Abstract
The practice of routine gastric residual aspiration in preterm infants remains controversial, with conflicting evidence regarding its impact on necrotizing enterocolitis (NEC). As front-line caregivers, nurses play a vital role in gastric aspiration procedures and must be informed by evidence. This quasi-experimental nursing study aimed to assess whether gastric aspiration is clinically relevant in reducing the risk of NEC in preterm infants.
A total of 250 preterm infants from two NICUs in Egypt were allocated to the gastric aspiration (n = 125) and non-aspiration (n = 125) groups. Feeding practices, gastric residuals, and incidence/severity of NEC were compared between groups according to modified Bell’s criteria. Risk factors were analyzed using multivariate regression. There were no significant baseline differences between the groups. The gastric residual attributes and feeding outcomes did not differ substantially from aspiration. The overall incidence of NEC was 14–15%, with no significant differences in the odds of onset or progression of NEC by stage between the groups. Lower gestational age and birth weight emerged as stronger predictors of NEC. Routine gastric aspiration does not appear to directly prevent or reduce the severity of NEC in this population. Although gastric residuals retain clinical importance, study findings question assumptions that aspiration protects against NEC and informs nursing practice. Evidence-based feeding protocols must continually evolve through ongoing research on modifiable risk factors for this devastating intestinal disease in preterm infants.
Similar content being viewed by others
Introduction
Preterm birth, defined as delivery before 37 weeks of gestation, poses a significant challenge in neonatal nursing due to underdeveloped organ systems and increased susceptibility to complications [1,2,3,4]. The care of preterm infants requires a collaborative, interdisciplinary approach involving neonatal nurses, physicians, and other healthcare professionals to optimize outcomes and mitigate the risk of complications. The immature organ systems of preterm infants leave them vulnerable to a myriad of complications, necessitating specialized care in neonatal intensive care units (NICUs) [5,6,7,8].
Among the many challenges faced by preterm infants, achieving optimal nutrition is recognized as a critical factor in ensuring their growth, development, and general health [9,10,11,12,13,14]. However, providing adequate nutrition is complicated by the prevalence of feeding difficulties in this population [15, 16], such as poorly coordinated sucking and swallowing reflexes [17], as well as the looming risk of serious complications, particularly necrotizing enterocolitis (NEC) [5, 18,19,20,21,22]. In addition to poorly coordinated sucking and swallowing reflexes, preterm infants often experience feeding intolerance, which can manifest as increased gastric residuals and may prompt the use of gastric residual aspiration to assess feeding readiness and prevent complications [23].
NEC is a life-threatening gastrointestinal emergency that disproportionately affects preterm infants, with potentially devastating consequences [15, 24]. The condition is characterized by inflammation and necrosis of the intestinal tissue, leading to high rates of morbidity and mortality [25,26,27]. Despite advances in neonatal care, the precise etiology of NEC remains elusive [28,29,30,31], although several risk factors have been identified, including prematurity, formula feeding, and aberrant gut microbial colonization [23, 32]. The complex multifactorial nature of NEC underscores the importance of early detection and prompt intervention to mitigate its impact on preterm infants [33, 34]. In this context, neonatal nurses play a crucial role in preventing and managing NEC through meticulous monitoring, clinical evaluation, and implementing evidence-based feeding protocols [35,36,37].
Nurses in NICUs are familiar with the prevalence of NEC and its significant effects on the care and results of premature infants [38,39,40]. Routine practices such as measuring the residual volume of the stomach (GRV) for the diagnosis and prevention of complications associated with NEC highlight the crucial and practical role of nursing in neonatal care [28,29,30,31]. One of the most common practices in NICUs around the world is the routine aspiration of gastric residuals prior to feeding as a means of assessing feeding tolerance, securing feeding tube placement, and preventing potential complications [1, 41,42,43,44,45]. This procedure involves aspiration of the contents of the stomach through a feeding tube to assess the volume and characteristics of the residuals, which have been traditionally used to guide feeding decisions, despite ongoing debate about their clinical significance and reliability as indicators of digestive function and feeding readiness [42, 46, 47].
A growing body of research has sought to elucidate the relationship between gastric residual aspiration and the development of NEC, producing contradictory and inconclusive results [48, 49]. Some studies suggest that routine aspiration of gastric residuals can disrupt the delicate balance of the develo** gut microbiome, potentially increasing the risk of NEC [15, 50,51,52,53,54,55]. On the contrary, other investigations have failed to demonstrate a significant association between gastric residual aspiration and the incidence of NEC [54, 56]. This lack of consensus within the scientific community highlights the urgent need for more research to clarify the role of gastric residual aspiration in the pathogenesis and prevention of NEC [57,58,59].
Beyond clinical practice, this research has major implications for preterm infant nursing education and policy. This study’s contribution to stomach residual aspiration and NEC understanding helps change the curriculum of neonatal nursing programs, ensuring future nurses have the latest and most evidence-based procedures. This research can also influence clinical recommendations and methods in NICUs worldwide to standardize NEC prevention and care. In summary, this study is crucial to understanding the complex link between residual gastric aspiration and NEC in preterm infants. This research fills a gap in the literature to clarify how this frequent practice contributes to a potentially fatal condition. Our objective is to improve evidence-based neonatal care and provide preterm infants with the best treatment to support their growth, development, and well-being through a comprehensive and rigorous approach. Neonatal nurses must endeavor to understand the problems faced by our most fragile preterm infants, and this study is an essential step toward knowledge and excellence in care.
Materials and methods
Research question
Is there a difference in the incidence and severity of necrotizing enterocolitis (NEC) between preterm infants who undergo routine gastric residual aspiration and those who do not?
Hypothesis
Our central hypothesis is that gastric residual aspiration in preterm infants could influence the incidence of NEC. Specifically, we postulate that:
H1. Preterm Infants who undergo routine gastric residual aspiration have a reduced risk of develo** NEC compared to those who do not undergo aspiration.
H2. Routine assessment of gastric residuals may not provide significant clinical benefits in predicting or preventing complications such as NEC.
Design
A quasi-experimental design was used to achieve the objective of the study. Quasi-experiments aim to estimate the causal effects of an intervention on the target population without randomly assigning subjects to a group [60].
Settings
The first NICU is located at Cairo University Children’s Hospital (El Monira). With a capacity of 40 incubators, it offers complimentary advanced neonatal care to infants throughout Egypt. This unit is segmented into an intermediate care area that houses 15 incubators for secondary treatment and an intensive care section with 25 incubators dedicated to tertiary care. Additional amenities include isolation chambers, breastfeeding support, and outpatient clinics.
On the contrary, the second NICU is located on the fourth floor of the maternity wing of El Manial University Hospital. This unit, equipped with 35 incubators, also provides state-of-the-art neonatal care. It features an intermediate care section with 15 incubators, two intensive care zones (each containing 5 incubators) designed for diverse and infected neonates, immediate postnatal care with 10 incubators, a designated breastfeeding area, and a medication preparation facility.
Sample
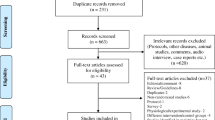
A priori power analysis was performed to determine the target sample size needed to detect a significant difference in the incidence of NEC between the gastric aspiration and non-aspiration groups. Based on previous studies, the incidence of NEC in preterm infants is approximately 10% [61, 62]. We hypothesized that the gastric aspiration intervention could reduce this incidence by 3%, which would be clinically significant given the available sample size. With a power of 80%, an alpha of 0.05, and the recruited sample size of 125 infants per group (250 infants in total), the study is sufficiently powered to detect a 3% reduction in the incidence of NEC between the groups. Power analysis was conducted using G*Power software (version 3.1.9.7) [63].
The software calculated a total sample size of 236 infants, rounded to 250 to account for potential attrition. Therefore, this study aimed to recruit a convenience sample of 250 preterm infants admitted to the NICU of El Manial University Hospital and Elmonira Pediatric Hospital, with a target of 125 infants assigned to each study group. This sample size provides adequate statistical power to detect a clinically significant difference of 3% in the incidence of NEC between the gastric residual aspiration and non-aspiration groups.
- The allocation procedure was as follows:
As infants were admitted to the NICU, they were screened for eligibility based on the predefined inclusion and exclusion criteria. Eligible infants underwent a 48-hour observation period before enrollment. After obtaining informed parental consent, the enrolled infants were allocated to either the gastric aspiration or non-aspiration group as they were recruited. The allocation was quasi-random based on the order of admission to the NICU, assigning approximately half to each study group in an alternating fashion throughout the recruitment period. For example, the first eligible enrolled infant was allocated to the aspiration group, the second to the non-aspiration group, the third to the aspiration group, and so on until the target sample size was achieved in both groups. This allocation order was not completely random but intended to distribute interventions evenly across the recruitment timeframe. The final group allocation was 125 infants in the gastric residual aspiration group and 125 infants in the non-aspiration group.
Eligibility criteria
Inclusion criteria
Subjects were considered if they were preterm infants born at less than 37 weeks of gestation receiving tube feeding (orogastric or nasogastric).
While the inclusion criteria encompassed all preterm infants born before 37 weeks, it is important to note that the study population primarily consisted of infants with lower gestational ages, as evidenced by the reported mean gestational age of 28.5 weeks. This is likely due to the fact that infants with lower gestational ages are more likely to require nasogastric feeding and are at a higher risk of develo** feeding-related complications, such as necrotizing enterocolitis. It is important to note that the risk of develo** NEC is inversely related to gestational age, with infants born at lower gestational ages being at a higher risk compared to those born at later gestational ages [64,65,
Table 7 explores the association between feeding practices and the development of necrotizing enterocolitis (NEC) in preterm infants who underwent residual gastric aspiration compared to those who did not. The percentage of infants with oral initial feeding (60% vs. 58%), time to full feed > 5 days (35% vs. 33%), use of formula milk use (60% vs. 58%), and feeding problems (30% vs. 28%) were similar between the two groups. The odds ratios for these feeding parameters were close to 1, and the p-values were > 0.05, indicating that there were no statistically significant differences in the association between feeding practices and the development of NEC based on the practice of gastric residual aspiration. These findings suggest that the feeding practices examined in this study did not significantly influence the relationship between gastric residual aspiration and NEC in preterm infants.
Discussion
This quasi-experimental study offered valuable information on the relationship between routine gastric residual aspiration and necrotizing enterocolitis (NEC) development in preterm infants. The findings suggest that aspiration of gastric residuals before feeding may not directly alter the risk or severity of NEC.
Feeding practices and residual characteristics
A thorough analysis of the core of the study revealed fascinating insights into food practices and residual gastric properties. The mere act of gastric residual aspiration does not appear to profoundly affect feeding behavior, residual volume, or consistency. Our findings are in contrast to those of [69], who found that routine gastric residual aspiration was associated with a delay in the time to reach full enteral feeding in preterm infants. In their randomized controlled trial, infants in the aspiration group took longer to achieve full feeds compared to those in the no-aspiration group (median 11 days vs. 9 days, p = 0.01). The authors suggested that the practice of routine gastric residual aspiration may disrupt the normal gastrointestinal tract development and lead to feeding intolerance.
Residual gastric aspiration could affect the progression of feeding in preterm infants. On the other hand, our findings are consistent with those of [50], who found no significant differences in feeding practices between infants undergoing gastric residual aspiration and those who did not. Although it is tempting to view gastric residuals as mere indicators of food intolerance, one might wonder if their role extends beyond this. They may be early signs or precursors to more severe conditions such as NEC.
No difference in NEC incidence with aspiration
Our central finding was that the incidence of NEC did not differ significantly between the gastric aspiration and non-aspiration groups. Approximately 15% of the infants developed some stage of NEC in both arms. Previous studies have been inconsistent, with some noting a higher NEC with aspiration [61, 65] while others did not find any difference [50,51,52,53]. Although the multivariate analysis (Table 5) suggested a possible association between gastric residual aspiration and NEC, with an odds ratio of 1.20 (95% CI: 0.98–1.47), the relationship did not reach statistical significance at the conventional 0.05 level (p = 0.08). It is important to note that the confidence interval for the odds ratio was wide and included 1, indicating that the true effect size could be smaller or larger than the point estimate. Furthermore, the interpretation of our results should be made with caution, given the limitations of our study design and sample size. Larger, well-controlled studies are needed to more definitively establish the relationship between gastric residual aspiration and NEC in preterm infants. While our findings suggest a possible association, they do not provide conclusive evidence that routine aspiration intrinsically increases the risk of NEC.
However, the etiology of NEC is complex. Although aspiration did not emerge as an independent risk factor in our regression analysis, elements such as lower gestational age and birth weight did. This agrees with [70], confirming the multifactorial nature of NEC. Our findings add to the evidence that routine gastric aspiration does not appear to directly precipitate the onset of NEC in preterm infants, but many factors are at play.
No difference in NEC severity with aspiration
Furthermore, we analyzed the outcomes of NEC by stage severity. The percentages of infants with mild, moderate, or advanced NEC were comparable. Previous studies have not examined the severity in detail [51, 69]. This suggests that routine aspiration practice may not worsen progression or exacerbate NEC once present. However, larger samples are needed to confirm this due to the low frequency of advanced NEC.
The lack of difference in NEC severity between the aspiration and non-aspiration groups suggests that routine gastric residual aspiration may not be an effective strategy for mitigating the severity of NEC once it develops. This finding may have implications for the management of preterm infants with suspected or confirmed NEC.
No major impact of clinical outcomes on aspiration
Longitudinal stay, readmission, mortality, and other complications were similar regardless of gastric aspiration. Some researchers have proposed that aspiration can delay feeding progress and prolong hospitalization [71]. However, we found minimal differences in clinical outcomes. However, no long-term follow-up was conducted; potential outcomes remain unknown.
The mean length of stay in the NICU of approximately 27–28 days for infants with a mean gestational age of 28.5 weeks in our study is shorter than what has been reported in some other studies. This difference may be attributed to variations in clinical practices, discharge criteria, and the availability of local resources and support services. It is important to note that the length of stay reported in our study represents only the initial hospital stay and does not include any subsequent home care or readmissions. Future studies should consider exploring factors that influence the duration of hospital stay for preterm infants in different settings and the potential impact of these variations on long-term outcomes. While shorter hospital stays may be beneficial for infants and their families, it is crucial to ensure that early discharge does not compromise the provision of appropriate care and support for preterm infants at risk of develo** NEC or other complications.
No difference in residual volume/frequency with aspiration
Interestingly, residual volume and frequency did not differ significantly between the aspiration and non-aspiration groups. Some have hypothesized that aspiration could alter gastric motility and residuals [72], but we did not find significant effects. A minimal comparison of residuals between groups has been studied. While aspiration did not appear to impact residual characteristics, the clinical utility of measuring volumes warrants further scrutiny.
Limitations, future directions, and contrasting perspectives
Our study has several limitations that should be considered when interpreting the results. The quasi-experimental design, which lacks randomization, may have introduced selection bias and limited the generalizability of our findings. Furthermore, our study may have been subject to other biases, such as patient variability and nurses’ expertise in performing routine gastric aspiration. To address these limitations, future studies should employ randomized patient selection, standardized protocols for gastric residual aspiration, and stratification of patients based on key characteristics.
Although our study sheds light on the potential non-significance of gastric residual aspiration in the context of NEC, it is paramount to approach this insight with caution. The multifaceted nature of NEC underscores the likelihood that a confluence of factors, rather than a singular determinant, dictates its onset and progression. Moreover, while our findings are comprehensive, they do not provide a conclusive argument for the clinical relevance of gastric residual aspiration. Some might argue that, while aspiration may not directly influence NEC, its indirect implications, such as affecting the gut microbiota, cannot be overlooked. Future studies should also explore the potential indirect effects of gastric residual aspiration on the development of NEC, such as its impact on the gut microbiome and immune function, as these factors may play a crucial role in the pathogenesis of NEC.
The importance of evidence-based feeding protocols in reducing the incidence of NEC in preterm infants has been highlighted in a recent systematic review by Jasani and Patole [73]. The authors found that standardized feeding regimens, which include strategies such as slow advancement of enteral feeds and the use of human milk, can significantly reduce the risk of NEC in this vulnerable population. Our findings and the evidence presented in this systematic review underscore the need for neonatal healthcare providers to adopt and adhere to evidence-based feeding protocols to improve outcomes for preterm infants.
In conclusion, while our study suggests that routine gastric aspiration may not alter NEC or clinical outcomes in preterm infants, we caution against generalized conclusions given the multifaceted nature of NEC etiology. More data are needed to shape evidence-based feeding guidelines and improve neonatal care.
Conclusions
This quasi-experimental study provides valuable information on the relationship between gastric residual aspiration and necrotizing enterocolitis (NEC) among preterm infants. Our findings suggest that aspirating gastric residuals before feeding may not substantially alter the risk or severity of develo** NEC. Several crucial observations support this conclusion. First, the gastric residual aspiration and non-aspiration groups in this study exhibited remarkably similar baseline characteristics. This homogeneity in gestational age, birth weight, feeding practices, and other variables lends credibility by minimizing potential confounders. Furthermore, the mere act of aspirating gastric residuals did not appear to profoundly affect residual volume, consistency, feeding progression, or other results. More importantly, the overall incidence and severity of the staging of NEC were comparable between the two groups.
Our regression analysis revealed that lower gestational age and birth weight played a more pronounced role in NEC development than gastric aspiration. This is consistent with other recent research demonstrating the complex and multifactorial nature of the etiology of NEC. While our study provides evidence to downplay the importance of routine gastric aspiration, it is only one piece of the complex puzzle behind the pathogenesis of NEC. Based solely on these findings, we cannot conclusively determine that gastric residuals lack clinical utility. However, this research highlights the need to holistically examine the multiple risk factors contributing to NEC using robust multicenter studies. In summary, although gastric residuals remain important clinical markers, our findings call into question the premise that routine aspiration directly reduces the risk of NEC in preterm infants. This has implications for the development of evidence-based nutrition guidelines. More research is needed incorporating advances in neonatal care to deepen our understanding of the etiology and prevention of NEC.