Abstract
Background
Lysyl oxidase-like 2 (LOXL2) belongs to a family of the LOX secretory enzyme, which involves the cross-linkage of extracellular matrix (ECM) proteins. Here, we aimed to analyze the correlation between serum LOXL2 and pelvic adhesion in chronic pelvic inflammatory disease (PID).
Methods
A total of 143 patients with PID and 130 healthy controls were included in this study. The serum levels of LOXL2 were measured using enzyme-linked immunosorbent assay (ELISA) kits. The patients were divided into non-adhesion group (102 cases) and adhesion group (41 cases).
Results
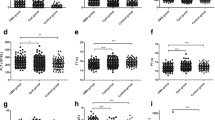
It was found that the serum level of LOXL2 expression was elevated in PID patients compared with healthy controls, and was elevated in PID patients with pelvic adhesion compared to patients without adhesion. In all PID patients, serum LOXL2 level was positively correlated with matrix metalloproteinases-9 (MMP-9), transforming growth factor-β (TGF-β1), whole blood viscosity (WBV) at low shear rate (LSR), WBV at high shear rate (HSR), and hematocrit (HcT). Multivariate logistic regression analysis showed that serum LOXL2 level was an independent risk factor for pelvic adhesion in PID patients (OR = 1.058; 95% CI = 1.030–1.086, P < 0.001).
Conclusions
Serum LOXL2 level not only predicts the presence of PID, but serum LOXL2 concentration is also associated with the presence of pelvic adhesions.
Similar content being viewed by others
Background
Pelvic inflammatory disease (PID) is an inflammatory disease caused by infection of microorganisms in the upper genital tract, such as endocervix, endometrium, fallopian tubes and ovaries. PID is characterized by infiltration of neutrophils and T-lymphocytes in the inflammatory lesions and a high neutrophil cell count in blood. PID can be divided into different types based on manifestations, such as endometritis, sal**itis and peritonitis [1]. Chronic infection and inflammation in PID can lead to several sequelae, such as chronic pelvic pain, endometriosis, and ectopic pregnancy [2]. Furthermore, genital tract infections lead to oviduct damage and its healing process involves scarring and adhesion formation, and this pelvic adhesion consequently increases the risk of tubal factor infertility and ectopic pregnancy [3, 26], and endometriosis is also associated with pelvic adhesion and fibrosis [27, 28]. Therefore, we speculate that LOXL2 may also play an important role in the formation of pelvic adhesion and fibrosis in PID, and more studies are needed to validate this hypothesis in PID tissue and animal model.
A large quantity of serum fibrotic markers has been found as possible biomarkers for PID, and our study showed that serum MMP-9, TGF-β1 and ICAM-1 levels were markedly higher in PID patients with pelvic adhesion. MMP-9 is a member of matrix metalloproteinases (MMPs), and is a key proteinase involved in normal matrix remodeling. MMPs can be produced by inflammatory cells to repair damaged ECM, while excessive MMPs can also promote the ECM degradation [29]. The level of plasma MMP-9 was elevated in PID patients compared with healthy controls [30]. TGF‑β1 signaling pathway is implicated in the fibrosis process, and serum TGF‑β1 level was increased in rats with chronic pelvic inflammatory disease (CPID) [31]. Our study supports this report in PID patients and further found its association with pelvic adhesion. TGF-β has potent fibrotic activity as TGF-β can induce endometrial fibrosis in human endometrial carcinoma cells [32]. ICAM-1 is a adhesion molecule that stimulates leukocyte adhesion. Serum ICAM-1 levels were higher in endometriosis patients compared to healthy women [33]. ICAM-1 can also promote human endometriotic stromal cells proliferation and adhesion [34]. Our previous study showed that ICAM-1 expression in uterus or fallopian tube were markedly increased in PID mice [35]. This study added MMP-9, TGF-β1 and ICAM-1 as new serum biomarkers of PID patients, and further showed their positive correlations with LOXL2. MMP-9 and TGF-β1 are both downstream molecules of LOXL2 in matrix remodeling and fibrogenesis of fibrosis associated diseases [8, 36]. Whether and how LOXL2 modulates MMP-9 and TGF-β1 remain totally unknown in PID and deserves further investigation.
There were some limitations to the study. Firstly, the sample size is small and our findings should be validated in study with larger sample. Secondly, the source of serum LOXL2 in PID patients is still unclear, and should be confirmed in biopsy tissue of PID patients. Thirdly, as an indicator for pelvic adhesion, prospective studies should be conducted to observe the predictive effect of LOXL2 on progression and recurrence of pelvic adhesion. Fourthly, an animal model of PID is needed to elucidate the possible mechanisms of LOXL2 in process of pelvic adhesion and fibrosis.
Conclusions
Serum LOXL2 levels are increased in PID patients, and also strongly correlated with pelvic adhesion, and fibrosis-related markers. Although these relationship are correlative and not causative, LOXL2 may be a disease-driver in pathogenesis of fibrotic process in PID in animal models. Our study provides LOXL2 as a biomarker for prediction of pelvic adhesion in PID patients.
Availability of data and materials
Data and materials are available upon request to the corresponding author.
Abbreviations
- ECM:
-
Extracellular matrix
- ELISA:
-
Enzyme-linked immunosorbent assay
- HcT:
-
Hematocrit
- HSR:
-
High shear rate
- LOX:
-
Lysyl oxidase
- LOXL2:
-
Lysyl oxidase like 2
- LSR:
-
Low shear rate
- MMP-9:
-
Matrix metalloproteinases-9
- PID:
-
Pelvic inflammatory disease
- TCM:
-
Traditional Chinese medicine
- TGF-β1:
-
Transforming growth factor-β
- WBV:
-
Whole blood viscosity
References
Curry A, Williams T, Penny ML. Pelvic inflammatory disease: diagnosis, management, and prevention. Am Fam Physician. 2019;100(6):357–64.
Chayachinda C, Rekhawasin T. Reproductive outcomes of patients being hospitalised with pelvic inflammatory disease. J Obstet Gynaecol. 2017;37(2):228–32.
Tao X, Ge SQ, Chen L, Cai LS, Hwang MF, Wang CL. Relationships between female infertility and female genital infections and pelvic inflammatory disease: a population-based nested controlled study. Clinics (Sao Paulo). 2018;73:e364.
Zhang Q, **ng X, Liu S, **e X, Liu X, Qian F, et al. Intramural ectopic pregnancy following pelvic adhesion: case report and literature review. Arch Gynecol Obstet. 2019;300(6):1507–20.
Liu L, Yang H, Guo Y, Yang G, Chen Y. The impact of chronic endometritis on endometrial fibrosis and reproductive prognosis in patients with moderate and severe intrauterine adhesions: a prospective cohort study. Fertil Steril. 2019;111(5):1002-1010.e2.
Moon HJ, Finney J, Ronnebaum T, Mure M. Human lysyl oxidase-like 2. Bioorg Chem. 2014;57:231–41.
Vallet SD, Ricard-Blum S. Lysyl oxidases: from enzyme activity to extracellular matrix cross-links. Essays Biochem. 2019;63(3):349–64.
Cosgrove D, Dufek B, Meehan DT, Delimont D, Hartnett M, Samuelson G, et al. Lysyl oxidase like-2 contributes to renal fibrosis in Col4α3/Alport mice. Kidney Int. 2018;94(2):303–14.
Sayed N, Khurana A, Saifi MA, Singh M, Godugu C. Withaferin A reverses bile duct ligation-induced liver fibrosis by modulating extracellular matrix deposition: role of LOXL2/Snail1, vimentin, and NFκB signaling. BioFactors. 2019;45(6):959–74.
Chen L, Li S, Li W. LOX/LOXL in pulmonary fibrosis: potential therapeutic targets. J Drug Target. 2019;27(7):790–6.
Zhao Y, Tang K, Tianbao X, Wang J, Yang J, Li D. Increased serum lysyl oxidase- like 2 levels correlate with the degree of left atrial fibrosis in patients with atrial fibrillation. Biosci Rep. 2017;37(6):BSR20171332.
Workowski KA, Berman S, Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(12):1–110.
Sercelik A, Besnili AF. The contribution of whole blood viscosity to the process of aortic valve sclerosis. Med Princ Pract. 2018;27(2):173–8.
Laganà AS, Garzon S, Götte M, Viganò P, Franchi M, Ghezzi F, Martin DC. The pathogenesis of endometriosis: molecular and cell biology insights. Int J Mol Sci. 2019;20(22):5615.
Laganà AS, Triolo O, Salmeri FM, Granese R, Palmara VI, Frangež HB, Bokal EV, Sofo V. Natural Killer T cell subsets in eutopic and ectopic endometrium: a fresh look to a busy corner. Arch Gynecol Obstet. 2016;293(5):941–9.
Laganà AS, Salmeri FM, Vitale SG, Triolo O, Götte M. Stem cell trafficking during endometriosis: May epigenetics play a pivotal role? Reprod Sci. 2018;25(7):978–9.
Ahmad G, Mackie FL, Iles DA, O’Flynn H, Dias S, Metwally M, Watson A. Fluid and pharmacological agents for adhesion prevention after gynaecological surgery. Cochrane Database Syst Rev. 2014;7:CD001298.
Torres-De La Roche LA, Campo R, Devassy R, Di Spiezio Sardo A, Hooker A, Koninckx P, et al. Adhesions and anti-adhesion systems highlights. Facts Views Vis Obgyn. 2019;11(2):137–49.
Puente A, Fortea JI, Cabezas J, Arias Loste MT, Iruzubieta P, Llerena S, et al. LOXL2-A new target in antifibrogenic therapy? Int J Mol Sci. 2019;20(7):1634.
Mahjour F, Dambal V, Shrestha N, Singh V, Noonan V, Kantarci A, et al. Mechanism for oral tumor cell lysyl oxidase like-2 in cancer development: synergy with PDGF-AB. Oncogenesis. 2019;8(5):34.
T** G, White ES, Faiz A, Sicard D, Tschumperlin DJ, Mahar A, et al. Lysyl oxidases regulate fibrillar collagen remodelling in idiopathic pulmonary fibrosis. Dis Model Mech. 2017;10(11):1301–12.
Chien JW, Richards TJ, Gibson KF, Zhang Y, Lindell KO, Shao L, et al. Serum lysyl oxidase-like 2 levels and idiopathic pulmonary fibrosis disease progression. Eur Respir J. 2014;43(5):1430–8.
Fu Q, Bai Y, Liu Y, Zhou J, Zheng Y. The serum level and significance of lysyl oxidase-like 2 in patients with rheumatoid arthritis-associated interstitial lung disease. Clin Rheumatol. 2018;37(1):193–8.
Zhao Y, Tang K, Tianbao X, Wang J, Yang J, Li D. Increased serum lysyl oxidase- like 2 levels correlate with the degree of left atrial fibrosis in patients with atrial fibrillation. Biosci Rep. 2017;37(6):BSR20171332.
Ruiz LA, Báez-Vega PM, Ruiz A, Peterse DP, Monteiro JB, Bracero N, et al. Dysregulation of lysyl oxidase expression in lesions and endometrium of women with endometriosis. Reprod Sci. 2015;22(12):1496–508.
Tai FW, Chang CY, Chiang JH, Lin WC, Wan L. Association of pelvic inflammatory disease with risk of endometriosis: a nationwide cohort study involving 141,460 individuals. J Clin Med. 2018;7(11):E379.
Goldberg JM, Falcone T, Diamond MP. Current controversies in tubal disease, endometriosis, and pelvic adhesion. Fertil Steril. 2019;112(3):417–25.
Ganieva U, Nakamura T, Osuka S, Nakanishi N, Kasahara Y, et al. Involvement of transcription factor 21 in the pathogenesis of fibrosis in endometriosis. Am J Pathol. 2020;190(1):145–57.
Robert S, Gicquel T, Victoni T, Valença S, Barreto E, Bailly-Maître B, et al. Involvement of matrix metalloproteinases (MMPs) and inflammasome pathway in molecular mechanisms of fibrosis. Biosci Rep. 2016;36(4):e00360.
Wang PH, Tsai HT, Tee YT, Lin LY, Yang SF, Hsieh YS. Significant elevation of plasma matrix metalloproteinase-9 level and its ratio to matrix metalloproteinase- 2 in patients with pelvic inflammatory disease. Fertil Steril. 2009;92(5):1679–84.
Zhang LJ, Zhu JY, Sun MY, Song YN, Rahman K, Peng C, et al. Anti-inflammatory effect of Man-Pen-Fang, a Chinese herbal compound, on chronic pelvic inflammation in rats. J Ethnopharmacol. 2017;208:57–65.
Lee CJ, Hong SH, Yoon MJ, Lee KA, Choi DH, Kwon H, et al. Eupatilin treatment inhibits transforming growth factor beta-induced endometrial fibrosis in vitro. Clin Exp Reprod Med. 2020;47(2):108–13.
Li R, Qiu Y. Diagnostic value of serum ICAM-1 for endometriosis: a meta-analysis. Medicine (Baltimore). 2018;97(31):e11760.
Kim KH, Lee EN, Park JK, Lee JR, Kim JH, Choi HJ, et al. Curcumin attenuates TNF-α-induced expression of intercellular adhesion molecule-1, vascular cell adhesion molecule-1 and proinflammatory cytokines in human endometriotic stromal cells. Phytother Res. 2012;26(7):1037–47.
Tang B, Wu K, Meng Q, Wang F. Comparison of the analgesic and anti- inflammatory effects of xiaoyuningkun decoction with Cynanchum paniculatum and Fukeqian** in a mouse model of pelvic inflammatory disease. Med Sci Monit. 2019;25:9094–102.
Wen X, Liu Y, Bai Y, Li M, Fu Q, Zheng Y. LOXL2, a copper-dependent monoamine oxidase, activates lung fibroblasts through the TGF-β/Smad pathway. Int J Mol Med. 2018;42(6):3530–41.
Acknowledgements
Not applicable.
Funding
This study was supported by Leading talents of health system in Pudong New Area (No. PWR12019-04) and Key subjects of TCM gynecology in Pudong New District (No. PWZxk2017-03). The funders provided financial support for the data collection and analysis of this study.
Author information
Authors and Affiliations
Contributions
Chan **e performed the experiment, collected data and wrote the manuscript; Bixin Tang designed the study and revised the manuscript; Kunlun Wu and Qingyi Meng performed the experiment and analyzed the data. Fang Wang performed the statistical analysis and revised the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The medical ethics committee of Shanghai Pudong New Area Gongli hospital approved this study protocol, and informed consent was obtained from all patients. All the experimental protocol for involving humans was by the guidelines of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interest
The authors declared no conflict of interest with other people or organizations.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
**e, C., Tang, B., Wu, K. et al. Increased serum LOXL2 concentration in pelvic inflammatory disease with pelvic adhesion. BMC Women's Health 22, 59 (2022). https://doi.org/10.1186/s12905-022-01640-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-022-01640-1