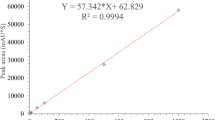
Abstract
Background
The lipopeptide herbicolin A (HA) secreted by the biocontrol agent Pantoea agglomerans ZJU23 is a promising antifungal drug to combat fungal pathogens by targeting lipid rafts, both in agricultural and clinical settings. Improvement of HA production would be of great significance in promoting its commercialization. This study aims to enhance the HA production in ZJU23 by combining fermentation optimization and strain engineering.
Results
Based on the results in the single-factor experiments, corn steep liquor, temperature and initial pH were identified as the significant affecting factors by the Plackett–Burman design. The fermentation medium and conditions were further optimized using the Box-Behnken response surface method, and the HA production of the wild type strain ZJU23 was improved from ~ 87 mg/mL in King’s B medium to ~ 211 mg/mL in HA induction (HAI) medium. A transposon library was constructed in ZJU23 to screen for mutants with higher HA production, and two transcriptional repressors for HA biosynthesis, LrhA and PurR, were identified. Disruption of the LrhA gene led to increased mRNA expression of HA biosynthetic genes, and subsequently improved about twofold HA production. Finally, the HA production reached ~ 471 mg/mL in the ΔLrhA mutant under optimized fermentation conditions, which is about 5.4 times higher than before (~ 87 mg/mL). The bacterial suspension of the ΔLrhA mutant fermented in HAI medium significantly enhanced its biocontrol efficacy against gray mold disease and Fusarium crown rot of wheat, showing equivalent control efficacies as the chemical fungicides used in this study. Furthermore, HA was effective against fungicide resistant Botrytis cinerea. Increased HA production substantially improved the control efficacy against gray mold disease caused by a pyrimethanil resistant strain.
Conclusions
This study reveals that the transcriptional repressor LrhA negatively regulates HA biosynthesis and the defined HAI medium is suitable for HA production. These findings provide an extended basis for large-scale production of HA and promote biofungicide development based on ZJU23 and HA in the future.
Graphical Abstract

Similar content being viewed by others
Background
Food production needs to increase by 60% to meet the demands of approximately 10 billion people around the world by 2050 [1]. The fast-growing human population requires an increased crop yield, along with a reduction in crop loss caused by crop pathogens and pests. An expert-based assessment of crop losses on an individual pathogen and pest basis for wheat, rice, maize, potato and soybean, as five major crops, globally indicated that the average of crop losses caused by pathogens and pests are about 22.5% for all five crops [2]. Wheat is the staple crop for an estimated 35% of the world population [3]. A critical approach to meeting the increased demand is better management of fungal diseases, which can be responsible for 15%–20% yield losses per annum, such as rusts, blotches and Fusarium head blight (FHB) [4]. For example, the annual average occurrence of FHB, which is mainly caused by Fusarium graminearum in China, affects more than 4.5 million hectares, approximately 20% of the total planted area of wheat, and has caused serious yield losses [5]. Furthermore, FHB pathogens contaminate grains with various mycotoxins, especially deoxynivalenol (DON), which poses a health threat to humans and livestock. Currently, chemical fungicides are still the most effective approach to control FHB. However, fungicide resistant F. graminearum isolates have been detected in fields after long-term intensive use of fungicides [6, 7]. Moreover, treatments with some fungicides at sub-lethal concentrations stimulate mycotoxin production [8,9,10]. There is an urgent need to develop and apply new approaches to control FHB and mycotoxin contamination.
Biological control by living microorganisms is considered to be a suitable, alternative strategy for the control of plant diseases. Several antagonistic microorganisms were identified as biocontrol agents (BCAs) to combat plant diseases and achieved comparatively good efficiency [11]. Moreover, more than 30 species of microbes have been reported to specifically inhibit the mycelial growth of F. graminearum, or/and provided successful control efficiencies against FHB and mycotoxin reduction under greenhouse and/or field conditions [12,4: Fig. S3a). The ORF2643 gene encodes a putative LysR-type DNA-binding transcriptional repressor, LrhA (Additional file 4: Fig. S3b). Its homologues were previously reported to repress biosynthesis of secondary metabolisms in Photorhabdus and Xenorhabdu [43,44,45]. The two identified genes were named purR and lrhA for further investigation.
To confirm the roles of PurR and LrhA on HA production, we constructed single deletion mutants, double deletion mutants, and corresponding complementation strains of the two genes. The mRNA expression levels of genes in the biosynthetic gene cluster AcbA-AcbJ [21]. Lipopeptides constitue a specific class of microbial secondary metabolites and harbor diverse biological functions, especially antimicrobial and anticancer activities [19, 20, 57,58,59,60,61,62]. Lipopeptides can be employed for pharmaceutical, food-related, agricultural, and environmental protection applications [63, 64]. In the present study, we demonstrated that increased HA yields in the ZJU23 deletion mutant result in control effects of fungal pathogens that are comparable to synthetic fungicides. This highlights its applicability for crop protection. HA belongs to the cyclic lipopeptides and its structure consists of a peptide ring of eight amino acids where a fatty acid chain, a dehydrobutyric acid, and a sugar moiety are attached [Quantification of HA via HPLC analysis Quantitative analysis of HA was performed as previously described [13]. Briefly, 1 mL of the fermentation supernatant was collected and freeze-dried. The precipitate formed was resuspended with 1 mL methanol for HA extraction and the crude samples were subjected to HPLC analysis (Agilent Technologies 1100 Infinity) under the following conditions: C18 reversed-phase column [Agilent ZORBAX RX-C18 column (250 × 4.6 mm)] eluted with methanol/H2O (A/B) (1 mL/min, 30–90% A in 30 min, followed by 90–100% A in 10 min). The peak areas were used to quantify the production of HA according to the standard sample. To quantify HA more precisely, the extracted samples were analyzed by liquid chromatography-mass spectrometry (LC–MS). A transposon mutagenesis library and gene deletion mutants of ZJU23 were constructed as described previously [13]. Briefly, transposon mutants were generated by conjugation of recipient ZJU23 resistant to rifampicin and donor E. coli SM10λ-pir. Each transposon was screened in an inhibition zone assay against F. graminearum. The selected transposons were re-sequenced by Bei**g Novogene Bioinformatics Technology Co., Ltd. to localize the insertion by comparing it with the wild type strain ZJU23. Gene deletion mutants were generated by using the λ-red recombinase method. The transformants were confirmed by PCR. Double mutant strains were generated using single mutants as a background strain as indicated. Complementation constructructions were generated as described previously [68]. The primers designed in this study for mutant strains and complementation constructions are listed in the Additional file 1: Table S4. The primer pair M13-F and M13-R were used for identification of pBBR-LrhA and pBBR-PurR plasmid construction [69]. For real-time quantitative PCR (RT-qPCR), all strains were cultured with HI medium at 25 °C and 180 rpm for 18 h. Total RNA purification was performed by using RNAprep Pure Cell/ Bacteria Kit (TIANGEN, DP430) and reverse transcription was done using HiScript II Q RT SuperMix for qPCR (+ gDNA wiper) (Vazyme, R223-01) according to the manufacturer's instructions. RT-qPCR was performed via qRT-PCR using ChamQ SYBR qPCR Master Mix (Vazyme, Q311-02). The experiments were performed in independent biological triplicates and 16S rRNA of ZJU23 was used as the internal control. The mRNA fold change was estimated by the threshold cycle (Ct) values of 2−(ΔΔCt). The primers used for qRT-PCR assays are listed in Additional file 1: Table S4. Biocontrol experiments with a fermentation suspension produced by ZJU23 in KB and ΔLrhA in HAI against gray mold caused by B. cinerea and Fusarium crown rot caused by F. pseudograminearum were conducted. The gray mold assay was performed as previously described [70]. The corresponding fermentation broth, pyrimethanil (50 mg/L) or water were sprayed on the tomato or apple surface. After 1 h air-drying, fresh mycelial plugs (6 mm in diameter) were inoculated. Pyrimethanil (50 mg/L) and clean water were used as fungicide treatment and negative control. Disease lesions were observed and measured 72 h after inoculation with the pathogenic fungus. The Fusarium crown rot assay was performed as described previously but with specific modifications [71]. F. pseudograminearum F303 conidia (1 × 106 CFU/mL) were mixed with soil at 1:10 (W/W) for three days. Then wheat seeds (cultivar: Jimai 22) were planted into conidia-inoculated soil. Non-inoculated soil was used as a control. At the seventh day, 50 mL of the fermentation suspension were sprinkled on the wheat root. Tebuconazole (150 mg/L) and clean water were used as fungicide treatment and negative control. After 21 days, the roots were washed and the browning areas of the wheat stem base were observed. The disease index (DI) was calculated as previously described [72] but with modifications. Five evaluation classes ranging from 0 to 4, were applied for the disease index. The disease index corresponds to the percentage of the browning length of the first stem node (0 = 0, 1 = 1–25%, 2 = 26–50%, 3 = 51–75%, and 4 ≥ 75%). The disease index was calculated as follows in Eq. (2): The control efficacy was calculated as follows in Eq. (3): All experiments in this study were repeated three times. Data presented are the mean ± standard errors. Differences between two groups were analyzed by Student’s t-test. Multiple comparisons were analyzed by one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) multiple-range test.Construction of transposon mutants and gene deletion mutants
RNA preparation and quantitative reverse transcription PCR (qRT-PCR)
Evaluation of biocontrol efficacy of the fermentation suspension
Statistical analysis
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and the additional files].
Abbreviations
- PBD:
-
Plackett–Burman design
- BBD:
-
Box-Behnken design
- RSM:
-
Response surface methodology
- HA:
-
Herbicolin A
References
Ristaino JB, Anderson PK, Bebber DP, Brauman KA, Cunniffe NJ, Fedoroff NV, Finegold C, Garrett KA, Gilligan CA, Jones CM, et al. The persistent threat of emerging plant disease pandemics to global food security. P Natl Acad of Sci USA. 2021;118: e2022239118.
Savary S, Willocquet L, Pethybridge SJ, Esker P, McRoberts N, Nelson A. The global burden of pathogens and pests on major food crops. Nat Ecol Evol. 2019;3:430–9.
Grote U, Fasse A, Nguyen TT, Erenstein O. Food security and the dynamics of wheat and maize value chains in Africa and Asia. Front Sustain Food S. 2021;4: 617009.
Figueroa M, Hammond-Kosack KE, Solomon PS. A review of wheat diseases-a field perspective. Mol Plant Pathol. 2018;19:1523–36.
Chen Y, Kistler HC, Ma Z. Fusarium graminearum trichothecene mycotoxins: biosynthesis, regulation, and management. Annu Rev Phytopathol. 2019;57:15–39.
Liu Y, Chen X, Jiang J, Hamada MS, Yin Y, Ma Z. Detection and dynamics of different carbendazim-resistance conferring β-tubulin variants of Gibberella zeae collected from infected wheat heads and rice stubble in China. Pest Manag Sci. 2014;70:1228–36.
de Chaves MA, Reginatto P, da Costa BS, de Paschoal RI, Teixeira ML, Fuentefria AM. Fungicide Resistance in Fusarium graminearum Species Complex. Curr Microbiol. 2022;79:62.
Duan YB, Lu F, Zhou ZH, Zhao HH, Zhang J, Mao YS, Li MX, Wang JX, Zhou MG. Quinone outside inhibitors affect DON biosynthesis, mitochondrial structure and toxisome formation in Fusarium graminearum. J Hazard Mater. 2020;398:122908.
Tang GF, Chen Y, Xu JR, Kistler HC, Ma ZH. The fungal myosin I is essential for Fusarium toxisome formation. Plos Pathog. 2018;14:e1006827.
Audenaert K, Callewaert E, Hofte M, De Saeger S, Haesaert G. Hydrogen peroxide induced by the fungicide prothioconazole triggers deoxynivalenol (DON) production by Fusarium graminearum. BMC Microbiol. 2010;10:112.
Zhou Y, Wang H, Xu S, Liu K, Qi H, Wang M, Chen X, Berg G, Ma Z, Cernava T, Chen Y. Bacterial-fungal interactions under agricultural settings: from physical to chemical interactions. Stress Biology. 2022;2:22.
Legrand F, Picot A, Cobo-Diaz JF, Chen W, Le Floch G. Challenges facing the biological control strategies for the management of Fusarium Head Blight of cereals caused by F. graminearum. Biol Control. 2017;113:26–38.
Xu S, Liu Y-X, Cernava T, Wang H, Zhou Y, **a T, Cao S, Berg G, Shen X-X, Wen Z, et al. Fusarium fruiting body microbiome member Pantoea agglomerans inhibits fungal pathogenesis by targeting lipid rafts. Nat Microbiol. 2022;7:831–43.
Wang J, Xu CY, Sun QM, Xu JR, Chai YR, Berg G, Cernava T, Ma ZH, Chen Y. Post-translational regulation of autophagy is involved in intra-microbiome suppression of fungal pathogens. Microbiome. 2021;9:131.
Chen Y, Wang J, Yang N, Wen ZY, Sun XP, Chai YR, Ma ZH. Wheat microbiome bacteria can reduce virulence of a plant pathogenic fungus by altering histone acetylation. Nat Commun. 2018;9:3429.
Jiao XR, Takishita Y, Zhou GS, Smith DL. Plant associated rhizobacteria for biocontrol and plant growth enhancement. Front Plant Sci. 2021;12: 634796.
Gu Q, Yang Y, Yuan Q, Shi G, Wu L, Lou Z, Huo R, Wu H, Borriss R, Gao X, Elliot MA. Bacillomycin D produced by Bacillus amyloliquefaciens Is Involved in the antagonistic interaction with the plant-pathogenic fungus Fusarium graminearum. Appl Environ Microb. 2017;83:e01075-e11017.
Hu WQ, Gao QX, Hamada MS, Dawood DH, Zheng JW, Chen Y, Ma ZH. Potential of Pseudomonas chlororaphis subsp aurantiaca Strain Pcho10 as a biocontrol agent against Fusarium graminearum. Phytopathol. 2014;104:1289–97.
Meena KR, Kanwar SS. Lipopeptides as the antifungal and antibacterial agents: applications in food safety and therapeutics. Biomed Res Int. 2015;2015: 473050.
Carolina CF, Kumar PS, Ngueagni PT. A review on new aspects of lipopeptide biosurfactant: types, production, properties and its application in the bioremediation process. J Hazard Mater. 2021;407: 124827.
Cesare GBD, Cristy SA, Garsin DA, Lorenz MC, Alspaugh JA. Antimicrobial peptides: a new frontier in antifungal therapy. MBio. 2020;11:e02123-e12120.
Dang YL, Zhao FJ, Liu XS, Fan X, Huang R, Gao WX, Wang SF, Yang C. Enhanced production of antifungal lipopeptide iturin A by Bacillus amyloliquefaciens LL3 through metabolic engineering and culture conditions optimization. Microb Cell Fact. 2019;18:68.
Maget-Dana R, Peypoux F. Iturins, a special class of pore-forming lipopeptides: biological and physicochemical properties. Toxicology. 1994;87:151–74.
Tao Y, Bie X-M, Lv F-X, Zhao H-Z, Lu Z-X. Antifungal activity and mechanism of fengycin in the presence and absence of commercial surfactin against Rhizopus stolonifer. J Microbiol. 2011;49:146–50.
Ongena M, Jacques P, Toure Y, Destain J, Jabrane A, Thonart P. Involvement of fengycin-type lipopeptides in the multifaceted biocontrol potential of Bacillus subtilis. Appl Microbiol Biot. 2005;69:29–38.
Singh V, Haque S, Niwas R, Srivastava A, Pasupuleti M, Tripathi CKM. Strategies for fermentation medium optimization: an in-depth review. Front Microbiol. 2017;7:02087.
Mnif I, Bouallegue A, Mekki S, Ghribi D. Valorization of date juice by the production of lipopeptide biosurfactants by a Bacillus mojavensis BI2 strain: bioprocess optimization by response surface methodology and study of surface activities. Bioproc Biosyst Eng. 2021;44:2315–30.
Khan MM, Kim YK, Cho SS, ** Y-Y, Suh J-W, Lee DY, Yoo JC. Response surface optimization of culture conditions for cyclic lipopeptide MS07 from Bacillus siamensis reveals diverse insights targeting antimicrobial and antibiofilm activity. Processes. 2020;8:744.
Plackett RL, Burman JP. The design of optimum multifactorial experiments. Biometrika. 1946;33:305–25.
Box GEP, Behnken DW. Some new three level designs for the study of quantitative variables. Technometrics. 1960;2:455–75.
Myers RH, Montgomery DC. Response surface methodology: process and product optimization using designed experiments. New York: Wiley; 1995.
Abbasi MN, Fu J, Bian X, Wang H, Zhang Y, Li A. Recombineering for genetic engineering of natural product biosynthetic pathways. Trends Biotechnol. 2020;38:715–28.
Lee SY, Kim HU, Park JH, Park JM, Kim TY. Metabolic engineering of microorganisms: general strategies and drug production. Drug Discov Today. 2009;14:78–88.
Muneeswari R, Iyappan S, Swathi KV, Vinu R, Ramani K, Sekaran G. Biocatalytic lipoprotein bioamphiphile induced treatment of recalcitrant hydrocarbons in petroleum refinery oil sludge through transposon technology. J Hazard Mater. 2022;431: 128520.
Nguyen KN, Kim Y, Maibunkaew S, Park J, Nguyen MT, Oh D-B, Kwon O. Enhanced production of 1-deoxynojirimycin in Bacillus subtilis subsp. inaquosorum by random mutagenesis and culture optimization. Biotechnol Bioproc E. 2021;26:265–76.
Luo S, Chen X-A, Mao X-M, Li Y-Q. Transposon-based identification of a negative regulator for the antibiotic hyper-production in Streptomyces. Appl Microbiol Biot. 2018;102:6581–92.
Greiner M, Winkelmann G. Fermentation and isolation of herbicolin A, a peptide antibiotic produced by Erwinia Herbicola Strain A 111. Appl Microbiol Biot. 1991;34:565–9.
King EO, Ward MK, Raney DE. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Cli Med. 1954;44:301–7.
Liu ES, Li MX, Abdella A, Wilkins MR. Development of a cost-effective medium for submerged production of fungal aryl alcohol oxidase using a genetically modified Aspergillus nidulans strain. Bioresour Technol. 2020;305: 123038.
Liu X, Wang J, Duan L, Song Y, Hu X, Wei J. Enhancing the production of butyric acid from sludge fermentation with an emphasis on zinc, cobalt, cuprum, ferrum and manganese. Environ Earth Sci. 2015;73:5057–66.
Zhu WY, Niu K, Liu P, Fan YH, Liu ZQ, Zheng YG. Enhanced O-succinyl-L-homoserine production by recombinant Escherichia coli ΔIJBB*TrcmetL/pTrc-metAfbr-Trc-thrAfbr-yjeH via multilevel fermentation optimization. J Appl Microbiol. 2020;130:1960–71.
Aydin M, Lucht N, König WA, Lupp R, Jung G, Winkelmann G. Structure elucidation of the peptide antibiotics herbicolin A and B. Liebigs Annalen Chemie. 1985;1985:2285–300.
Tobias NJ, Heinrich AK, Eresmann H, Wright PR, Neubacher N, Backofen R, Bode HB. Photorhabdus-nematode symbiosis is dependent on hfq-mediated regulation of secondary metabolites. Environ Microbiol. 2017;19:119–29.
Neubacher N, Tobias NJ, Huber M, Cai XF, Glatter T, Pidot SJ, Stinear TP, Lutticke AL, Papenfort K, Bode HB. Symbiosis, virulence and natural-product biosynthesis in entomopathogenic bacteria are regulated by a small RNA. Nat Microbiol. 2020;5:1481–9.
Shanks R, Stella NA, Lahr RM, Aston MA, Liu X. Suppressor analysis of eepR mutant defects reveals coordinate regulation of secondary metabolites and serralysin biosynthesis by EepR and HexS. Microbiology. 2017;163:280–8.
Winkelmann G, Lupp R, Jung G. Herbicolins-New peptide antibiotics from Erwinia herbicola. J Antibiot. 1980;33:353–8.
Kona RP, Qureshi N, Pai JS. Production of glucose oxidase using Aspergillus niger and corn steep liquor. Bioresource Technol. 2001;78:123–6.
Liggett RW, Koffler H. Corn steep liquor in microbiology. Bacteriol Rev. 1948;12:297–311.
Strieker M, Marahiel MA. The Structural Diversity of Acidic Lipopeptide Antibiotics. ChemBioChem. 2009;10:607–16.
Kiran GS, Thomas TA, Selvin J, Sabarathnam B, Lipton AP. Optimization and characterization of a new lipopeptide biosurfactant produced by marine Brevibacterium aureum MSA13 in solid state culture. Bioresour Technol. 2010;101:2389–96.
Dos Santos LFM, Coutte F, Ravallec R, Dhulster P, Tournier-Couturier L, Jacques P. An improvement of surfactin production by B. subtilis BBG131 using design of experiments in microbioreactors and continuous process in bubbleless membrane bioreactor. Bioresour Technol. 2016;218:944–52.
Pickens LB, Tang Y, Chooi YH. Metabolic engineering for the production of natural products. Annu Rev Chem Biomol Eng. 2011;2:211–36.
Baltz RH, Miao V, Wrigley SK. Natural products to drugs: daptomycin and related lipopeptide antibiotics. Nat Prod Rep. 2005;22:717–41.
Mao D, Bushin LB, Moon K, Wu Y, Seyedsayamdost MR. Discovery of scmR as a global regulator of secondary metabolism and virulence in Burkholderia thailandensis E264. P Natl Acad of Sci USA. 2017;114:E2920-8.
Déziel E, Gopalan S, Tampakaki AP, Lépine F, Padfield KE, Saucier M, **ao G, Rahme LG. The contribution of MvfR to Pseudomonas aeruginosa pathogenesis and quorum sensing circuitry regulation: multiple quorum sensing-regulated genes are modulated without affecting lasRI, rhlRI or the production of N-acyl-L-homoserine lactones. Mol Microbiol. 2005;55:998–1014.
Mukherjee A, Cui Y, Ma W, Liu Y, Chatterjee AK. hexA of Erwinia carotovora ssp carotovora strain Ecc71 negatively regulates production of RpoS and rsmB RNA, a global regulator of extracellular proteins, plant virulence and the quorum-sensing signal, N-(3-oxohexanoyl)-L-homoserine lactone. Environ Microbiol. 2000;2:203–15.
Zhao P, Xue Y, Gao W, Li J, Zu X, Fu D, Feng S, Bai X, Zuo Y, Li P. Actinobacteria-Derived peptide antibiotics since 2000. Peptides. 2018;103:48–59.
Zhao P, Xue Y, Li X, Li J, Zhao Z, Quan C, Gao W, Zu X, Bai X, Feng S. Fungi-derived lipopeptide antibiotics developed since 2000. Peptides. 2019;113:52–65.
Zhao P, Xue Y, Gao W, Li J, Zu X, Fu D, Bai X, Zuo Y, Hu Z, Zhang F. Bacillaceae-derived peptide antibiotics since 2000. Peptides. 2018;101:10–6.
Heydari H, Golmohammadi R, Mirnejad R, Tebyanian H, Fasihi-Ramandi M, Moosazadeh MM. Antiviral peptides against coronaviridae family: a review. Peptides. 2021;139: 170526.
Xue Y, Zhao P, Quan C, Zhao Z, Gao W, Li J, Zu X, Fu D, Feng S, Bai X, et al. Cyanobacteria-derived peptide antibiotics discovered since 2000. Peptides. 2018;107:17–24.
Lee Y, Phat C, Hong S-C. Structural diversity of marine cyclic peptides and their molecular mechanisms for anticancer, antibacterial, antifungal, and other clinical applications. Peptides. 2017;95:94–105.
Biniarz P, Łukaszewicz M, Janek T. Screening concepts, characterization and structural analysis of microbial-derived bioactive lipopeptides: a review. Crit Rev Biotechnol. 2017;37:393–410.
Coutte F, Lecouturier D, Dimitrov K, Guez J-S, Delvigne F, Dhulster P, Jacques P. Microbial lipopeptide production and purification bioprocesses, current progress and future challenges. Biotechnol J. 2017;12:1600566.
Sambrook J, Edward FF, Tom M. Molecular cloning: a laboratory manual. No. Ed. 2. Cold spring harbor laboratory press;1989.
Washington JA. Evaluation of two commercially available media for detection of bacteremia. Appl Microbiol. 1972;23:956–9.
Hattori T. Plate count of bacteria in soil on a diluted nutrient broth as a culture medium. Rep Inst Agric Res. 1976;27:23–30.
Chen Y, **a J, Su ZH, Xu GG, Gomelsky M, Qian GL, Liu FQ. Lysobacter PilR, the regulator of type IV pilus synthesis, controls antifungal antibiotic production via a cyclic di-GMP pathway. Appl Environ Microb. 2017;83:e03397-e13316.
Berdoulay M, Salvado J. Genetic characterization of microbial communities living at the surface of building stones. Lett appl microbiol. 2009;49:311–6.
Yin Y, Wu S, Chui C, Ma T, Jiang H, Hahn M, Ma Z. The MAPK kinase BcMkk1 suppresses oxalic acid biosynthesis via impeding phosphorylation of BcRim15 by BcSch9 in Botrytis cinerea. Plos Pathog. 2018;14: e1007285.
Zhang J, Zhu W, Goodwin PH, Lin Q, **a M, Xu W, Sun R, Liang J, Wu C, Li H, et al. Response of Fusarium pseudograminearum to Biocontrol Agent Bacillus velezensis YB-185 by phenotypic and transcriptome analysis. J Fungi. 2022;8:763.
Smiley RW, Gourlie JA, Easley SA, Patterson LM. Pathogenicity of fungi associated with the wheat crown rot complex in Oregon and Washington. Plant Dis. 2005;89:949–57.
Acknowledgements
Not applicable.
Funding
This research was supported by National Key Research and Development Program of China (2022YFD1400100), the National Natural Science Foundation (31922074), the Natural Science Foundation of Zhejiang Province (Z23C140008), China Agriculture Research System (CARS-3-1-15) and the Fundamental Research Funds for the Central Universities (2021FZZX001-31).
Author information
Authors and Affiliations
Contributions
YC initiated, coordinated and supervised the project. HW, YZ, SX, BZ performed the experiments. H.W and YC collected and analyzed the data. HW and YC wrote the manuscript. YC, ZM and TC revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
The component of different media used for basic medium screening. Table S2. List of levels and factors in the Plackett–Burman design experiments. Table S3. Strains and plasmids used in this study. Table S4. PCR primers used in this study.
Additional file 2: Fig. S1.
Selection of a base medium for HA production.
Additional file 3: Fig. S2.
Effects of different components in the medium and various fermentation parameters on the HA production.
Additional file 4: Fig. S3.
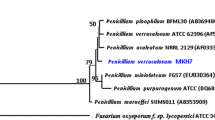
Phylogenetic analysis based on the amino acid sequences of proteins encoded by ORF22 a and ORF2643 b in P. agglomerans ZJU23.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, H., Zhou, Y., Xu, S. et al. Enhancement of herbicolin A production by integrated fermentation optimization and strain engineering in Pantoea agglomerans ZJU23. Microb Cell Fact 22, 50 (2023). https://doi.org/10.1186/s12934-023-02051-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12934-023-02051-z