Abstract
Background
Senescence is accompanied by a progressive decrease in male reproductive performance, mainly due to oxidative stress and endothelial dysfunction. Alpha lipoic acid (ALA) is a potent antioxidant, that diffuses freely in aqueous and lipid phases, possessing anti-inflammatory and anti-apoptotic properties. This study aimed to examine the effects of supplemental dietary ALA on testicular hemodynamics (TH), circulating hormones, and semen quality in aged goats. Twelve Baladi bucks were divided into two groups (n = 6 each); the first fed a basic ration and served as a control group (CON), while the second received the basic ration supplemented with 600 mg ALA/ kg daily for consecutive eight weeks (ALA).
Results
There were improvements in testicular blood flow in the ALA group evidenced by a lower resistance index (RI) and pulsatility index (PI) concurrent with higher pampiniform-colored areas/pixel (W3-W6). There were increases in testicular volume and decreases in echogenicity (W3-W5; ALA vs. CON). Compared to the CON, ALA-bucks had higher serum concentrations of testosterone, estradiol, and nitric oxide (W3-W5). There were enhancements in semen traits (progressive motility, viability, morphology, and concentration, alanine aminotransferase enzyme) and oxidative biomarkers (catalase, total antioxidant capacity, and malondialdehyde).
Conclusions
ALA dietary supplementation (600 mg/kg diet) improved aged bucks’ reproductive performance by enhancing the testicular volume, testicular hemodynamics, sex steroids, and semen quality.
Similar content being viewed by others
Introduction
Males with superior genetic traits are demanded to be in action to the end of their lives, improving animal production outcomes. However, senescence progressively deteriorates organ functions and homeostasis [1]. Aged males suffer a dramatic decrease in reproductive hormones, semen quality, sperm cell concentration, and prolificacy. Many factors integrate into aging pathophysiology including pituitary-testicular axis alterations, hormonal imbalance, vascular impairment, and lipid peroxidation [2,3,4].
Oxidative stress (OS) is primarily blamed for senescence-associated reproductive dysfunction in male animals [3]. However, the male testicular tissue is equipped with an antioxidant army (enzymatic + non-enzymatic) [5], and advancement in age ruins its synthesis, regeneration, and power, jeopardizing the whole reproductive performance [6,7,8]. According to several studies, OS has detrimental impacts on animal reproductive performance and functions, including decreased sperm quality and fertility potential [6]. Spermatozoa have low antioxidant protection and large levels of polyunsaturated fatty acids (PUFAs), making them vulnerable to reactive oxygen species (ROS) damage. Alpha-lipoic acid (ALA) is a coenzyme used in mitochondrial metabolism. The reduced form of ALA known as dihydro-lipoic acid (DHLA) is a powerful antioxidant for the mitochondria [9]. ALA and DHLA function as powerful redox couple that can scavenge a range of ROS, including hydroxyl radicals and hypochlorous acid. Numerous reports have demonstrated the effects of dietary ALA supplementation on the antioxidant capabilities of mice and broilers, which also help to improve the quality of meat [10, 11]. Due to its potent antioxidant effects, ALA influences cardiovascular and cognitive health, anti-aging, detoxification, anti-inflammation, anti-cancer, and neuroprotection [11]. ALA decreases oxidative stress, improves neurocognitive performance in rats and dogs, and acts as an anti-inflammatory in mice [12,13,14].
Since ALA has diversiform actions in the perspective of endothelial functions and OS, as well as energy production, it was hypothesized that dietary ALA could mitigate aging-induced OS and enhance testicular functions. The hypothesis was tested by monitoring the testicular vascularization, echogenic, and biometric changes, reproductive hormones, and semen quality following ALA dietary supplementation in aged bucks.
Materials and methods
Ethical committee approval statement
The study was approved by the institutional animal use committee (Vet CU 2009-2022-492) at the Faculty of Veterinary Medicine, Cairo University.
Animals and housing
Twelve aged Baladi bucks with an average body condition score of 3 with body weight (55 ± 1.5 kg) and age (6.4 ± 0.4 years) were conducted in this experiment. Bucks were routinely scanned by B-mode and colored Doppler ultrasound to exclude any bucks suffering any andrological and cardiovascular diseases. They were housed in a paddock enjoying daylight with a 25 m2 shaded sector. Animals were provided a daily balanced diet depending on NRC requirements as the normal diet consisted of 500 g/animal every day in the form of pelleted concentrates that contain crude protein (15–19%) with 1.34 kg /animal every day in the form of roughage with fresh water available all day.
Experimental design, location, and time points
The present study was performed at the small ruminant research farm belonging to the Department of Theriogenology, Faculty of Veterinary Medicine, Cairo University. Bucks were divided into two main groups, the group I (n = 6, control bucks with age and weight of 6.4 ± 0.4 years and 55 ± 1.6 kg respectively) received the basic diet only; group II (n = 6, ALA bucks with age and weight of 6.2 ± 0.4 years and 50 ± 1.5 kg, respectively) received the basic diet supplemented with ALA (600 mg/kg diet; Thiotacid compound 600, Eva Pharma co., Egypt) for eight wks (W1-W8; goat spermatogenesis) [15, 16]. Bucks were examined (ultrasound, semen, and biochemical analyses) on the first day of supplementation (W 0) and once/week (W1-W8).
Testicular ultrasonography
Biometry and Echogenicity
B-mode linear probe (7.5 MHz; EXAGO, Echo Control Medical, France) was used to measure testicular volume and echogenicity (TE). Testicular volume was estimated following the equation for ellipsoid = 4/3π abc, where (a) for testicular length/2, (b) for width/2, and (c) for height/2 [17], while both TE [pixel heterogeneity (PH) and testicular echotexture (TE)] were assessed using the Adobe Photoshop CC programme as previously published [18, 19].
Testicular vascular perfusion (TVP)
TVP was measured using color-Doppler ultrasound (7.5 MHz; EXAGO, Echo Control Medical, France). Bucks were controlled without analgesics and the scrotal hair (including the spermatic cord area) was clipped and shaven. The STA was visualized (B-mode; at the proximal end of the testis) meshed with the pampiniform plexus followed by activating the color mode showing the blue area (testicular direction) and the red area (probe direction) [20]. Pulsed-wave Doppler mode was then activated for recording the arterial cardiac cycle as previously described [21]. The device settings were as follows: window gate (0.5 mm), color maps (2), brightness (56%), pulse repetition frequency (3000), and insonation angle (< 60°). The examined Doppler parameters were resistance (RI) and pulsatility indices (PI).
Hormonal and nitric oxide assay
Just before the ultrasound examination (9:00 AM), blood samples were collected (jugular vein) into vacutainer tubes (4 ml), centrifuged (15 min at 3000 rpm), harvested, and preserved at − 18 °C for hormonal analysis in the sera. Follicle-stimulating hormone (FSH; ng/mL), luteinizing hormone (LH; ng/mL), testosterone (T; ng/mL), and estradiol (E2; pg/mL) were measured with an intra- and inter-assay variation coefficient of 10 and 12%, respectively using commercial ELISA kits (Sun Long Biotech Co., LTD CHINA). NO metabolites (nitrate and nitrites) were measured photometrically (spectrophotometer; 540 nm) by commercial kits (Bio-diagnostics co., Tahrir st., Giza, Egypt) for representing the serum NO concentrations with an inter-assay variation coefficient of 6.9% and a sensitivity of 0.225 µmol/l [22, 23].
Semen picture assessment
Semen was extracted from clean, sterile, prewarmed (37 oC) falcon tubes using an electro-ejaculator and kept at that temperature for further examination. Each sample was divided into two portions, one for the evaluation of sperm quality and the other for the biochemical study of seminal plasma (SP). SP was extracted using a sterile pipette, centrifuged at 2000 g for 15 min at 4 oC, and then kept at -20 oC for subsequent analysis [24].
Sperm traits
Sperm progressive movement (SPM, %) was examined by placing a 10 µl diluted (1:30 v/v, sodium citrate dihydrate 2.9%) semen drop on a prewarmed (37 oC) clean, dry, and sterile glass slide, cover-slipped, and visualized utilizing phase-contrast microscopy (heated stage: 37 oC; magnification: 400 x). At different representable microscopic fields, the percent of forward and rectilinear motile spermatozoa was recorded. Two slides/buck were examined and averaged for data verification.
Supra-vital eosin-nigrosin staining method was adopted for the evaluation of sperm viability (SV) and normal morphology (NS). In 100 ml double distilled water, 1.67 g eosin, 10 g nigrosin, and 2.9% sodium citrate dihydrate were dissolved (boiling water bath) and filtered (three times) [24]. A diluted semen sample was mixed with a triple amount of the stain, smeared, and air-dried. For SV %, a sum of 300 sperm/slide in different microscopic areas was examined for the stained (partially or completely; dead) and unstained (viable) spermatozoa and recorded in percent. For NS, the SV slide was examined for different sperm abnormalities including head (size and shape) and tail (protoplasmic droplets, coiled, tapered, bent) defects. The results are expressed as the percentage of normal spermatozoa. Sperm cell concentration (SCC, 109 sperm/ml) was examined using an improved Neubauer hemocytometer following a previous report [25].
SP oxidative biomarkers and ALT activity
To collect the seminal plasma, the semen samples were spun at a force of 2000 g at a temperature of 4 °C for 15 min. Afterward, the resultant supernatant was gathered was kept at a temperature of -20 °C until it was time for further analysis. Enzymatic activity of catalase (CAT) (U/L), Alanine aminotransferase (ALT) (U/ml), and levels of malondialdehyde (MDA) (mM/ml) and total antioxidant capacity (TAC) (mM/L) were analyzed utilizing a spectrophotometer (commercial colorimetric kits) with the guidance of the manufacturer’s instructions (Bio-diagnostics co., Tahrir st., Giza, Egypt), specifically at a wavelength of 520, 505, 534, and 510 nm, respectively.
Statistical analysis
Firstly, Shapiro-Wilk and Levene tests were used to determine data normal distribution and homogeneity, respectively. Since the ultrasound parameters didn’t differ between the right and left testis, the data of both testes were pooled and the means for each time point were shown. The differences between control and ALA males (treatment effect) in testicular vascularization values (PI, RI, and CA), circulating hormones (T, E2, FSH, and LH) and NOMs, semen traits (SPM, SV, NS, and SCC), and SP biomarkers (MDA, TAC, CAT, and ALT) during the examined time points (W0-W7; time effect) with the interaction between treatment and time were analyzed using repeated measure two- way ANOVA using the statistical package SPSS 20.
Results
B-mode ultrasonographic measurements
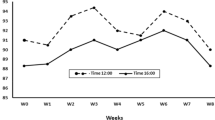
ALA dietary supplementation affected both testicular volume (cm3) as well as testicular echotexture, as testicular volume increased significantly (P < 0.05) from week 3 (53.24 ± 1.22) to week 6 (55.66 ± 1.85). In addition, the time and interaction between time with treatment had shown a significant (P < 0.05) difference in testicular volume in the ALA group compared to the control group (Fig. 1A). echogenic changes of the testicular parenchyma (TE and PH) were affected by treatment and time*treatment interaction (P < 0.05) by declination from week 3 (74.14 ± 1.02 for TE; 18.66 ± 0.33 for PH) till week 5 (72.31 ± 1.22 for TE; 18.01 ± 0.21 for PH) as shown in (Fig. 1B).
Testicular volume (A) and testicular echotexture (B) in the form of pixel heterogeneity (PH, SdNPVs) and testicular echogenicity (TE, NPVs). a, b values are significantly different at P < 0.05 compared with the control and α lipoic acid (ALA) males, while the * value is significantly different at P < 0.05 between the two groups at the same time point. T = treatment, and W = weeks
Testicular blood flow alterations
Values of Doppler indices (resistance and pulstatility [RI, PI]) and CA of the STA were affected by supplementation, weeks, and supplementation*weeks (P < 0.05; Table 1). Instantly, RI values decreased significantly (P < 0.05) in the ALA-supplemented males from W3 (0.71 ± 0.02) to W6 (0.61 ± 0.02) compared to the controls (0.91 ± 0.01 to 0.89 ± 0.01, respectively). In addition, PI means of the STA declined significantly (P < 0.05) from W3 (0.45 ± 0.02) until W6 (0.42 ± 0.02) compared to the control group (0.71 ± 0.02 to 0.71 ± 0.02). The colored areas (testicular direction) elevated significantly (P < 0.05) in the ALA-supplemented bucks from W3 (987.25 ± 23.65) until W6 (1165.02 ± 17.36) compared to the controls (595.21 ± 33.62 to 788.32 ± 11.87).
Hormonal levels and semen picture alterations
Serum concentrations of testosterone (T), estradiol (E2), and nitric oxide (NO) were affected by supplementation, weeks, and supplementation*weeks (P < 0.05; Fig. 2A, B, and C). In detail, serum levels of T and E2 elevated (P < 0.05) during W3 (4.02 ± 0.04 ng/mL for T and 22.54 ± 1.44 pg/mL for E2) till W5 (4.21 ± 0.04 ng/mL for T and 27.23 ± 1.34 pg/mL for E2). In addition, NO levels increased (P < 0.05) from W3 (56.23 ± 1.33 µmol/L) to W5 (61.21 ± 1.32 µmol/L). The ALA supplementation did not affect FSH and LH (Fig. 2D and E).
Serum levels of testosterone (T; ng/mL; A), estradiol (E2; pg/mL; B), nitric oxide (NOMs, µmol/L; C), follicle-stimulating hormone (FSH; ng/mL; D), and luteinizing hormones (LH; ng/mL; E) in male bucks administrated α-lipoic acid (ALA) compared to control group. Values are presented as means ± SEM. a, b values are significantly different at P < 0.05 compared with the control and ALA males, while the * value is significantly different at P < 0.05 between the two groups at the same time point. T = treatment, and W = weeks
The semen parameters (SPM, SV, NS, and SCC) in ALA-supplemented and control bucks is presented in Table 2. There were effects (P < 0.05) of supplementation, week, and supplementation*weeks interaction in SPM, SV, and SCC; while NS was only affected (P < 0.05) by supplementation and supplementation*week interaction. Precisely, ALA supplementation increased significantly (P < 0.05) the percentage of SPM, SV, and NS (W5-W8), while SSC elevated (P < 0.05; W6-W8).
SP oxidative biomarkers and ALT activity
Data of SP levels of CAT, TAC, ALT, and MDA in the ALA-supplemented and control bucks through the study time frame (W0-W8) are presented in Table 3. The supplementation*week effects showed significant (P < 0.05) differences in MDA, TAC, CAT, and ALT, while the time effect showed a significant (P < 0.05) difference in CAT and ALT activities. In detail, TAC activity increased significantly starting from week 4 and this pattern sustained till week 8, same pattern was recorded in catalase activity at week 5 and continue till end of expermint (week 8). MDA activity starts to decrease significantly beginning from week 6 and this pattern sustained till week 8, while ALT level start to decline starting from week 5 and continue till week 8.
Discussion
Males possessing superior genetic merits have pivotal impacts on animal breeding success even at senescence. Therefore, this work aimed to alleviate the adverse effects of senescence on testicular vascularization and subsequent steroidogenic and spermatogenic functions by dietary supplementation of ALA. These outcomes may help the animal breeding industry to improve the use of superior males till the last day of their lives. Present work reported a decrease in RI and PI values of the STA concurrent with an increase in pampiniform plexus CA in the ALA-supplemented (W3-W6) compared to the unsupplemented group. Lower RI and PI, and higher CA values indicate an improvement in the blood flow within the STA by decreasing the arterial resistance and ultimately higher blood perfusion [26,27,28]. Despite the actual mechanisms by which ALA enhanced the TBF were not examined here, different explanations could ease the vision. To begin with, ALA protects vascular and endothelial functions by influencing the NO-mediated vasodilation pathway and being an antithrombotic agent [29]. This evidence supports the higher levels of serum NOMs levels observed in the present study. Aging is mainly accompanied by vascular and endothelial irregularities due to oxidant/antioxidant imbalance. In addition, ALA enhances vascular homeostasis by altering endothelial responsiveness [30] and modulating angiogenic factors [31]. Furthermore, being a potent ROS scavenging molecule, ALA increases NO bioavailability through coupling with superoxide anions (main endothelial deteriorative radical) [32].
ALA-supplemented bucks showed higher TV measurements and lower echogenicity through W3 to W5 compared to the controls, indicating a testicular flourishment. Surprisingly, the changes in TV and TE were parallel to the improvement in testicular hemodynamics. Higher TBF is positively correlated with TV and negatively with TE [33]. Indeed, a study reported an increase in the TV of varicocelised rats by oral ALA supplementation through reversing the negative impacts of varicocele on testicular tissue [34]. In addition, supplemental dietary ALA improved testicular histomorphometric parameters evidenced by higher epithelial height and wider seminiferous tubules in senile breeder roosters [35].
ALA-supplemented bucks possessed higher serum concentrations of T and E2 (W3-W5) compared to controls. These results are corroborated by a recent study on aged breeder roosters [35] that reported a caspase 3 downregulation concurrent with upregulation of Nrf2 mRNA in the testicular tissue which affirms the anti-apoptotic properties of ALA [35]. ALA-antioxidant capacity is beyond special, as it not only compulsively scavenges free radicals but also can regenerate endogenous antioxidant defense (glutathione, vitamins A, C, and E) being a super-protector for the steroids’ producing cells (Leydig and Sertoli) [36]. Though the FSH and LH serum levels were not significantly altered by ALA supplementation, the T and E2 improvements should be interpreted cautiously as the local role of ALA in the testes couldn’t be negated [37, 38].
Assessment of semen traits unveils the actual andrological properties of males [39]. ALA-bucks semen quality witnessed improvements in sperm progressive motility, viability, morphology, and concentration as well as SP oxidative biomarkers (CAT, TAC, and MDA). These results are supported by many studies on men [40, 41], rodents [37], and Roosters [35]. Goat spermatozoa possess higher levels of PUFAs making them highly susceptible to membrane damage via lipid peroxides [42, 43]. ALA can make a protective shield covering the spermatozoa making them less injured by ROS [4]. ALA is a mitochondrial coenzyme integrating into ATP production that affects sperm motility and hyperactivity [44].
In the current investigation, ALA supplementation caused a significant reduction in MDA and elevation in TAC, similar findings were reported in infertile men received ALA supplementation [41]. ALA showed to reduce production of ROS and inhibit lipid peroxidation which in turn, provides a protective effect for goat sperms [45].
In the present study, catalase activity start to elevate from week 5, in one study investigate the effect of ALA supplementation on antioxidant enzymes on semen in cashmere goat, there was elevation of CAT level [46]. The higher enzymatic activity (reported herein) of CAT and TAC and lower MDA and ALT ascertain the protective role of ALA against oxidative stress and membrane lipid peroxidation. Many studies have reported improvements in the seminal antioxidant defense by ALA supplementation in different stress conditions and species either in vivo or in vitro [35, 37, 47]. Research on the oxidation of phospholipids within mammalian sperm indicates that this oxidative process results in damage to the membrane, ultimately causing a decrease in the ability of sperm to move effectively [48]. In one human study, the concentration of total antioxidant capacity in the seminal fluid of men with asthenozoospermia was found to be considerably less compared to that of healthy individuals. Furthermore, a direct association was observed between diminished TAC concentrations and decreased movement of sperm [49]. Measuring the activity of alanine transaminase (ALT) is deemed a valuable indicator of the integrity of the sperm plasma membrane. This is due to the fact that sperm cells with compromised membranes tend to discharge ALT into the seminal fluid [50]. The vascular supply is expressed by Lower RI and PI, and higher blood flow expressed by elevation of colored area with increase the voulme of the main artery [28, 51,52,53], as ALA is recomeended to improve the sexual activity, fertility potential, and testicular blood flow.
Conclusion
Alpha lipoic acid dietary supplementation ameliorated the adverse impacts of senescence of testicular blood flow, sex hormones (testosterone and estradiol), semen quality, and oxidative biomarkers. Newly proposed studies are demanded to investigate the effect of dietary ALA on advanced sperm evaluation techniques, sexual activity, and fertility potential.
Data availability
The raw data support outcomes of the present study is available by the corresponding author.
References
Childs BG, Durik M, Baker DJ, Van Deursen JM. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med. 2015;21:1424–35.
Hedia M, El-Shalofy A. Ageing affects plasma steroid concentrations and testicular volume, echotexture and haemodynamics in rams. Andrologia. 2022;54:e14309.
Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, et al. Oxidative stressin, aging, and diseases. Clin Interv Aging. 2018;13:757.
Behnamifar A, Rahimi S, Torshizi MAK, Sharafi M, Grimes J. Effects of dietary alpha-lipoic acid supplementation on the seminal parameters and fertility potential in aging broiler breeder roosters. Poult Sci. 2021;100:1221–38.
De Luca MN, Colone M, Gambioli R, Stringaro A, Unfer V. Oxidative stress and male fertility: role of antioxidants and inositols. Antioxidants. 2021;10:1283.
Pintus E, Ros-Santaella JL. Impact of oxidative stress on male reproduction in domestic and wild animals. Antioxidants. 2021;10:1154.
da Rosa Filho RR, Angrimani DS, Brito MM, Nichi M, Vannucchi CI, Lucio CF. Susceptibility of epididymal sperm against reactive oxygen species in dogs. Animal Biotechnol. 2021;32:92–9.
Guthrie H, Welch G. Effects of reactive oxygen species on sperm function. Theriogenology. 2012;78:1700–8.
Silvestri S, Orlando P, Armeni T, Padella L, Brugè F, Seddaiu G, et al. Coenzyme Q10 and α-lipoic acid: antioxidant and pro-oxidant effects in plasma and peripheral blood lymphocytes of supplemented subjects. J Clin Biochem Nutr. 2015;57:21–6.
Chen H, Cangello D, Benson S, Folmer J, Zhu H, Trush MA, et al. Age-related increase in mitochondrial superoxide generation in the testosterone-producing cells of Brown Norway rat testes: relationship to reduced steroidogenic function? Exp Gerontol. 2001;36:1361–73.
Gorąca A, Huk-Kolega H, Piechota A, Kleniewska P, Ciejka E, Skibska B. Lipoic acid–biological activity and therapeutic potential. Pharmacol Rep. 2011;63:849–58.
SUH JH, Shigeno ET, Morrow JD, COX JB, ROCHA AE. Oxidative stress in the aging rat heart is reversed by dietary supplementation with (R)-α‐lipoic acid. FASEB J. 2001;15:700–6.
Zicker SC, Hagen TM, Joisher N, Golder C, Joshi DK, Miller EP. Safety of long-term feeding of dl-alpha-lipoic acid and its effect on reduced glutathione: oxidized glutathione ratios in beagles. Veterinary Therapeutics: Res Appl Veterinary Med. 2002;3:167–76.
Milgram NW, Head E, Zicker SC, Ikeda-Douglas C, Murphey H, Muggenberg BA, et al. Long-term treatment with antioxidants and a program of behavioral enrichment reduces age-dependent impairment in discrimination and reversal learning in beagle dogs. Exp Gerontol. 2004;39:753–65.
Zhang H, Yang G, Li H, Wang L, Fu T, Li G, et al. Effects of dietary supplementation with alpha-lipoic acid on apparent digestibility and serum metabolome alterations of sheep in summer. Trop Anim Health Prod. 2021;53:1–13.
Wang D, Zhou L, Zhou H, Hou G, Shi L. Effects of dietary α-lipoic acid on carcass characteristics, antioxidant capability and meat quality in Hainan black goats. Italian J Anim Sci. 2017;16:61–7.
El-Sherbiny H, Abdelnaby E, El-Shahat K, Salem N, Ramadan E, Yehia S et al. Coenzyme Q10 Supplementation enhances testicular volume and hemodynamics, reproductive hormones, sperm quality, and seminal antioxidant capacity in goat bucks under summer hot humid conditions. Vet Res Commun 2022.
Brito L, Barth A, Wilde R, Kastelic J. Testicular ultrasonogram pixel intensity during sexual development and its relationship with semen quality, sperm production, and quantitative testicular histology in beef bulls. Theriogenology. 2012;78:69–76.
El-Sherbiny H, Shahat A, Hedia M, El-Shalofy A. Effect of sexual maturation on testicular morphometry and echotexture and their association with intratesticular blood flow in ossimi rams. Indian J Small Ruminants (the). 2022;28:85–90.
Abdelnaby EA. Testicular haemodynamics, plasma testosterone and oestradiol concentrations, and serum nitric oxide levels in the Egyptian buffalo bull after a single administration of human chorionic gonadotropin. Reprod Domest Anim. 2022;57:754–60.
Fadl AM, Abdelnaby EA, El-Sherbiny HR. Supplemental dietary zinc sulphate and folic acid combination improves testicular volume and haemodynamics, testosterone levels and semen quality in rams under heat stress conditions. Reprod Domest Anim. 2022;57:567–76.
El-Sherbiny HR, El-Shalofy AS, Samir H. Exogenous L-carnitine administration ameliorates the adverse effects of heat stress on testicular hemodynamics, echotexture, and total antioxidant capacity in rams. Front Veterinary Sci. 2022;9:860771.
Hashem NM, El-Sherbiny HR, Fathi M, Abdelnaby EA. Nanodelivery System for Ovsynch Protocol improves ovarian response, ovarian blood Flow Doppler velocities, and Hormonal Profile of goats. Animals. 2022;12:1442.
Hedia MG, El-Belely MS, Ismail ST, Abo El‐Maaty AM. Seasonal variation in testicular blood flow dynamics and their relation to systemic and testicular oxidant/antioxidant biomarkers and androgens in rams. Reprod Domest Anim. 2020;55:861–9.
Hansen C, Vermeiden T, Vermeiden J, Simmet C, Day B, Feitsma H. Comparison of FACSCount AF system, Improved Neubauer hemocytometer, Corning 254 photometer, SpermVision, UltiMate and NucleoCounter SP-100 for determination of sperm concentration of boar semen. Theriogenology. 2006;66:2188–94.
Abdelnaby EA, Yasin NA, Abouelela YS, Rashad E, Daghash SM, El-Sherbiny HR. Ovarian, uterine, and luteal vascular perfusions during follicular and luteal phases in the adult cyclic female rabbits with special orientation to their histological detection of hormone receptor. BMC Vet Res. 2022;18:1–18.
El-Sherbiny H, El-Shahat K, Abo EL, Abdelnaby E. Ovarian and uterine haemodynamics and their relation to steroid hormonal levels in postpartum Egyptian buffaloes. Bulgarian J Veterinary Med. 2022;25:262–73.
Samir H, El-Shalofy A, El-Sherbiny HR. Effects of a single dose of long-acting FSH on testicular blood flow, testicular echotexture, and circulating testosterone, estradiol, and nitric oxide in rams during the non-breeding season. Domest Anim Endocrinol 2023:106765.
Heitzer T, Finckh B, Albers S, Krohn K, Kohlschütter A, Meinertz T. Beneficial effects of α-lipoic acid and ascorbic acid on endothelium-dependent, nitric oxide-mediated vasodilation in diabetic patients: relation to parameters of oxidative stress. Free Radic Biol Med. 2001;31:53–61.
Okudan N, Atalik KN, Gökbel H, Canbilen A, Kara I. Alpha lipoic acid treatment improved endothelium-dependent relaxation in diabetic rat aorta. Yakugaku Zasshi. 2011;131:739–44.
Dworacka M, Iskakova S, Krzyżagórska E, Wesołowska A, Kurmambayev Y, Dworacki G. Alpha-lipoic acid modifies circulating angiogenic factors in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2015;107:273–9.
Budin SB, Othman F, Louis S, Bakar MA, Radzi M, Osman K, et al. Effect of alpha lipoic acid on oxidative stress and vascular wall of diabetic rats. Rom J Morphol Embryol. 2009;50:23–30.
El-Sherbiny HR, Fathi M, Samir H, Abdelnaby EA. Supplemental dietary curcumin improves testicular hemodynamics, testosterone levels, and semen quality in Baladi bucks in the non-breeding season. Theriogenology. 2022;188:100–7.
Shaygannia E, Tavalaee M, Akhavanfarid GR, Rahimi M, Dattilo M, Nasr-Esfahani MH. Alpha‐lipoic acid improves the testicular dysfunction in rats induced by varicocele. Andrologia. 2018;50:e13085.
Ye N, Lv Z, Dai H, Huang Z, Shi F. Dietary alpha-lipoic acid supplementation improves spermatogenesis and semen quality via antioxidant and anti-apoptotic effects in aged breeder roosters. Theriogenology. 2021;159:20–7.
Tibullo D, Li Volti G, Giallongo C, Grasso S, Tomassoni D, Anfuso CD, et al. Biochemical and clinical relevance of alpha lipoic acid: antioxidant and anti-inflammatory activity, molecular pathways and therapeutic potential. Inflamm Res. 2017;66:947–59.
Guzel EE, Tektemur NK, Tektemur A. Alpha-lipoic acid may ameliorate testicular damage by targeting dox-induced altered antioxidant parameters, mitofusin-2 and apoptotic gene expression. Andrologia. 2021;53:e13990.
Sheikholeslami S, Khodaverdian S, Dorri-Giv M, Hosseini SM, Souri S, Abedi-Firouzjah R, et al. The radioprotective effects of alpha-lipoic acid on radiotherapy-induced toxicities: a systematic review. Int Immunopharmacol. 2021;96:107741.
El-Sherbiny H, Abdelnaby E, Samir H, Fathi M. Addition of autologous platelet rich plasma to semen extender enhances cryotolerance and fertilizing capacity of buffalo bull spermatozoa. Theriogenology. 2022;194:104–9.
Ibrahim SF, Osman K, Das S, Othman AM, Majid NA, Rahman MPA. A study of the antioxidant effect of alpha lipoic acids on sperm quality. Clinics. 2008;63:545–50.
Haghighian HK, Haidari F, Mohammadi-Asl J, Dadfar M. Randomized, triple-blind, placebo-controlled clinical trial examining the effects of alpha-lipoic acid supplement on the spermatogram and seminal oxidative stress in infertile men. Fertil Steril. 2015;104:318–24.
Fadl AM, Abdelnaby EA, El-Sherbiny HR. INRA82 extender enhances semen quality in ram under cooled and cryopreserved stages. Asian Pac J Reprod. 2022;11:100–4.
Fadl A, Abdelnaby E, El-seadawy I, Kotp M, El-Maaty AMA, El-Sherbiny H. Eco-friendly synthesized zinc Oxide nanoparticles Improved Frozen-thawed semen quality and antioxidant capacity of rams. J Adv Veterinary Res. 2022;12:259–64.
Grasso S, Bramanti V, Tomassoni D, Bronzi D, Malfa G, Traini E, et al. Effect of lipoic acid and α-glyceryl‐phosphoryl‐choline on astroglial cell proliferation and differentiation in primary culture. J Neurosci Res. 2014;92:86–94.
Ma H, Quan F, Chen D, Zheng Y, Zhang B, Wang Y, Zhang Y. Protective function of alpha-lipoic acid on sperm motility and mitochondrial function during goat sperm-mediated gene transfer. Small Ruminant Res. 2011;99(2–3):191–8.
Ren F, Feng T, Dai G, Wang Y, Zhu H, Hu J. Lycopene and alpha-lipoic acid improve semen antioxidant enzymes activity and cashmere goat sperm function after cryopreservation. Cryobiology. 2018;84:27–32.
Najafi A, Kia HD, Hamishehkar H. Does alpha-lipoic acid–loaded nanostructured lipid carriers improve post-thawed sperm quality and ameliorate apoptosis-related genes of rooster sperm? Poult Sci. 2021;100:357–65.
Baumber J, Ball BA, Gravance CG, Medina V, Davies-Morel MC. The effect of reactive oxygen species on equine sperm motility, viability, acrosomal integrity, mitochondrial membrane potential, and membrane lipid peroxidation. J Androl. 2000;21:895–902.
Bidmeshkipour A, Hosseinzadeh Colagar A, Gholinezhad Chari M, Biparva P. Seminal plasma total antioxidant capacity and vitamin-C levels in asthenozoospermia: a case-control study. Tehran Univ Med J. 2010;67:835–42.
Taha T, Abdel-Gawad E, Ayoub M. Monthly variations in some reproductive parameters of Barki and Awassi rams throughout 1 year under subtropical conditions 2. Biochemical and enzymatic properties of seminal plasma. Anim Sci. 2000;71:325–32.
Abdelnaby EA, Alhaider AK, El-Maaty AMA, Ragab RSA, Seida AA, El-Badry DA. Ovarian and uterine arteries blood flow velocities waveform, hormones and nitric oxide in relation to ovulation in cows superstimulated with equine chorionic gonadotropin and luteolysis induction 10 and 17 days after ovulation. BMC Vet Res. 2023;19(1):205. https://doi.org/10.1186/s12917-023-03692-3. PMID: 37833782; PMCID: PMC10571355.
Madbouly H, El-Shahat KH, Fathi M, Abdelnaby EA. Hemodynamic changes in late advanced pregnant Zaraibi goats during the peripartum period. BMC Vet Res. 2023;19(1):194. https://doi.org/10.1186/s12917-023-03745-7. PMID: 37803319; PMCID: PMC10559465.
Abdelnaby EA, Alhaider AK, Ghoneim IM, Salem NY, Ramadan ES, Farghali HA, Khattab MS, AbdElKader NA, Emam IA. Effect of pyometra on vascularity alterations, oxidative stress, histopathology and inflammatory molecules in feline. Reprod Biol. 2024;24(1):100855. Epub 2024 Jan 22. PMID: 38262266.
Acknowledgements
Authors are grateful to the Open access funding provided by the Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank.
Funding
The article open access funding provided by the Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB) with institutions.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Elshymaa A. Abdelnaby, and Hossam R. El-Sherbiny: the notion, Methodology, data curation, Doppler examination, writing and reviwing. Mohamed Fathi, Ali Salama, and Haney Samir: hormonal analysis, and paper editing. Noha Y. Salem, Shimaa G. Yehia and Eman S. Ramadan: antioxidant analysis, conceptualization, reviewing and editing. Ibrahim A. Emam: reviewing and editing. All authors accepted and revised the final version of the paper.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animals were treated after the acceptance of the ethical approval from the Veterinary Medicine Cairo University Institutional Animal Care (Vet CU 2009-2022-492). All methods were performed in accordance with relevant guidelines and regulations. All methods are reported in accordance with ARRIVE guidelines.
Consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Conflict of interest
All authors declare any conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Abdelnaby, E.A., Fathi, M., Salem, N.Y. et al. Outcomes of dietary alpha-lipoic acid on testicular vascularization, steroid hormones, and seminal quality in aged Baladi bucks. BMC Vet Res 20, 293 (2024). https://doi.org/10.1186/s12917-024-04134-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-024-04134-4