Abstract
Background
L-cysteine is an essential chemical building block in the pharmaceutical-, cosmetic-, food and agricultural sector. Conventionally, L-cysteine production relies on the conversion of keratinous biomass mediated by hydrochloric acid. Today, fermentative production based on recombinant E. coli, where L-cysteine production is streamlined and facilitated by synthetic plasmid constructs, is an alternative process at industrial scale. However, metabolic stress and the resulting production escape mechanisms in evolving populations are severely limiting factors during industrial biomanufacturing. We emulate high generation numbers typically reached in industrial fermentation processes with Escherichia coli harbouring L-cysteine production plasmid constructs. So far no genotypic and phenotypic alterations in early and late L-cysteine producing E. coli populations have been studied.
Results
In a comparative experimental design, the E. coli K12 production strain W3110 and the reduced genome strain MDS42, almost free of insertion sequences, were used as hosts. Data indicates that W3110 populations acquire growth fitness at the expense of L-cysteine productivity within 60 generations, while production in MDS42 populations remains stable. For the first time, the negative impact of predominantly insertion sequence family 3 and 5 transposases on L-cysteine production is reported, by combining differential transcriptome analysis with NGS based deep plasmid sequencing. Furthermore, metabolic clustering of differentially expressed genes supports the hypothesis, that metabolic stress induces rapid propagation of plasmid rearrangements, leading to reduced L-cysteine yields in evolving populations over industrial fermentation time scales.
Conclusion
The results of this study implicate how selective deletion of insertion sequence families could be a new route for improving industrial L-cysteine or even general amino acid production using recombinant E. coli hosts. Instead of using minimal genome strains, a selective deletion of certain IS families could offer the benefits of adaptive laboratory evolution (ALE) while maintaining enhanced L-cysteine production stability.
Similar content being viewed by others
Background
The amino acid L-cysteine, harbouring a thiol group, provides a high redox activity in cell metabolism, plays a crucial role in protein folding, functions as a catalytic residue of several enzymes and serves as a building block of 5-L-glutamyl-L-cysteinylglycine (GSH) and as a donor compound of sulphur, which is required for the synthesis of Fe/S clusters, biotin, coenzyme A and thiamine [1, 2].
Besides the essential function in metabolism, L-cysteine is also of considerable industrial importance, with applications ranging from pharmaceutical products and cosmetics over food production to feed additives in livestock farming [3, 4].
To date, the cheapest and thereby most prevalent means of L-cysteine production involves chemical hydrolysis of—and extraction from—keratinous biomass, such as feathers, pig bristles and animal hair by means of electrolysis [5]. Up to 27 tons of hydrochloric acid are required to obtain 100 kg of a racemic mixture of cysteine from 1.000 kg raw material [5, 6]. In order to circumvent negative impacts upon the environment associated with hydrochloric waste disposal, alternative technologies such as fermentation and enzymatic conversion have been explored and rapidly gained significance since their implementation. In 2004, 12% of the globally manufactured L-cysteine global originated from fermentation [7].
The enzymatic conversion of DL-2-amino-∆2-thiazoline-4-carboxylic acid (D-ATC) to L-cysteine with Pseudomonas spp. derived enzymes is limited by product inhibition [8, 9]. For biotechnological L-cysteine production, the bacteria C. glutamicum and E. coli harbouring optimised plasmids represent the dominant expression organisms. Since titres from C. glutamicum are low (approx. 950 mg/L), E. coli is the preferred host for L-cysteine production by fermentation [10].
However, there are still major obstacles in upscaling fermentation processes with engineered microorganisms. The stability of strains with synthetic production is highly fragile and presents a challenge when implementing bioprocesses on a large scale [11, 12]. The introduction of designed plasmid constructs and the upregulation of the genetic elements for recombinant L-cysteine production pose a defiance to the tightly regulated homeostasis within host cells [13, 14]. This metabolic load hinders the expression of other genes, thereby negatively affecting growth rate and promoting evolutionary pressure [15,16,17]. Furthermore, L-cysteine has an inhibitory to toxic effect on E. coli cell growth, depending on the concentration present in cells [18]. In microorganisms, several concepts are reported that can lead to a selection advantage and thus to both phenotypic and genotypic variation within populations [19, Full size image
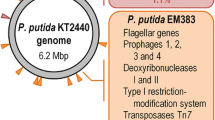
The distribution of the different IS families was very similar for all three production plasmids. This observation was the same for EGPs compared to LGPs (Fig. 6B). Nevertheless, the total number of reads that could be mapped to IS doubled on average when comparing W3110 EGPs with LGPs, while the number remained the same for MDS42 populations. This indicates, that transpositions of different IS families propagated in similar frequencies. For plasmids extracted from W3110 populations, we most frequently identified reads that could be mapped to the IS3 and IS5 family, which supports the result of a high expression of insJK, an IS3 family transposase, in LGPs of W3110_pCYS. In addition, reads mapped to the ISAs1 family were very abundant. Among plasmids from MDS42 populations, the reads could be mapped mainly to sequences belonging to IS200- and IS110 families. Due to deletion of most IS, the few transposition events in MDS42 populations are in line with the stable L-cysteine production and growth rates, rendering E. coli MDS42 as a stable host for industrial L-cysteine fermentations.