Abstract
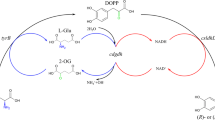
Dopamine is high-value compound of pharmaceutical interest, but its industrial scale production mostly focuses on chemical synthesis, possessing environment pollution. Bio-manufacturing has caused much attention for its environmental characteristic. Resting cells were employed to as biocatalysts with extraordinary advantages like offering stable surroundings, the inherent presence of expensive cofactors. In this study, whole-cell bioconversion was employed to convert dopa to dopamine. To increase the titer and yield of dopamine production through whole-cell catalysis, three kinds of aromatic amino acid transport protein, AroP, PheP and TyrP, were selected to be co-expressed. The effects of the concentration of L-dopa, pyridoxal-5’- phosphate (PLP), reaction temperature and pH were characterized for improvement of bioconversion. Under optimal conditions, dopamine titer reached 1.44 g/L with molar yield of 46.3%, which is 6.62 times than that of initial conditions. The catalysis productivity of recombinant E. coli co-expressed L-dopa decarboxylase(DDC) and AroP was further enhanced by repeated cell recycling, which maintained over 50% of its initial ability with eight consecutive catalyses. This study was the first to successfully bioconversion of dopamine by whole-cell catalysis. This research provided reference for whole-cell catalysis which is hindered by cell membrane.
Similar content being viewed by others
Introduction
Dopamine [2-(3,4-Dihydroxyphenyl-D3)ethylamine or 3,4-dihydroxytyramine] is a high-value compound in pharmaceutics and materials [1]. As a neurotransmitter, dopamine plays a key role in learning and motivation [2]. As therapeutic agents, dopamine was taken by Arvid Carlsson for Parkinson’s disease [3]. Dopamine and its derivative norepinephrine and epinephrine are key therapeutic uses, like in emergency of COVID-19 [4, 5]. As a monomer of polydopamine (PDA), PDA materials have been applied in various fields [6,26]. Therefore, the dominating reason was the membrane permeability to L-dopa that caused a descend in catalysis efficiency by whole-cell catalysis compared to crude extract catalysis.
To solve the membrane permeability, engineering modification on cells was conducted, such as expressing transport protein. In fact, five systems of aromatic amino acids transport had been reported in E. coli [27]. Due to structural similarity to L-dopa, we selected phenylalanine, tyrosine transport protein for further research. Additionally, a general aromatic transport protein was also employed into research. General transport system was coded by aroP [28] and the tyrosine- and phenylalanine-specific systems were coded by tyrP and pheP in this study [29].The results in Fig. 1(B) obviously illustrated that cell membrane did inhibit transfer of L-dopa and dopamine, resulting in poor performance of bioconversion. To decrease the substrate diffusion barrier, an efficient transport system was constructed that three kinds of transport protein, AroP, TyrP and PheP contributed to transfer substrate into cell. AroP, TyrP and PheP were aromatic amino acid symporter in E. coli. AroP and PheP belong to the amino acid-polyamine-organocation (APC) superfamily (https://www.uniprot.org/uniprot/P15993). PheP belongs to the amino acid/polyamine transporter 2 family (https://www.uniprot.org/uniprot/P0AAD4) [27, 28].
In order to investigate the influences of transport proteins on catalysis efficiency, the coding genes of three transport proteins were cloned into pRSFDuet and co-transformed with pET28a-DDC into BL21(DE3) to form recombinant cell BL21-AD-aroP, BL21-AD-tyrP and BL21-AD-pheP. In Fig. 2, all three transport protein had enhanced the production of dopamine and the most obvious result was BL21-AB-arop that titer and yield of which had up to 74 mg/L and 19%, respectively was nearly 4 times than that of whole-cell catalysis. According to Shang et al., AroP exhibited high affinity to phenylalanine and tyrosine similar with L-dopa [30]. Taking transport protein to enhance production is an effective strategy. The yield of L-tryptophan has been improved by 12.6% through modification of tryptophan transport system by Liu et al. [31]. The highest cadaverine production at that time was obtained by Ma et al. using recombinant E. coli co-overexpressing CadA and CadB which was a lysine/cadaverine transport antiporter [32].
Considering that transport protein was used to help transportation, pRSFDuet-pelB harboring a signal peptide pelB from pET22b, which can enhance transcript and translation levels of genes and direct protein translocation, was used as a vector to expression of AroP protein [33, 34]. BL21(DE3) harboring pRSFDuet-pelB-aroP and pET28a-DDC was constructed as BL21-AB-aroP. As signal peptide, PelB was expected to help the AroP periplasm secretion. However, PelB did not achieve the desired effect and the yield of dopamine was half of BL21-AD-aroP (Fig. 3). The result was contrary to that reported by Ma et al., where CadB fused to pelB increased the cadaverine production [32].Unlike CadB structure that expression and translocation of CadB to cell envelope contributed to L-lysine/cadaverine exchange and enhance production of cadaverine [35], aroP is highly hydrophobic with transmembrane potential so AroP fused with pelB may make activity decreased [30]. Thus, BL21-AD-aroP was determined to further study to enhance bioconversion of dopamine from L-dopa.
Optimizing BL21-AD-aroP catalysis conditions
BL21-AD-aroP harboring pET28a-DDC and pRSFDuet-aroP conding dopa decarboxylase DDC and transport protein AroP, respectively. In order to increase the titer and yield of dopamine catalyzed by BL21-AD-aroP, the catalysis conditions including reaction temperature and pH and concentration of L-dopa and PLP were examined. Six pH gradients, 5.7, 6.0, 6.5, 7.0, 7.5 and 8.0, were set to study the effect of pH on catalysis. The bioconversion lasted for 1 h at 45 ℃ with 4 g/L substrate. Similar to results of temperature, the titer of dopamine was more in the presence of AroP (Fig. 4A). The highest titer was found at pH 7.5 instead of 7.0. The phenomenon would be related to nature of AroP because in absence of AroP, the peak value was at pH 7.0. In addition, the most suitable pH for DDC was 7.5 in our previous study [12]. After optimizing conditions of reaction, the highest yield was up to 46.2% and the productivity of catalysis under optimal conditions had been raised 8.66 times against the initial condition.To investigated effects of temperature on whole-cell catalysis, the reaction temperature ranges from 40 ℃ to 70 ℃, every 5 degrees as a group with system consisting of 4 g/L dopa and pH of 7.0. PBS buffer and catalysis lasted for 1 h. In general, whole-cell catalysis with AroP was superior to that without AroP and effect at 45 ℃ was the most salient. The titer of dopamine by expressing AroP was 3.5 times of that of without expressing AroP. The highest yield was up to 45.1% (Fig. 4B). In addition, the concentration of substrate, L-dopa, was also investigated. The concentration of L-dopa was set as 1, 2, 3 and 4 g/L, respectively. The reactions were performed under conditions of pH = 7.0, 40 ℃ and 1 mM PLP. The results were shown in Fig. 4C that titer slightly increased but yield gradually decreased with improvement of dopa concentration. The same phenomenon was also found in purified catalysis [12]. The phenomenon further reflected that although AroP had been overexpressed, the reaction rate was quite low in whole-cell transformation which means a huge room for improvement of bioconversion of dopamine.
PLP as a key cofactor for dopa decarboxylase, was essential in the reaction. Extra 0, 0.2, 0.4, 0.6, 0.8 and 1 mM PLP was added into system consisting of 1 g/L dopa and pH = 7.0 PBS buffer and catalysis was at 40 ℃ for 1 h. According to Fig. 4D, additional PLP did not enhance the titer and yield of biotransformation and inhibited the catalysis instead. PLP synthesized by E. coli itself was enough for catalysis and extra addition PLP could be adverse. To account for this result, the initial cellular PLP was enough for poor activity of whole-cell catalysis and extra PLP made the environment more adverse leading to decrease the productivity [36].
Cycle catalyzing by BL21-AD-aroP
Although the yield of crude extract catalysis was high, there was a problem of rapid loss of enzyme activity leading to discontinuous catalysis. On the contrary, although whole-cell catalysis was slow and yield was not high, it can be reused to improve the utilization rate of the enzyme. Thus, cells in catalysis system was recycled by centrifugation after bioconversion and was used for the next round of catalysis. Total 8 rounds of catalysis with 2 g/L substrate was taken to investigate the performance of cell cycle catalysis and the reactions took place at 40, 45 and 50 ℃, respectively. The results were shown in Fig. 5 that catalytic property under three temperature conditions was different. Under condition of 50 ℃, the titer of first catalysis was the highest but titer nearly disappeared at 8th catalysis, and catalytic performance was the fastest decrease among the three groups. The performance at 45 ℃ was similar to 50 ℃ but dopamine was still up to 0.1 g/L in the 8th catalysis, which was one quarter of the 1st catalysis. Compared to 45 and 50 ℃, the titer of first catalysis was the lowest but the 8th titer was the highest. Thus, cells at 40 ℃ possessed the most stable catalytic performance. The total titers and yield of dopamine were 1.91, 1.91 and 1.71 g/L and 11.9%, 11.9% and 10.7%, respectively in 8 rounds catalysis under 40, 45 and 50 ℃. Here, we attempted to co-express transport protein AroP and DDC for whole-cell catalyzing dopa to dopamine. Under optimal condition, 4 g/L of dopa was converted into 1.85 g/L of dopamine with a yield of 46.2%. After 8 rounds of cyclic catalysis, the catalytic performance of the resting cells remained above 40% catalytic performance of initial under the optimal condition. Given reaction lasted for only 1 h, the accumulation of dopamine did not reach the peak value. The titer and yield would further increase as time goes on.
To increase utilization of enzyme in bioconversion, immobilization was the first choice and had an excellent outcome. Zhou et al. reported that the immobilized ChBD-CadA can catalyze 200 g/L L-lysine to cadaverine of 135.6 g/L within 120 min and possess more than 57% activity after four cycles of use [37]. Compared to immobilized enzymes catalysis, whole-cell catalysis circumvented the need of process and materials of immobilization. At the same time, cell membrane shield enzyme from adverse surroundings and enable the resting cell to cycle catalysis [13]. But to overcome the disadvantages that poor utilization of cells limiting the further application of whole-cell bioconversion, repeated cell recycling is a conventional solution to get the utmost out of whole-cell catalysis [16]. Ying et al. reported that the titer of L-pipecolic acid reached 17.25 g/L under repeated cell recovery, which was 2.7 times of that without repeated cell recovery [16]. Cell recovering was not applied in whole-cell catalysis but also in fermentation. According to Ma, succinic acid productivity and mass yield was up to 1.81 g/L h and 0.85 g/g, respectively after three times of recycling cell [38]. In our study, we succeeded in cycle bioconversion by whole-cell catalysis that the catalysis activity was remained over 50% at 40 °C after eight batches of catalyses.
Conclusion
In this study, we co-expressed dopa decarboxylase (DDC) and transport protein AroP in E. coli BL21(DE3) to enhance the titer and yield of dopamine production through whole-cell catalysis. The presence of permeability limited the efficiency of whole-cell catalysis. To solve the problem of permeability, AroP, PheP and TyrP were selected and expressed in E. coli BL21(DE3). AroP was the optimal transport protein whose coding gene was cloned into expression vector pRSFDuet. Additionally, reaction conditions were investigated to further enhance the efficiency of whole-cell catalysis. The best condition was conducted under 50 °C at pH 7.5 with 4 g/L of L-dopa. Compared with the initial catalytic results, the optimized productivity increased by 8.66 times. With the aim of maximization of cells utilization, repeated cell recovery was studied that the catalysis activity preserved over 50% at 40 °C after eight batches of catalyses. To the best of our knowledge, we are the first that successfully synthesized dopamine from L-dopa by whole-cell catalysis. This work also provides reference for whole-cell catalysis which is hindered by permeability.
Data Availability
All data generated or analyzed during this study will be available from the first author (Siyuan Gao, 201,962,118,011@njtech.edu.cn) for anyone who wishes to access the data.
Abbreviations
- PLP:
-
Pyridoxal-5’- phosphate
- DDC:
-
L-dopa decarboxylase
- LB:
-
Luria-Bertani
- Amp:
-
Ampicillin
- Kan:
-
Kanamycin
- PDA:
-
Polydopamine
- CTAB:
-
Cetyl trimethyl ammonium bromide
- DMSO:
-
Dimethyl sulfoxide
- HPLC:
-
High-performance liquid chromatography
- APC:
-
Acid-polyamine-organocation
References
Kurt AG, Aytan E, Ozer U, Ates B, Geckil H. Production of L-DOPA and dopamine in recombinant bacteria bearing the Vitreoscilla hemoglobin gene. Biotechnol J. 2009;4:1077–88.
Berke JD. What does dopamine mean? Nat Neurosci. 2018;21:787–93.
Carlsson A. A half-century of neurotransmitter research: impact on neurology and psychiatry. Biosci Rep. 2001;21:691–710.
Nataf S. An alteration of the dopamine synthetic pathway is possibly involved in the pathophysiology of COVID-19. J Med Virol. 2020;92:1743–4.
Khalefah MM, Khalifah AM. Determining the relationship between SARS-CoV-2 infection, dopamine, and COVID-19 complications. J Taibah Univ Med Sci. 2020; November.
Liu Z, Qu S, Weng J. Application of polydopamine in surface modification of biomaterials. Biotechnol Bioeng. 1999;64:54–60.
Geng W, Wang L, Jiang N, Cao J, **ao Y-X, Wei H, et al. Single cells in nanoshells for the functionalization of living cells. Nanoscale. 2018;10:3112–29.
Wang G, Wang L, Liu P, Yan Y, Xu X, Tang R. Extracellular silica Nanocoat confers Thermotolerance on Individual cells: a case study of material-based functionalization of living cells. ChemBioChem. 2010;11:2368–73.
Anderson WA, Moo-Young M, Legge RL. Development of a multienzyme reactor for dopamine synthesis: I. Enzymology and kinetics. Biotechnol Bioeng. 1992;39:781–9.
Gao S, Guo Y, Ma C, Ma D, Chen K, Ouyang P et al. Characterization and application of a recombinant dopa decarboxylase from Harmonia axyridis for the efficient biosynthesis of dopamine. Chin J Chem Eng. 2021.
Ren LQ, Wienecke J, Hultborn H, Zhang M. Production of dopamine by aromatic l-Amino acid decarboxylase cells after spinal cord Injury. J Neurotrauma. 2016;33:1150–60.
Wachtmeister J, Rother D. Recent advances in whole cell biocatalysis techniques bridging from investigative to industrial scale. Curr Opin Biotechnol. 2016;42:169–77.
Wang X, Cai P, Chen K, Ouyang P. Efficient production of 5-aminovalerate from L-lysine by engineered Escherichia coli whole-cell biocatalysts. J Mol Catal B Enzym. 2016;134:115–21.
Ying H, Chen X, Cao H, **ong J, Hong Y, Bai J, et al. Enhanced uridine diphosphate N-acetylglucosamine production using whole-cell catalysis. Appl Microbiol Biotechnol. 2009;84:677–83.
Ying H, Wang J, Wang Z, Feng J, Chen K, Li Y, et al. Enhanced conversion of L-lysine to L-pipecolic acid using a recombinant Escherichia coli containing lysine cyclodeaminase as whole-cell biocatalyst. J Mol Catal B Enzym. 2015;117:75–80.
Chen RR. Permeability issues in whole-cell bioprocesses and cellular membrane engineering. Appl Microbiol Biotechnol. 2007;74:730–8.
Ni Y, Chen RR. Accelerating whole-cell biocatalysis by reducing outer membrane permeability barrier. Biotechnol Bioeng. 2004;87:804–11.
Liu Y, Hama H, Fujita Y, Kondo A, Inoue Y, Kimura A, et al. Production of S-lactoylglutathione by high activity whole cell biocatalysts prepared by permeabilization of recombinant Saccharomyces cerevisiae with alcohols. Biotechnol Bioeng. 1999;64:54–60.
Fontanille P, Larroche C. Optimization of isonovalal production from alpha-pinene oxide using permeabilized cells of Pseudomonas rhodesiae CIP 107491. Appl Microbiol Biotechnol. 2003;60:534–40.
Silveira M, Jonas R. The biotechnological production of sorbitol. Appl Microbiol Biotechnol. 2002;59:400–8.
Zhao W, Huang J, Peng C, Hu S, Ke P, Mei L, et al. Permeabilizing Escherichia coli for whole cell biocatalyst with enhanced biotransformation ability from L-glutamate to GABA. J Mol Catal B Enzym. 2014;107:39–46.
Matsumoto T, Takahashi S, Kaieda M, Ueda M, Tanaka A, Fukuda H, et al. Yeast whole-cell biocatalyst constructed by intracellular overproduction of Rhizopus oryzae lipase is applicable to biodiesel fuel production. Appl Microbiol Biotechnol. 2001;57:515–20.
Sroga GE, Dordick JS. A strategy for in vivo screening of subtilisin E reaction specificity in E. coli periplasm. Biotechnol Bioeng. 2002;78:761–9.
Shimazu M, Mulchandani A, Chen W. Cell surface display of organophosphorus hydrolase using ice nucleation protein. Biotechnol Prog. 2001;17:76–80.
Mirbagheri M, Nahvi I, Emtiazi G, Darvishi F. Enhanced production of citric acid in Yarrowia lipolytica by Triton X-100. Appl Biochem Biotechnol. 2011;165:1068–74.
Sarsero JP, Wookey PJ, Gollnick P, Yanofsky C, Pittard AJ. A new family of integral membrane proteins involved in transport of aromatic amino acids in Escherichia coli. J Bacteriol. 1991;173:3231–4.
Brown KD. Formation of aromatic amino acid pools in Escherichia coli K-12. J Bacteriol. 1970;104:177–88.
West TP. Isolation and characterization of an Escherichia coli B mutant strain defective in uracil catabolism. Can J Microbiol. 1998;44:1106–9.
Honoré N, Cole ST, Erratum. Nucleotide sequence of the arop gene encoding the general aromatic amino acid transport protein of Escherichia coli k-12: homology with yeast transport proteins. Nucleic Acids Res. 1990;18:1332.
Liu Q, Cheng Y, **e X, Xu Q, Chen N. Modification of tryptophan transport system and its impact on production of L-tryptophan in Escherichia coli. Bioresour Technol. 2012;114:549–54.
Ma W, Cao W, Zhang H, Chen K, Li Y, Ouyang P. Enhanced cadaverine production from L-lysine using recombinant Escherichia coli co-overexpressing CadA and CadB. Biotechnol Lett. 2015;37:799–806.
Lei SP, Lin HC, Wang SS, Higaki P, Wilcox G. Characterization of the Erwinia carotovora peh gene and its product polygalacturonase. Gene. 1992;117:119–24.
Sletta H, Tøndervik A, Hakvåg S, Vee Aune TE, Nedal A, Aune R, et al. The presence of N-terminal secretion signal sequences leads to strong stimulation of the total expression levels of three tested medically important proteins during high-cell-density cultivations of Escherichia coli. Appl Environ Microbiol. 2007;73:906–12.
Soksawatmaekhin W, Uemura T, Fukiwake N, Kashiwagi K, Igarashi K. Identification of the cadaverine recognition site on the cadaverine-lysine antiporter CadB. J Biol Chem. 2006;281:29213–20.
Ma W, Cao W, Zhang B, Chen K, Liu Q, Li Y, et al. Engineering a pyridoxal 5’ -phosphate supply for cadaverine production by using Escherichia coli whole-cell biocatalysis. Sci Rep. 2015;5:1–10.
Zhou N, Zhang A, Wei G, Yang S, Xu S, Chen K, et al. Cadaverine Production from L-Lysine with chitin-binding protein-mediated lysine decarboxylase immobilization. Front Bioeng Biotechnol. 2020;8:1–11.
Ma JF, Jiang M, Chen KQ, Xu B, Liu SW, Wei P, et al. Succinic acid production with metabolically engineered E. coli recovered from two-stage fermentation. Biotechnol Lett. 2010;32:1413–8.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Key Research and Development Program of China (Grant No. 2021YFC2100800) and National Natural Science Foundation of China (U21B2097). The funding body played no role in the design of the study and collection, analysis, interpretation of data, and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
SG and XW conceived the experiments, supervised the work, and confirmed the manuscript. SG, and DM conducted the experiment, analyzed the data and drafted the manuscript. YW and AZ were responsible for catalysis. KC analyzed the data and revised the manuscript. All of the authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gao, S., Ma, D., Wang, Y. et al. Whole-cell catalyze L-dopa to dopamine via co-expression of transport protein AroP in Escherichia coli. BMC Biotechnol 23, 33 (2023). https://doi.org/10.1186/s12896-023-00794-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12896-023-00794-6