Abstract
Background
Lameness has been associated with compromised animal welfare and reduced productivity in dairy cattle herds worldwide. However, little is known about the prevalence of claw lesions in the dairy buffalo population in Egypt. Furthermore, the optimum measurements for claw trimming in buffalo are unknown. A cross-sectional cadaver study was conducted where 135 pair buffalo hind feet were collected from 4 slaughterhouses and examined for the presence of claw lesions. The proportion and associated 95% confidence interval (CI) of each type of lesion were calculated. A separate set of healthy claws (n = 26) underwent ultrasonography (US) and computed tomography (CT). The agreement between US and CT measurements was assessed using Passing-Bablok regression and intraclass correlation coefficient. The CT measurements were used to calculate trimming recommendations.
Results
At least one lesion was identified in 242 claws (89.6%, 95% CI = 85.4–93.0). In healthy claws, poor to moderate agreement was identified between US and CT measurements which could be due a sample size of the study. The average ± standard deviation (SD) minimum recommended external wall length of the lateral and medial claws in heifers was 7.1 ± 0.36 cm and 7.5 ± 0.35 cm, respectively. The average ± SD minimum recommended external wall length in buffaloes over five years of age was 8.2 ± 0.27 cm and 8.4 ± 0.39 cm for the lateral and medial claws, respectively.
Conclusions
The study found a high prevalence of claw lesions in buffalo in Egypt, the clinical significance of which requires further elucidation. Recommended measurements will help guide claw trimming in buffalo to minimise lesions.
Similar content being viewed by others
Background
Buffalo production is important for food security in Egypt, contributing approximately 28% and 38% of the raw milk and red meat production, respectively [1]. The official buffalo population in Egypt was 1,348,000 heads in 2020 [1]. Historically, buffaloes have been raised under a mixed crop-livestock farming system [2]. However, commercial buffalo farms with semi-intensive and intensive modern production systems have become increasingly available in Egypt in recent years [3, 4]. Most studies on buffaloes in Egypt have focused on endemic infectious diseases, such as foot and mouth disease (FMD) and tropical theileriosis [5,6,7], or on improving reproductive performance and productivity, such as studying the usefulness of crossbreeding with Italian Mediterranean buffalo [4, 8, 9].
Lameness is associated with reduced welfare and impaired productivity in dairy cattle worldwide [10, 11]. A recent study in Egypt conducted on 55 commercial dairy cattle farms reported an average within-herd lameness prevalence of 43.1% and a 100% herd-level prevalence [12]. Relatively little research has been conducted to investigate the impact of lameness on the buffalo population worldwide. A study by Guccione et al. [13] reported foot lesions in 229 of 1297 (17.7%) multiparous Italian Mediterranean buffaloes subjected to routine trimming on four free-stall dairy buffalo farms in Italy. These lesions were associated with clinical lameness in 206 buffaloes (90.0% of animals with lesions; 15.9% overall). Earlier studies have reported minimal impact of lameness on dairy buffaloes [14, 15]. These studies reported zero prevalence of lameness in three dairy buffalo farms in Italy and attributed this reduced prevalence to a lack of genetic predisposition to lameness and the lower feeding regimen in buffalo compared with cattle. In Egypt, no previous study has documented foot lesions in buffaloes or investigated the prevalence of lameness in this population of animals. FMD, a leading infectious cause of foot lesions in cattle and buffalo, is endemic in Egypt, with multiple outbreaks reported annually [16]. The perceived low impact of lameness in dairy buffaloes and the presence of other endemic, economically important diseases to which research resources have been allocated could be the reason why lameness research in buffaloes has not drawn much attention.
Routine claw trimming has been established as a component of a lameness prevention plan for dairy cattle herds [17,18,19,20], with 82.4% of dairy farmers practicing routine trimming in the UK [21]. Functional claw trimming reduces the risk of lameness development by improving foot balance between the lateral and medial claws, increasing the contact area with the ground [22], resulting in improved grip [23], and reducing external weight from the typical sole ulcer site at the axial sole [17, 24]. It also contributes to the early detection and treatment of subclinical claw lesions before they develop into clinical lameness [19]. A lack of routine trimming has been associated with claw horn disruption lesions, such as white line fissures and sole ulceration [25].
Because of the high propensity for over-trimming, proper claw trimming is equally as important as preventive trimming itself [25]. Trimming to a minimum sole thickness of 5 mm at the tip of the 3rd phalanx has been standardized in dairy cattle to prevent compression damage to the corium, particularly at the level of the flexor tubercle, and to prevent the development of a thin sole [25,26,27]. Furthermore, trimming the claws to an average length of 7.2 – 9 cm has been recommended to achieve a 5 mm minimal sole thickness in dairy cattle [25, 27, 28]. Over-trimming can result in a thin sole with subsequent compression of the subsolar soft tissue and the development of claw lesions such as toe ulcers [29]. These claw measurement recommendations are lacking for buffaloes. Therefore, the objectives of the current study were to 1) document foot lesions in buffalo hind feet collected from local abattoirs, 2) use data obtained from computed tomography (CT) examination to standardize claw-trimming measurements in Egyptian buffalo, 3) investigate the utility of ultrasonography as an objective method to measure the sole and solar soft tissue thickness at the ground surface of the hind claws, and 4) investigate the agreement between measurements taken by CT and ultrasonography examination.
Results
During the study period (November –December 2019), we examined 270 hind feet of 135 buffaloes. They were recruited from four abattoirs located in three Egyptian governorates (Cairo, Al Sharqiya, and Dakahlia). The feet were examined during 8 visits to these abattoirs. The buffaloes examined included 62 males (45.9%; 95% confidence interval [CI] = 37.3 – 54.7) and 73 females (54.1%; 95% CI = 45.3 – 62.7). The age distribution of examined animals was unknown, however, fattened male buffaloes are generally slaughtered at 2–3 years of age and by law, female buffaloes are not slaughtered before 5 years of age. At least one lesion was identified in 242 feet (89.6%, 95% CI = 85.4 – 93.0). The maximum number of lesions identified in a single foot was 11, which were identified in four feet (1.5%, 95% CI = 0.41 – 3.8). Table 1 presents the lesions identified, their prevalence, and associated 95% CIs.
The association between lesions identified at ≥ 5% prevalence (scissor claws, interdigital dermatitis, heel horn erosion, diffuse sole haemorrhage, white line fissure, double sole) and sex of the animals (male or female) were explored using multiple correspondence analysis, and the results are presented in Fig. 1. The first two dimensions explained 45.1% of the total variation in the data. There was a strong association between the presence of heel horn erosion, double sole, white line fissure, and female buffaloes. In contrast, male buffaloes were strongly correlated with scissor claws and diffuse sole haemorrhage.
Multiple correspondence analysis of the relationship between sex of the buffalo (a proxy for age) and claw lesions identified at ≥ 5% prevalence. The first two dimensions explained 45.1% of variation in the data. There is correlation between double sole, heel erosion, white line fissure, interdigital dermatitis, and female buffaloes and between scissor claws and sole haemorrhage and male buffaloes
Healthy claws from buffalo heifers (n = 14) and buffaloes > 5 years of age (n = 12) underwent US and CT examinations. Summary statistics of claw measurements obtained and the results of the Shapiro–Wilk test of normal distribution are presented in Table 2. Measurements obtained using ultrasonography (M1, M2, M3) and CT (M1, M2, M3, M4, M5, claw angle) are presented for the two study groups and for the medial and lateral claws separately Figs. 2 and 3. Some of the results obtained did not follow a normal distribution (P < 0.05). The mean ± standard deviation (SD) of dorsal wall thickness in the old age group was 8.1 ± 0.72 and 8.6 ± 1.1 mm for the lateral and medial claws, respectively. In heifers, the mean dorsal wall thickness was 6.6 ± 0.34 and 6.9 ± 0.38 for the lateral and medial claws, respectively. The mean internal wall length (measure M5) was 7.4 ± 0.23 cm and 7.5 ± 0.34 cm for the lateral and medial claws, respectively in the old age group and 6.6 ± 0.34 and 6.9 ± 0.38 for the lateral and medial claws, respectively in heifers.
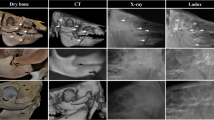
Ultrasonography image obtained from the ground surface of the claw showing locations of measurements taken for the corium and sole thickness. The 3 hyper-reflective lines from the top down are the sole ground surface, sole-corium junction, and the ventral surface of the 3rd phalanx. Location M1 is at the most apical margin of the 3rd phalanx, M2 is at the deepest concavity of the 3rd phalanx and M3 is at the region of the flexor tubercle
Approximately mid-sagittal computed tomography image of a boffola hind claw. The figure shows the locations of measurements taken. M1 is at the most apical margin of 3rd phalanx, M2 is at the deepest concavity of the 3rd phalanx, M3 is at the region of the flexor tubercle, M4 is the dorsal wall thickness measured at 3 different locations along the dorsal surface of the 3rd phalanx, M5 is the internal wall length measured from the most proximal border of the wall to the tip of the wall corium
The internal wall length measured in the CT studies was adjusted to calculate the minimal external wall length if the claws were trimmed to a point or a step. This considered the dorsal wall thickness, claw angle, and minimum sole thickness of 5 mm at the tip of 3rd phalanx (Fig. 4). These calculations indicated that the average ± SD minimum external wall length of the lateral and medial claws in the heifers should be 7.1 ± 0.36 cm and 7.5 ± 0.35 cm, respectively if the claws are trimmed to a step. These measurements increased to a mean ± SD of 7.7 ± 0.37 cm and 8.2 ± 0.34 cm for the lateral and medial claws, respectively, if the claws are trimmed to a point in the same age group. In the old age group, the average minimum recommended external wall length was 8.2 ± 0.27 cm and 8.4 ± 0.39 cm for the lateral and medial claws, respectively if they are trimmed to a step and 8.9 ± 0.30 cm and 9.1 ± 0.43 cm for the lateral and medial claws, respectively if they are trimmed to a point. The results of the calculations are listed in Table 3.
Calculation of the recommended minimal external wall length. Segment “a” is added to the internal wall length if the claw is trimmed to a step and segment “a + c” is added if the claw is trimmed to a point. Segment “a” is calculated as a = b / (tangent of claw angle) where “b” is the dorsal wall sickness. Segment “c” is calculated as c = d / (sine claw angle) where “d” is the minimum recommended sole thickness of 5 mm at the tip of the 3rd phalanx
The results of the Passing–Bablok regression and ICC for the agreement between measurements taken using ultrasonography and CT are presented in Table 4 and Figs. 5, 6 and 7. There was poor to moderate agreement between measurements.
Passing–Bablok regression plot for corium (a, b) and sole (c, d) thicknesses obtained from ultrasonography and computed tomography at the most apical margin of the 3rd phalanx (location M1). The thick blue line is the line of best fit and the dashed lines are 95% confidence interval. The intercept, slope and Pearson’s correlation coefficient are demonstrated on the plot
Passing–Bablok regression plot for corium (a, b) and sole (c, d) thicknesses obtained from ultrasonography and computed tomography at the deepest concavity of the 3rd phalanx (location M2). The thick blue line is the line of best fit and the dashed lines are 95% confidence interval. The intercept, slope and Pearson’s correlation coefficient are demonstrated on the plot
Passing–Bablok regression plot for corium (a, b) and sole (c, d) thickness obtained from ultrasonography and computed tomography at the flexor tubercle of the 3rd phalanx (location M3). The thick blue line is the line of best fit and the dashed lines are 95% confidence interval. The intercept, slope and Pearson’s correlation coefficient are demonstrated on the plot
Discussion
To our knowledge, this is the first study to provide information on claw lesions in the buffalo population in Egypt and to set recommendations about the optimum dorsal wall length of claws during trimming in two different age groups of buffalo. Furthermore, this study evaluated the use of ultrasonography as a cost-effective, non-invasive, and objective technique to guide claw trimming in buffaloes.
The current study reported a very high prevalence of foot lesions (89.6%) in buffalo feet. A study that evaluated post-mortem assessment of claws as a welfare indicator of feedlot cattle at slaughter reported a high prevalence of claw disorders, such as abnormally shaped claws (61%) and claw wall fissures (26.7%) [30], which is consistent with the current study. Hind foot lesions have also been reported to occur at a prevalence of 32% postmortem in another abattoir-based study of feedlot cattle [31]. Although these lesions are typically associated with compromised animal welfare, their clinical significance cannot be assessed using cadaver study designs. Lesions identified during routine trimming of Italian Mediterranean buffaloes (animal-level prevalence of 17.7%) were associated with clinical signs of lameness in 15.9% of the trimmed buffaloes. Further studies are required to assess the clinical significance of foot lesions in Egyptian buffaloes.
In the current study, foot lesions were examined in slaughtered buffalo which included females over 5 years of age and fattening males between 2–3 years of age. We found that sole haemorrhage was associated with feedlot/male buffalo. A study by Magrin et al. [32] reported a sole haemorrhage prevalence of 65% in the claws of veal calves fed high-carbohydrate diets. Another abattoir-based study of feedlot cattle reported that concrete slatted floors were significantly associated with increased risk of sole haemorrhage [33]. We did not collect information on the management practices of buffaloes in the current study; however, the high prevalence of sole haemorrhage could also be due to feeding a high-carbohydrate diet or managing these animals on solid concrete floors, which has been reported as a common management practice in Egypt [3]. Scissor claws were also more prevalent in feedlot/male buffaloes in the current study, which is consistent with previous studies on feedlot cattle [30, 31].
Double sole, heel horn erosion, and white line fissures were more prevalent in female buffaloes over 5 years age. Possible reasons for this relationship include a lack of routine trimming, poor farm hygiene, or previous infections with FMD. Lack of routine trimming has previously been associated with claw horn disruption lesions in cattle [25]. Poor farm hygiene is prevalent in Egypt and could be one possible reason for the increased prevalence of heel erosion observed in the current study [12, 34]. FMD is endemic in Egypt [5] and is frequently associated with the development of ulcerative lesions in the coronary band [35], with subsequent growth of abnormally weak horn, which may result in the development of a double sole [36].
In the current study, we used ultrasonography to determine the sole and solar soft tissue thickness (combined digital cushion and corium thickness) of the hind claws of female buffaloes from two age groups. Images were successfully obtained from all animals using a linear rectal ultrasound probe, consistent with previous studies on cattle [37, 38]. The probe frequency had to be decreased to 4 MHz in claws with greater sole thickness in older buffaloes, which is consistent with the results of Tsuka et al. [39], who reported that better images were obtained at lower ultrasound probe frequencies. Ultrasonography has been validated to provide objective measurements of the claws in cattle, and has been used to study, for example, the effect of changes in sole soft-tissue thickness and/or echogenicity on the development of claw horn disruption lesions [37, 40, 41], to objectively guide claw trimming [39, 42], and to diagnose pedal bone fractures [43]. Here, we confirmed the utility of ultrasonography for examining the claws of buffaloes. Further research utilizing this diagnostic technique in buffaloes is warranted. Solar soft tissue measurements obtained at M2 were consistently greater than those obtained at other locations in both age groups, which agrees with previous cattle studies [39].
The current study reported a poor-to-moderate agreement between measurements obtained by ultrasonography and CT. Kofler et al. [44] reported greater correlation between ultrasound- and CT-measured sole horn thickness (r = 0.83 – 0.89) than between ultrasound- and CT-measured solar soft tissue thickness (r = 0.51 – 0.64) which was not observed in the present study. A recent study reported strong correlation (r = 0.91 – 0.92) between CT- and ultrasound-measured sole horn thickness in cattle [39]. The reduced agreement between measurements obtained by ultrasonography and CT reported here compared to the later study could be due to a small sample size of the present study, in which we examined only 26 hind buffalo feet [45].
In the current study, we used trigonometry to calculate the minimum recommended dorsal wall length, considering the dorsal wall thickness, claw angle, and internal wall length measured using CT and assuming a minimal sole thickness at the apex of 3rd phalanx of 5 mm [27]. In heifers, an average minimum dorsal wall length of 7.5 and 8.2 cm if the claws were trimmed to a step or to a point, respectively was calculated. A study that investigated current practices of preventative claw trimming in UK dairy herds reported that only 5.9% of respondents practised preventative claw trimming in pre-calving heifers [21]. A randomized controlled trial that evaluated the effectiveness of routine foot trimming of heifers 3 weeks pre-calving and 100 days post-calving in reducing the first lactation lameness and improving milk production reported that preventative trimming in heifers pre- or post-calving was not associated with lameness prevalence, time to first lameness, 305-day lactation milk yield, or the type of lesions identified during dry-off claw trimming compared with only performing locomotion scoring [46]. Another study that investigated the usefulness of routine early lactation trimming in heifers reported that trimming was more effective in lame heifers at the time of trimming than in non-lame heifers in terms of milk productivity [19]. These findings suggest that dairy heifers should be selected for trimming based on the results of regular locomotion scoring, which could be more economically effective than routine trimming in this group of animals [19, 47, 48].
The average dorsal wall length in older buffaloes in the current study was 8.4 and 9.1 cm if the claws were trimmed to a step or to a point, respectively. Age-related changes in claw dimensions in cattle have been previously reported [27, 49], which is consistent with the findings of the current study. Earlier studies on Holstein Friesian cattle described the optimum dorsal wall length during the 1st step of claw trimming as 7.5 cm [50]. A recent study reported correlation between the dorsal wall length and sole thickness at the apex of the 3rd phalanx where a sole thickness of 3.8 and 4.0 mm of the medial and lateral claws, respectively were correlated with a dorsal wall length of < 7 cm. Furthermore, a sole thickness of 7 mm at the apex of 3rd phalanx was correlated with a dorsal wall length of 7.98 and 7.84 cm for the medial and lateral claws, respectively [25]. Notably, this previous study reported that the medial claw should be trimmed to approximately 1.4 mm longer than the lateral claw, which is consistent with our findings. Based on our findings, trimming to a dorsal wall length of 7.5 cm in older buffaloes will result in over-trimming in all animals. This agrees with a study by Archer et al. [27] who reported that trimming to a fixed dorsal wall length of 7.5 cm without considering the age, size of the animals and the size of the claws would result in over trimming in 97% and 96% of Holstein Friesian cattle aged ≥ 4 years and < 4 years, respectively.
Conclusion
This study found a high prevalence of claw lesions in slaughtered buffalo. However, further studies on live animals are necessary to fully understand the impact of lameness on dairy buffaloes in Egypt. As this was an abattoir-based study, we could not elucidate the clinical significance of the identified lesions. The findings of the current study suggest a lack of routine trimming in this buffalo population, which could be due to the perceived reduced importance of routine trimming, or the less cooperative nature of this species compared with cattle. The study also provides recommendations on the minimal dorsal wall length during trimming in two age groups and reported poor to moderate agreement between ultrasonography and CT.
Methods
Abattoir visits and claw examination
Abattoir visits were arranged with abattoir managers, and dates for each visit were agreed upon in advance. Because buffalo feet are expensive edible carcass parts in Egypt, and the project was not funded to purchase feet for claw examination, all examinations were performed within abattoirs. Brief trimming was performed for each examined claw using a hoof knife and hoof nippers, and lesions were recorded on an examination sheet (Additional file 1). Because most lesions causing lameness in farm animals are associated with hind feet [51], only hind feet were examined in the current study. All examinations were performed by a single operator (the first author) to ensure consistency. An assistant was assigned to each visit to help record the lesions identified during the examination. Pictorial descriptions and definitions of the claw lesions were obtained from the ICAR Claw Health Atlas [52]. The slaughter law in Egypt prohibits the routine slaughtering of female buffaloes before the age of five. Therefore, two age groups were included in this part of the study: fattening buffaloes were males aged 2–3 years, and culled adult female buffaloes over 5 years of age. Sample size calculations to detect the presence of at least one claw lesion in 30% of the examined feet indicated that 300 feet were required to be examined in the study, assuming a buffalo population of 4000 would be sent for slaughter at the study area during the study period and a 95% confidence level. Sample size calculations were performed using the Epi Info software (Centre for Disease Control (CDC), Atlanta, Georgia, USA).
Claw measurement study
Feet from two age groups were included in this study. These included apparently healthy feet purchased from government-controlled abattoirs, which belonged to adult female buffaloes over 5 years of age (n = 12), and apparently healthy feet collected from small private non-government-controlled abattoirs which belonged to young female buffaloes approximately 2–3 years of age (n = 14). These young female buffaloes were slaughtered for reasons exempt from the female age restriction (failed to conceive, clinical emergency such as upper limb fractures). Following slaughter and exsanguination, the hind feet were separated at the tarsometatarsal joints and transported to the laboratory, where they were stored at -20 °C until further ultrasonography and CT examinations.
Ultrasonography examination
Prior to the ultrasonographic evaluation, all feet were thawed overnight at room temperature. Brief trimming was performed using a hoof knife to remove dirt and any apparently loose horn, which might have created air pockets impeding the ultrasound beam transmission. Measurements were performed using a veterinary ultrasound machine and a multifrequency linear rectal probe (Sonoscape A5 ultrasonography machine, SonoScape, China). The probe frequency was set to 6–7 MHz for most examinations, and the focus and gain were adjusted until an image of optimum quality was obtained. The probe frequency was decreased to 4 MHz in claws with greater sole thickness to obtain an image of optimum quality. The solar surface of the claws was not trimmed flat before the ultrasound scan. Therefore, a copious amount of coupling gel was applied to fill any concavity on the solar surface to ensure proper probe contact with the claw horn. The solar surface of each claw was scanned along an imaginary mid-longitudinal line from the apex of the claw to the heel region. The thicknesses of the sole and corium were measured at three locations. A location that was perpendicular to the apical margin of the third phalanx (M1), a location opposite to the deepest concavity the 3rd phalanx (M2) and a location that was opposite to the flexor tubercle of the 3rd phalanx (Fig. 2) [44]. Measurements were performed in triplicate at each of the three sites.
Computed tomography examination
Thawed distal hind limbs underwent CT examination using an Optima CT660 clinical CT scanner (GE Medical Systems) at 120 kV and 50 mA. Scans were taken at slice thickness and spacing between slices of 0.62 mm resulting in approximately 700 images per leg (from proximal metatarsal to the tip of the claws). Images were reconstructed and visualized using RadiAnt DICOM Viewer program (Medixant, Poznan, Poland). Images were reconstructed in the axial, coronal, and sagittal planes, and approximately midsagittal images were used to obtain measurements. Lateral and medial claws were evaluated. The images were calibrated to represent the actual measurements of the specimens. Measurements were taken at the same M1, M2, and M3 sites as in the ultrasonographic examination. Additionally, dorsal wall thickness (M4) was evaluated at three different sites along the dorsal surface of the 3rd phalanx. Internal wall length was measured as the distance from the proximal limit of the wall to the tip of the corium distally (M5) [27]. Figure 3. shows an approximately midsagittal CT image with the sites of the measurements identified. The claw angle was measured as the angle formed by two straight lines, one on the dorsal surface of the wall and the other on the ground surface of the sole (Fig. 4).
The minimum recommended external dorsal wall length for each claw was estimated by adjusting the internal dorsal wall length, considering the value of the claw angle, dorsal wall thickness, and recommended minimum sole thickness of 5 mm at the tip of the 3rd phalanx [27, 49]. Trigonometry was used to perform calculations for each of the claws included in the study (Fig. 4).
Statistical methods
The results of the claw examination were transferred to a custom-built Microsoft Access database. The data were exported to a Microsoft Excel spreadsheet for statistical analysis. The number, proportion, and 95% confidence interval (CI) of the proportions were calculated for each type of claw lesions included in the examination sheet (asymmetric claws, concave dorsal wall, corkscrew claws, scissor claws, digital dermatitis, interdigital/superficial dermatitis, axial horn fissure, heel horn erosion, horizontal fissure, vertical fissure, interdigital hyperplasia, interdigital phlegmon, diffuse sole haemorrhage, circumscribed sole haemorrhage, coronary swelling, sole ulcer, heel ulcer, toe ulcer, toe necrosis, thin sole, white line fissure, white line abscess, double sole). The relationship between buffalo sex and claw lesions identified at a proportion of ≥ 5% was examined using multiple correspondence analysis (MCA). The first two dimensions resulting from the MCA were plotted. The sex of buffaloes was used as a proxy for age, where female buffaloes were over five years of age and male buffaloes were approximately 2–3 years of age. The statistical package FactoMineR [53] was used to conduct MCA. Summary statistics (mean, standard deviation, median, interquartile range) were calculated for each claw measurement obtained from ultrasonography and CT examinations of the feet. Measurements obtained from the claws of the older buffaloes (> 5 years of age) and buffalo heifers are presented separately. The Shapiro–Wilk test was used to assess whether a measurement followed a normal distribution. The agreement between measurements (corium and sole thickness at M1, M2, and M3 sites) obtained using ultrasonography and CT examinations was assessed using the Passing–Bablok regression [54] and calculation of the intraclass correlation coefficient for agreement. The statistical packages mcr [55] and irr [56] were used to conduct the Passing–Bablok regression and to calculate the ICC for agreement, respectively. All the analyses were performed in R software version 4.2.2 [57].
Availability of data and materials
The datasets generated during and/or analysed during the current study are available in the figshare repository; https://doi.org/10.6084/m9.figshare.23566278.
References
FAOSTAT: Crop and livestock products. https://www.fao.org/faostat/en/#data/QCL. Accessed 20 Oct. 2022.
Fahim NH, Abdel-Salam SAM, Mekkawy W, A. I, Abou-Bakr S, El-Sayed M, Ibrahim MAM: Delta and upper Egypt buffalo farming systems: A survey comparison. Egyptian J Anim Prod 2018, 55(2):95–106.
Ghoneim EM, Omar S, El-Dahshan E. Measuring Welfare of Egyptian Buffaloes in Different Management Systems. J Animal and Poultry Prod Mansoura Univ. 2018;9(10):407–14.
Nasr M. The impact of cross-breeding Egyptian and Italian buffalo on reproductive and productive performance under a subtropical environment. Reprod Domest Anim. 2017;52(2):214–20.
Hagag NM, Hassan AM, Zaher MR, Elnomrosy SM, Shemies OA, Hussein HA, Ahmed ES, Ali MH, Ateay M, Abdel-Hakim MA, et al. Molecular detection and phylogenetic analysis of newly emerging foot-and-mouth disease virus type A, Lineage EURO-SA in Egypt in 2022. Virus Res. 2022;323:198960.
Ibrahim HM, Galon EMS, Tumwebaze MA, Byamukama B, Liu M, Mohammed-Geba K, Sheir SK, Galal-Khallaf A, Latif H, Morsi DS, et al. Serological Survey of Babesia bigemina and Babesia bovis in Cattle and Water Buffaloes from Menoufia Province. Egypt Acta Parasitol. 2021;66(4):1458–65.
El Damaty HM, Yousef SG, El-Balkemy FA, Nekouei O, Mahmmod YS, Elsohaby I. Seroprevalence and risk factors of tropical theileriosis in smallholder asymptomatic large ruminants in Egypt. Front Vet Sci. 2022;9:1004378.
Nasr MA. The impact of crossbreeding Egyptian and Italian buffalo on milk yield and composition under subtropical environmental conditions. J Dairy Res. 2016;83(2):196–201.
Ramadan SI. Effect of some genetic and non-genetic factors on productive and reproductive traits of Egyptian buffaloes. J Adv Vet Anim Res. 2018;5(4):374–80.
Puerto MA, Shepley E, Cue RI, Warner D, Dubuc J, Vasseur E. The hidden cost of disease: II. Impact of the first incidence of lameness on production and economic indicators of primiparous dairy cows. J Dairy Sci. 2021;104(7):7944–55.
Schenkenfelder J, Winckler C. To meet or not to meet welfare outcome thresholds: a case-control study in dairy cow herds. Animal. 2022;16(3):100461.
Salem SE, Mesalam A, Monir A. A cross-sectional study of the prevalence of lameness and digital dermatitis in dairy cattle herds in Egypt. BMC Vet Res. 2023;19(1):68.
Guccione J, Carcasole C, Alsaaod M, D’Andrea L, Di Loria A, De Rosa A, Ciaramella P, Steiner A. Assessment of foot health and animal welfare: clinical findings in 229 dairy Mediterranean Buffaloes (Bubalus bubalis) affected by foot disorders. BMC Vet Res. 2016;12(1):107.
Napolitano F, Grasso F, Bordi A, Tripaldi C, Saltalamacchia F, Pacelli C, De Rosa G. On-farm welfare assessment in dairy cattle and buffaloes: evaluation of some animal-based parameters. Ital J Anim Sci. 2005;4(3):223–31.
De Rosa G, Napolitano F, Grasso F, Pacelli C, Bordi A. On the development of a monitoring scheme of buffalo welfare at farm level. Ital J Anim Sci. 2005;4(2):115–25.
Soltan MA, Mahmoud MM, Hegazy Y, Abd-Eldiam MM. Emergence of foot and mouth disease virus, serotype O, Europe-South America topotype in Egypt, 2022. Transbound Emerg Dis. 2022;69(5):2409–11.
Manske T, Hultgren J, Bergsten C. The effect of claw trimming on the hoof health of Swedish dairy cattle. Prev Vet Med. 2002;54(2):113–29.
Hernandez JA, Garbarino EJ, Shearer JK, Risco CA, Thatcher WW. Evaluation of the efficacy of prophylactic hoof health examination and trimming during midlactation in reducing the incidence of lameness during late lactation in dairy cows. J Am Vet Med Assoc. 2007;230(1):89–93.
Maxwell OJ, Hudson CD, Huxley JN. Effect of early lactation foot trimming in lame and non-lame dairy heifers: a randomised controlled trial. Vet Rec. 2015;177(4):100.
Sadiq MB, Ramanoon SZ, Shaik Mossadeq WM, Mansor R, Syed-Hussain SS. A modified functional hoof trimming technique reduces the risk of lameness and hoof lesion prevalence in housed dairy cattle. Prev Vet Med. 2021;195:105463.
Pedersen SIL, Huxley JN, Hudson CD, Green MJ, Bell NJ. Preventive hoof trimming in dairy cattle: Determining current practices and identifying future research areas. Vet Rec. 2022;190(5):e1267.
van der Tol PP, van der Beek SS, Metz JH, Noordhuizen-Stassen EN, Back W, Braam CR, Weijs WA. The effect of preventive trimming on weight bearing and force balance on the claws of dairy cattle. J Dairy Sci. 2004;87(6):1732–8.
Phillips CJ, Chiy PC, Bucktrout MJ, Collins SM, Gasson CJ, Jenkins AC. Paranhos da Costa MJ: Frictional properties of cattle hooves and their conformation after trimming. Vet Rec. 2000;146(21):607–9.
Vidmar M, Hodnik JJ, Starič J. Review of guidelines for functional claw trimming and therapeutic approach to claw horn lesions in cattle. Trop Anim Health Prod. 2021;53(5):476.
Tsuka T, Murahata Y, Azuma K, Osaki T, Ito N, Okamoto Y, Imagawa T. Quantitative evaluation of the relationship between dorsal wall length, sole thickness, and rotation of the distal phalanx in the bovine claw using computed tomography. J Dairy Sci. 2014;97(10):6271–85.
Laven LJ, Margerison JK, Laven RA. Validation of a portable ultrasound machine for estimating sole thickness in dairy cattle in New Zealand. N Z Vet J. 2012;60(2):123–8.
Archer SC, Newsome R, Dibble H, Sturrock CJ, Chagunda MG, Mason CS, Huxley JN. Claw length recommendations for dairy cow foot trimming. Vet Rec. 2015;177(9):222.
Nuss K, Sauter-Louis C, Sigmund B. Measurements of forelimb claw dimensions in cows using a standardised sole thickness: a post-mortem study. Vet J. 2011;190(1):84–9.
van Amstel SR, Shearer JK, Palin FL. Moisture content, thickness, and lesions of sole horn associated with thin soles in dairy cattle. J Dairy Sci. 2004;87(3):757–63.
Bautista-Fernández M, Estévez-Moreno LX, Losada-Espinosa N, Villarroel M, María GA, De Blas I. Miranda-de la Lama GC: Claw disorders as iceberg indicators of cattle welfare: Evidence-based on production system, severity, and associations with final muscle pH. Meat Sci. 2021;177:108496.
Losada-Espinosa N, Estévez-Moreno LX, Bautista-Fernández M, Galindo F, Salem AZM. Miranda-de la Lama GC: Cattle welfare assessment at the slaughterhouse level: Integrated risk profiles based on the animal’s origin, pre-slaughter logistics, and iceberg indicators. Prev Vet Med. 2021;197:105513.
Magrin L, Brscic M, Cozzi G, Armato L, Gottardo F. Prevalence of gastrointestinal, liver and claw disorders in veal calves fed large amounts of solid feed through a cross-sectional study. Res Vet Sci. 2020;133:318–25.
Magrin L, Brscic M, Armato L, Contiero B, Lotto A, Cozzi G, Gottardo F. Risk factors for claw disorders in intensively finished Charolais beef cattle. Prev Vet Med. 2020;175:104864.
Saber AA, Hassan MA, El Nabtiti AS, Hassan AM, Mansour SR. Evaluation of field techniques to diagnose early subclinical mastitis in relation to hygiene score in a buffalo farm. The Egyptian Society for Environmental Sciences. 2017;16(1):53–60.
Misk NA, Misk TN, Rateb HZ. Assessment and topical treatment of lesions of foot and mouth disease in cattle. Assiut Vet Med J. 2015;61(145):75–81.
Bergsten C. Causes, Risk Factors, and Prevention of Laminitis and Related Claw Lesions. Acta Vet Scand. 2003;44(1):S157.
Fabbri G, Gianesella M, Morgante M, Armato L, Bonato O, Fiore E. Ultrasonographic alterations of bovine claws sole soft tissues associated with claw horn disruption lesions, body condition score and locomotion score in Holstein dairy cows. Res Vet Sci. 2020;131:146–52.
Laven LJ, Laven RA, Parkinson TJ, Lopez-Villalobos N, Margerison JK. An evaluation of the changes in distance from the external sole surface to the distal phalanx in heifers in their first lactation. Vet J. 2012;193(3):639–43.
Tsuka T, Nishimura R, Hishinuma M, Murahata Y, Yamashita M, Azuma K, Osaki T, Ito N, Okamoto Y, Imagawa T. Reliability of ultrasonographic measurements of bovine sole structures in relation to sole horn thickness, measured by computed tomography, and sole horn hardness. J Dairy Sci. 2019;102(11):10105–18.
Fabbri G, Magrin L, Gottardo F, Armato L, Contiero B, Gianesella M, Fiore E. Development of an equation to screen for solar hemorrhages from digital cushion ultrasound texture analysis in veal calves at slaughter. Front Vet Sci. 2022;9:899253.
Newsome RF, Green MJ, Bell NJ, Bollard NJ, Mason CS, Whay HR, Huxley JN. A prospective cohort study of digital cushion and corium thickness. Part 1. Associations with body condition, lesion incidence, and proximity to calving. J Dairy Sci. 2017;100(6):4745–58.
Bach K, Nielsen SS, Danscher AM, Capion N. Ultrasonographical examination of bovine claws through the sole horn on weight-bearing claws. J Dairy Sci. 2019;102(5):4364–75.
Laschinger J, Kofler J, Schieder K, Tichy A, Hund A. Ultrasonographic diagnosis of closed pedal bone fractures in bovine claws: An ex-vivo study in slaughterhouse specimens. Vet J. 2021;268:105591.
Kofler J, Kübber P, Henninger W. Ultrasonographic imaging and thickness measurement of the sole horn and the underlying soft tissue layer in bovine claws. Vet J. 1999;157(3):322–31.
Bates BT, Zhang S, Dufek JS, Chen FC. The effects of sample size and variability on the correlation coefficient. Med Sci Sports Exerc. 1996;28(3):386–91.
Mahendran SA, Huxley JN, Chang YM, Burnell M, Barrett DC, Whay HR, Blackmore T, Mason CS, Bell NJ. Randomised controlled trial to evaluate the effect of foot trimming before and after first calving on subsequent lameness episodes and productivity in dairy heifers. Vet J. 2017;220:105–10.
Randall LV, Green MJ, Chagunda MG, Mason C, Green LE, Huxley JN. Lameness in dairy heifers; impacts of hoof lesions present around first calving on future lameness, milk yield and culling risk. Prev Vet Med. 2016;133:52–63.
Capion N, Thamsborg SM, Enevoldsen C. Prevalence and severity of foot lesions in Danish Holstein heifers through first lactation. Vet J. 2009;182(1):50–8.
Nuss K, Paulus N. Measurements of claw dimensions in cows before and after functional trimming: a post-mortem study. Vet J. 2006;172(2):284–92.
Toussaint Raven E. Structure and Function. In: Toussaint Raven E, editor. Cattle Foot Care and Claw Trimming. Ipswich: UK: Farming Press,; 1989. p. 24–6.
Archer S, Bell N, Huxley J. Lameness in UK dairy cows: a review of the current status. In Pract. 2010;32(10):492–504.
Egger-Danner C, Nielsen P, Fiedler A, Müller K, Fjeldaas T, Döpfer D, Daniel V, Bergsten C, Cramer G, Christen AM et al: ICAR Health Atlas. ICAR Technical Series No 18 2014. https://www.icar.org/ICAR_Claw_Health_Atlas.pdf. Accessed Oct 2019.
Le S, Josse J, Husson F. FactoMineR: An R Package for Multivariate Analysis. J Stat Softw. 2008;25:1–18.
Passing H. Bablok: A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in clinical chemistry, Part I. J Clin Chem Clin Biochem. 1983;21(11):709–20.
Potapov S, Model F, Schuetzenmeister A: mcr: Method Comparison Regression_. R package version 1.3.3, 2023. https://CRAN.R-project.org/package=mcr.
Gamer M, Lemon j: irr: Various Coefficients of Interrater Reliability and Agreement_. R package version 0.84.1, 2019. https://CRAN.R-project.org/package=irr.
R Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2014. URL http://www.R-project.org/.
Acknowledgements
We are grateful to the abattoir managers who agreed to perform claw trimming and examinations within the abattoirs.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors declare that no funds, grants, or other support were received during the preparation of this manuscript. Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
SES contributed to the study design, data collection, statistical analysis, interpretation, and writing of the manuscript. WR contributed to the study design, data interpretation, and writing of the manuscript. MA and MAH contributed to the study design, data collection, and interpretation. SAE and EFE contributed to the study design and data interpretation. AM contributed to data collection and interpretation. TWM contributed to the study design and interpretation. AM contributed to study design, data collection, interpretation, and writing of the manuscript. All the authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Zagazig University Veterinary and Agriculture Research Ethics Committee (ZU-IACUC/2/F/450/2022). Informed consent was not required for this study. All methods were performed in accordance with the relevant guidelines and regulations. The methods used were reported in accordance with the STROBE guidelines (http://www.strobe-statement.org) for observational studies.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Claw examination sheet.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Salem, S.E., Refaai, W., Abd EL Raouf, M. et al. An abattoir study of the prevalence of foot lesions and claw measurements in water buffalo in Egypt. BMC Vet Res 20, 29 (2024). https://doi.org/10.1186/s12917-024-03877-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-024-03877-4