunother Cancer. 2021;9(2):A291.
Park CH, Byun SS, An JY, Ha SH, Han HR, Kang NR, et al. Abstract 1626: PMC309, a highly selective anti-VISTA antibody enhances T cell activation through blocking the interaction of T cells and myeloid derived suppressor cells (MDSC). Cancer Res. 2021;81(13):1626.
Thisted T, Mukherjee A, Malhotra K, Biesova Z, Kleschenko Y, Jiang Z-G, et al. 228 Antagonistic pH-selective VISTA antibody SNS-101 potentiates anti-PD-1/PD-L1-induced anti-tumor immunity. J Immunother Cancer. 2021;9(2):243.
Wu C, Cao X, Zhang X. VISTA inhibitors in cancer immunotherapy: a short perspective on recent progresses. RSC Med Chem. 2021;12(10):1672–9.
Rabadi D, Sajani AA, Noelle RJ, Lines JL. The role of VISTA in the tumor microenvironment. J Cancer Meta Treat. 2022;8(5):24.
Mahoney KM, Freeman GJ. Acidity changes immunology: a new VISTA pathway. Nat Immunol. 2020;21(1):13–6.
Acknowledgements
This review article is related to Najibeh Shekari’s Ph.D. thesis in immunology at the School of Medicine, Shahid Beheshti University of Medical Sciences (Registration number in Vice-Chancellor in Research Affairs: 610).
Funding
This study was supported by a grant from the Research Department of the School of Medicine, Shahid Beheshti University of Medical Sciences (Grant No: 30640), and the Immunology Research Center of Tabriz University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
NSh contributed to data gathering, writing the manuscript, and designing the tables and figures. DSh and HZ contributed to the edition of the manuscript. TK contributed to the manuscript revision. BB and SAJ contributed to the correspondence, final manuscript revision, and manuscript conforming before submission.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shekari, N., Shanehbandi, D., Kazemi, T. et al. VISTA and its ligands: the next generation of promising therapeutic targets in immunotherapy. Cancer Cell Int 23, 265 (2023). https://doi.org/10.1186/s12935-023-03116-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-023-03116-0