Abstract
Background
The development of synchronous multiple primary cancers is one of the major causes of death in patients with head and neck cancer. Herein, we report a case of synchronous intraductal papillary mucinous carcinoma (IPMC), invasive in a patient with maxillary gingival carcinoma.
Case presentation
A 73-year-old female visited our hospital complaining of a mass on the left side of the maxillary gingiva. Intraorally, an exophytic tumor, 50 × 25 mm in size, was found on the gingiva of the left maxillary posterior, and a diagnosis of squamous cell carcinoma was revealed by cytology. Emission tomography/ computed tomography with 18 Fluorodeoxyglucose-Positron (18FDG- PET/ CT) showed increased accumulation in the left maxillary gingiva, the left side of cervical lymph nodes, and the main pancreatic duct. The pancreatic ductal tumor was performed the biopsy at esophagogastroduodenoscopy (EGD) and resulted in a pathological diagnosis of IPMC, invasive. The patient was diagnosed as synchronous double primary cancers consisting of maxillary gingival carcinoma cT4aN2bM0 and IPMC, invasive cT3N0M0. She refused radical treatment, and died 11 months later.
Conclusion
18FDG- PET/ CT, EGD and multidisciplinary approach is required for the detection and determining the treatment strategy of synchronous double primary cancers.
Similar content being viewed by others
Background
Head and neck squamous cell carcinoma (HNSCC) is the sixth most prevalent cancer in the world [1], with a high rate of development of multiple primary carcinomas [2]. Multiple primary cancers were defined by Warren et al. as the following: 1) each tumor shows a certain malignant image, 2) each tumor is located in mutually distant sites, and 3) one is not a metastasis of the other [3]. Moertel et al. defined multiple primary cancers as being synchronous if they are detected within 6 months of the primary cancer [4]. The presence of synchronous multiple primary cancers is a major clinical problem and a leading cause of death in patients with head and neck cancer [5].
It has been reported that synchronous multiple primary cancers are highly prevalent in the head and neck region, upper gastrointestinal tract, and lungs but rare in the pancreas [6, 7]. Pancreatic carcinoma includes the following histological subtypes: pancreatic ductal adenocarcinoma, intraductal papillary mucinous carcinoma (IPMC), invasive, acinar cell carcinoma, neuroendocrine carcinoma. Among these subtypes, adenocarcinoma contributes to about 85% of primary pancreatic malignancies. IPMNs are characterized by papillary growths in the pancreatic ducts that produce mucus. They can be benign or progress to become malignant during the course of the disease, leading to high-grade dysplasia (HGD) or IPMC, invasive. Depending on the location of origin, IPMNs can be classified as main pancreatic ductal (MD-IPMN), branched ductal (BD-IPMN), or mixed types. The Fukuoka consensus criteria provide different parameters for surgical resection according to the classification of the IPMNs [8, 9].
Here we report a case of synchronous multiple primary cancers with maxillary gingival cancer and MD-IPMC.
Case presentation
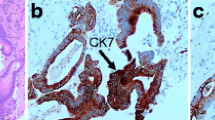
A 73-year-old female visited a local dental clinic complaining of a mass on the left side of the maxillary gingiva and was referred to our hospital in August 2020. Although the patient reported an unremarkable medical history, a blood test revealed an HbA1c of 6.9%, suggesting a diagnosis of diabetes mellitus. Extraorally, slightly enlarged lymph nodes were palpable bilaterally in the neck. Intraorally, an exophytic tumor, 50 × 25 mm in size, was found on the gingiva of the left maxillary posterior (Fig. 1). Contrast-enhanced computed tomography (CT) showed a mass with irregular bony destruction and contrast effects in the left side of the maxilla, extending to the base of the pterygoid muscle and the pterygoid process. Lymph nodes with internal defects were found in the left level IB and IIA area, suggesting multiple lymph node metastasis. However, there were no evidence of extranodal extension due to the defined marginal morphology. (Fig. 2). Contrast-enhanced magnetic resonance imaging (MRI) showed a mass in the left maxillary gingiva extending beyond the maxillary nodule to the base of the pterygoid process and the masticatory muscle space (Fig. 3). Emission tomography/ computed tomography with 18 Fluorodeoxyglucose-Positron (18 FDG-PET/CT) showed increased accumulation in the left maxilla (SUVmax 32.5) and the level IB (SUVmax 18.5) and level IIA (SUVmax 4.2) left cervical lymph nodes. In addition, there was an increased accumulation in the head of the pancreas (SUVmax 6.2) and the body of the pancreas (SUVmax 7.4), accompanied by a dilatation of the main pancreatic duct (MPD) (Fig. 4). No metastasis to the paratracheal lymph nodes or other organs was detected. The patient was referred to the Department of Gastroenterology for a detailed examination of the pancreatic tumor, and contrast-enhanced abdominal CT and MRI examinations were performed. A neoplastic lesion with a maximum diameter of 82 mm was observed in the pancreatic head. The tumor was in contact with the Vater papilla and the descending leg of the duodenum; it was suspected that the tumor had invaded the duodenum (Fig. 5). Esophagogastroduodenoscopy (EGD) showed that the tumor had indeed invaded the descending duodenum and perforated the intestinal wall (Fig. 6); a specimen was collected from the same area. Histopathologically, the diagnosis was mucinous carcinoma (Fig. 7). In combination with the imaging findings, the final diagnosis was cT3N0M0, StageIII MD-IPMC. On the other hand, cytodiagnosis of the tumor in the maxillary gingiva revealed SCC (Fig. 8), with a final diagnosis of cT4bN2bM0, Stage IVB SCC, based on the staging described by the 8th edition of Unio Internationalis Contra Cancrum.
We explained the necessity of histopathological examination for treatment planning and proposed resection of the left maxilla and radical neck dissection followed by resection of the pancreatic tumor, but the patient refused this treatment plan; thus, we pursued a supportive care strategy. The patient underwent palliative irradiation of 30 Gy/10Fr in the oral cavity and was subsequently followed up at another hospital. She died 11 months after her initial diagnosis due to respiratory failure caused by multiple lung metastases.
Discussion and conclusions
HNSCC patients present with a 5.3% occurrence of synchronous multiple cancers [10]. Based on the concept of “field cancerization”, HNSCC is considered a risk factor for multiple primary cancers in the head and neck, lung, and esophagus [11]. A previous review of HNSCC reported that the cumulative incidence of heterogeneous second primary cancers was 7.2% at six months, 17.9% at five years, and 23.1% at 10 years [7]. In this case, IPMC was found as a synchronous second primary cancer. The occurrence of synchronous cancer of the pancreas in patients with head and neck carcinoma is exceedingly rare, and to our knowledge, cases of multiple primary cancers with maxillary gingival carcinoma and MD-IPMC have not been reported. 18FDG-PET/CT and EGD are useful tools in the detection of multiple primary cancers in HNSCC patients. The sensitivity and specificity of 18FDG-PET/CT in the search for distant metastases and multiple cancers in HCSCC patients are very high, at 0.888 and 0.971, respectively [12]. The sensitivity of 18FDG-PET/CT in the detection of pancreatic cancer is also high, at 89.1% [13]. Even when limited to IPMNs, 18FDG-PET/CT has a sensitivity and specificity of 96.8 and 91.1%, respectively, demonstrating significant utility as a diagnostic tool [14]. Kamegaka et al. reported a triple cancer case; cancers in the middle part of the extrahepatic bile duct, the pancreas head, and the supraglottis [15]. The case was diagnosed with supraglottic carcinoma with hoarseness as the main complaint, and subsequently 18FDG-PET/CT examination was performed, which revealed the extrahepatic bile duct cancer and the pancreas head cancer. In a study comparing the frequency of detection of multiple cancers by 18FDG-PET/CT and EGD, 18FDG-PET/CT showed superior results, but it is considered that both are necessary because early esophageal and gastric cancers are difficult to be detected by 18FDG-PET/CT due to low FDG uptake [16, 17].
The frequency of invasive carcinoma or HGD in MD-IPMN is 61.6% (range, 36–100%) and the frequency of invasive carcinoma is 43.1%, while the mean frequency of invasive carcinoma or HGD in BD-IPMN is 25.5% (range, 6.3–46.5%) and the mean frequency of invasive carcinoma is 17.7% (range 1.4–36.7%) [8, 9]. In the case of MD-IPMN, surgical resection is highly recommended in the presence of high-risk stigmata such as MPD dilation over 10 mm, cystic lesions of the pancreatic head with obstructive jaundice, and the presence of a solid component with the contrast-enhancement [8, 9]. MPD dilation of 5–9 mm is considered to be a "worrisome feature" warranting close examination and follow-up rather than immediate resection [8, 9]. Although the treatment strategy differs depending on the site of origin, tumor diameter, invasion of surrounding tissues, and presence or absence of lymph node metastasis in the pancreas, patients may undergo pancreaticoduodenectomy, caudal pancreatectomy, or total pancreatectomy. For IPNC that are surgically resected at an early stage (T1N0), the 5-year survival rate is about 40%, and for IPMC that are more advanced or have lymph node metastases, the survival rate is about 20% [18]. This is comparable to the 5-year survival rate of about 27% in pancreatic adenocarcinoma patients who underwent surgical resection and postoperative adjuvant chemotherapy [19]. Although the mechanism is unknown, it has been suggested that the occurrence and malignancy risk of IPMN are related to diabetes mellitus, and this case also showed diabetes mellitus [20, 21]. In addition to diabetes mellitus, family history in the first degree, chronic pancreatitis, and history of insulin use have also been associated as risk factors, and these cases require careful follow-up.
In this case, the patient had a maxillary gingival carcinoma corresponding to T4bN2b in the head and neck region. Previous studies have reported 5-year survival rates of 25.4–41.0% for T4 patients who underwent surgery and 20.5–36.9% for those with positive cervical lymph nodes [22,23,24,25]. Although a multidisciplinary approach is required in determining the treatment strategy for patients with synchronous multiple cancers, treatment decisions should be guided on the following three principles [26]:
-
1)
Give priority to high-grade cancers.
-
2)
Treat more advanced stages of cancer first. If it is difficult to cure the more advanced cancer, the secondary cancer may not warrant treatment.
-
3)
Simultaneous treatment should be completed when possible.
Treatment outcomes have been reported in cases of head and neck cancer with synchronous esophageal carcinoma [27, 28]. Panosetti et al. have shown that synchronous carcinoma has a lower survival rate than metachronous carcinoma (18% vs. 55%), and radical or curative treatment of head and neck cancer cases complicated by IPMC, invasive is extremely difficult [29]. In this case, the maxillary gingival carcinoma was more advanced than the IPMC; hence, resection of the left maxilla and radical neck dissection followed by resection of the pancreatic carcinoma was treatment planned, but the patient declined and requested palliative care only. Although the prognosis of IPMC, invasive is better than that of pancreatic adenocarcinoma, the prognosis of stage IVB maxillary gingival cancer is poor.
In conclusion, it is important to identify synchronous multiple primary carcinomas in the treatment of HNSCC, and 18FDG-PET/CT is quite useful. Furthermore, it follows that a multidisciplinary approach is needed for these cancers.
Availability of data and materials
We include all data supporting the findings in the manuscript.
Abbreviations
- HNSCC:
-
Head and neck squamous cell carcinoma
- IPMNs:
-
Intraductal papillary mucinous neoplasms
- IPMC:
-
Intraductal papillary mucinous carcinoma
- HGD:
-
High-grade dysplasia
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- 18FDG-PET/CT:
-
Emission tomography/ computed tomography with 18 Fluorodeoxyglucose-Positron
- MPD:
-
Main pancreatic duct
- EGD:
-
Esophagogastroduodenoscopy
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917.
Vikram B. Changing patterns of failure in advanced head and neck cancer. Arch Otolaryngol. 1984;110:564–5.
Warren S, Gates O. Multiple primary malignant tumors: a survey of the literature and statistical study. Am J Cancer. 1932;16:1358–414.
Moertel CG, Dockerty MB, Baggenstoss AH. Multiple primary malignant neoplasms. Cancer. 1961;99:401–5.
Baxi SS, Pinheiro LC, Patil SM, Pfister DG, Oeffinger KC, Elkin EB. Causes of death in long-term survivors of head and neck cancer. Cancer. 2014;120:1507–13.
Morris LG, Sikora AG, Patel SG, Hayes RB, Ganly I. Second primary cancers after an index head and neck cancer: subsite-specific trends in the era of human papillomavirus-associated oropharyngeal cancer. J Clin Oncol. 2011;29:739–46.
Lee DH, Roh JL, Baek S, Jung JH, Choi SH, Nam SY, et al. Second cancer incidence, risk factor, and specific mortality in head and neck squamous cell carcinoma. Otolaryngol Head Neck Surg. 2013;149:579–86.
Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–97.
Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738–53.
Coca-Pelaz A, Rodrigo JP, Suárez C, Nixon IJ, Mäkitie A, Sanabria A, et al. The risk of second primary tumors in head and neck cancer: a systematic review. Head Neck. 2020;42:456–66.
Jégu J, Binder-Foucard F, Borel C, Velten M. Trends over three decades of the risk of second primary cancer among patients with head and neck cancer. Oral Oncol. 2013;49:9–14.
Xu GZ, Guan DJ, He ZY. 18FDG-PET/CT for detecting distant metastases and second primary cancers in patients with head and neck cancer. A meta-analysis. Oral Oncol. 2011;47:560–5.
Lemke AJ, Niehues SM, Hosten N, Amthauer H, Boehmig M, Stroszczynski C, et al. Retrospective digital image fusion of multidetector CT and 18F-FDG PET: clinical value in pancreatic lesions–a prospective study with 104 patients. J Nucl Med. 2004;45:1279–86.
Sultana A, Jackson R, Tim G, Bostock E, Psarelli EE, Cox TF, et al. What is the best way to identify malignant transformation within pancreatic IPMN: a systematic review and meta-analyses. Clin Transl Gastroenterol. 2015;6:e130.
Kametaka H, Makino H, Fukada T, Seike K, Koyama T, Kushihashi Y, et al. A case of synchronous triple cancer treated with multidisciplinary therapy - cancers in the middle part of the extrahepatic bile duct, the pancreas head, and the supraglottis. Gan To Kagaku Ryoho. 2014;41:2459–61.
Haerle SK, Strobel K, Hany TF, Sidler D, Stoeckli SJ. (18)F-FDG-PET/CT versus panendoscopy for the detection of synchronous second primary tumors in patients with head and neck squamous cell carcinoma. Head Neck. 2010;32:319–25.
Hanamoto A, Takenaka Y, Shimosegawa E, Ymamamoto Y, Yoshii T, Nakahara S, et al. Limitation of 2-deoxy-2-[F-18]fluoro-D-glucose positron emission tomography (FDG-PET) to detect early synchronous primary cancers in patients with untreated head and neck squamous cell cancer. Ann Nucl Med. 2013;27:880–5.
Kaiser J, Scheifele C, Hinz U, Leonhardt CS, Hank T, Koenig AK, et al. IPMN-associated pancreatic cancer: survival, prognostic staging and impact of adjuvant chemotherapy. Eur J Surg Oncol. 2022;48:1309–20.
McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;2018(24):4846–61.
Capurso G, Boccia S, Salvia R, Del Chiaro M, Frulloni L, Arcidiacono PG, et al. Risk factors for intraductal papillary mucinous neoplasm (IPMN) of the pancreas: a multicentre case-control study. Am J Gastroenterol. 2013;108:1003–9.
Morales-Oyarvide V, Mino-Kenudson M, Ferrone CR, Sahani DV, Pergolini I, Negreros-Osuna AA, et al. Diabetes mellitus in intraductal papillary mucinous neoplasm of the pancreas is associated with high-grade dysplasia and invasive carcinoma. Pancreatology. 2017;17:920–6.
Zhang WB, Wang Y, Mao C, Guo CB, Yu GY, Peng X. Cervical metastasis of maxillary squamous cell carcinoma. Int J Oral Maxillofac Surg. 2015;44:285–91.
Lin HW, Bhattacharyya N. Survival impact of nodal disease in hard palate and maxillary alveolus cancer. Laryngoscope. 2009;119:312–5.
Morris LG, Patel SG, Shah JP, Ganly I. High rates of regional failure in squamous cell carcinoma of the hard palate and maxillary alveolus. Head Neck. 2011;33:824–30.
Yang X, Song X, Chu W, Li L, Ma L, Wu Y. Clinicopathological characteristics and outcome predictors in squamous cell carcinoma of the maxillary gingiva and hard palate. J Oral Maxillofac Surg. 2015;73:1429–36.
Shiga K, Kobayashi T. Treatment strategy for the head and neck cancer patients with synchronous second primary malignancies. Japanese J Head Neck Cancer. 2008;34(1):14–8.
Park JW, Lee SW. Clinical outcomes of synchronous head and neck and esophageal cancer. Radiat Oncol J. 2015;33:172.
Kanamori K, Kurita D, Hirano Y, Ishiyama K, Oguma J, Masutomi K, et al. Does synchronous early head and neck cancer with esophageal cancer need treatment after preoperative chemotherapy? Gen Thorac Cardiovasc Surg. 2022;70:280–4.
Panosetti E, Luboinski B, Mamelle G, Richard JM. Multiple synchronous and metachronous cancers of the upper aerodigestive tract a nine-year study. Laryngoscope. 1989;99:1267–73.
Acknowledgements
This work was supported by JSPS KAKENHI.
Funding
Grant information: Grant-in-Aid for Young Scientists 22K17085.
Author information
Authors and Affiliations
Contributions
RI performed drafting of the manuscript, writing, and literature search. HM, AK, and TT contributed substantially to the conception, design, and patient data acquisition of the manuscript. KK and AM performed the biopsy and was involved in revising the manuscript with respect to intellectual content. FF performed the histopathological diagnosis and contributed to the interpretation of the data. All authors reviewed and approved the final manuscript prior to submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The patient provided written informed consent, and this case report was approved by Ethics Committee Tohoku University Hospital (the committee’s reference number 26261).
Consent for publication
We obtained written informed consent from the patient and her son for publication, including clinical findings and imaging data.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Iwama, R., Miyashita, H., Koketsu, A. et al. A case of synchronous double cancers consisting of maxillary gingival carcinoma and intraductal papillary mucinous carcinoma, invasive: case report. BMC Oral Health 23, 595 (2023). https://doi.org/10.1186/s12903-023-03253-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-023-03253-y