Abstract
Background
Allostatic load, the cumulative strain resulting from chronic stress responses, has been linked to disease occurrence and progression, yet research quantifying this relationship is limited. This study aimed to explore the relationship between allostatic load score (ALS) levels and the degree of hepatic steatosis and fibrosis.
Methods
Data from the National Health and Nutrition Examination Survey 2017–2020 were analyzed. The ALS was based on the statistical distribution, assigning one point for each biomarker if it was in the highest risk quartile, and then summing them to generate the ALS score (range, 0–8). The multivariate linear regression was employed to analyze the association between the controlled attenuation parameter (CAP) and liver stiffness measurement (LSM) with ALS. Additionally, multinomial logistic regression was used to investigate the association between ALS and the degree of hepatic steatosis and fibrosis.
Results
Participants had a weighted mean age of 52.69 years and 56.14% were female. In the multivariate linear regression analysis, ALS showed a significant positive correlation with CAP (β = 15.56, 95% CI: 14.50–16.62) and LSM (β = 0.58, 95% CI: 0.48–0.67). Age, healthy dietary level, and PIR had significant interactions with this positive correlation. In the multinomial logistic regression analysis, ALS exhibited a significant positive correlation with different degrees of hepatic steatosis and fibrosis. Consistency of the results was observed in sensitivity analyses using clinical thresholds of ALS.
Conclusions
Comprehensive clinical assessment targeting load adaptation may enhance the effectiveness of risk assessment in patients with hepatic steatosis and fibrosis.
Similar content being viewed by others
Introduction
Steatotic liver disease (SLD) represents a chronic hepatic disorder that afflicts over a quarter of the global population, with its incidence rapidly increasing [12]. Furthermore, recent investigations have shown that persistent psychological and physiological stress induce protracted hyperactivation and dysregulation within the body's neuroendocrine and immune systems, culminating in disruptions to glucose and lipid metabolism alongside chronic hepatic inflammatory responsiveness [13]. Evidence from cell, animal, and clinical studies suggests that high cortisol levels and psychosocial stress may be involved in SLD and liver fibrosis onset and progression [13, 14]. However, the relationship between ALS and the degree of hepatic steatosis and fibrosis remains unclear. Although prior studies have demonstrated the pervasiveness of stress in the aetiopathogenesis and treatment of SLD, we have advanced the field by identifying early stress states through ALS and thereby intervening in the psychosocial chronic stress response for more favorable and longer-lasting treatment outcomes.
Therefore, our study aims to investigate the association between ALS and hepatic steatosis and liver fibrosis in adults, utilizing a large sample of people from the National Health and Nutrition Examination Survey (NHANES) 2017–2020.
Methods
Data source
The National Health and Nutrition Examination Survey (NHANES), conducted by the National Center for Health Statistics (NCHS), is a nationally representative survey of the U.S. population that utilizes complex, multi-stage, and probability sampling methods to assess the health and nutritional status of the general population. The ethical approval for the administration of NHANES, including its strict procedures and protocols, was granted by the NCHS Research Ethics Review Board. Written informed consent was obtained from all participants.
Study population
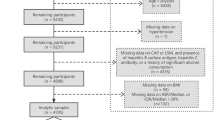
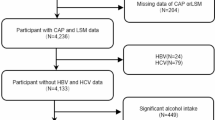
This study utilizes data from participants in the NHANES 2017–2020 cycle as the basis for analysis. We excluded 6,329 individuals aged below 20 years from the 15,560 eligible individuals, 2,281 individuals who lacked any missing biomarkers necessary for ALS calculation, 342 subjects with missing data for CAP or LSM, 35 subjects with positive hepatitis B antigen, 161 subjects with positive hepatitis C antibody or hepatitis C RNA samples, 1,510 significant alcohol drinkers (defined as consuming more than 3 drinks a day for men and 2 drinks a day for women), 658 individuals without demographic details (including age, sex, race/ethnicity, education level, poverty income ratio (PIR), and marital status), and individuals without information on smoking, physical activity, healthy diet level, ALT, AST, GGT, and ALP. After applying these exclusion criteria, a total of 4,307 participants were selected in the analysis (Fig. 1).
Definition of hepatic steatosis and liver fibrosis
This study relied on data obtained from the most recent NHANES 2017–2020 iteration, which implemented techniques such as ultrasound and vibration-controlled transient elastography (VCTE) to evaluate liver function. The measurements of elastography were conducted at the NHANES Mobile Examination Center using the FibroScan model 502 V2 Touch, which was equipped with either a medium (M) or extra-large (XL) wand (probe). Hepatic steatosis was determined using the median Controlled Attenuation Parameter (CAP), while liver fibrosis was assessed using the median Liver Stiffness Measurement (LSM).
Steatotic liver disease is an overarching term chosen in the latest multi-society Delphi consensus to encompass the various causes that can lead to hepatic steatosis [15], including metabolic dysfunction-associated steatotic liver disease (MASLD), metabolic alcohol-related liver disease (MetALD), alcohol-related Liver Disease (ALD), cryptogenic SLD, and specific etiologies of SLD (e.g., drug-induced, genetic, etc.).
Based on previous research findings, median CAP values of ≥ 274 dB/m and ≥ 302 dB/m have been identified as indicative of hepatic steatosis [4] and severe hepatic steatosis [23], thus age was evaluated from birth until baseline and categorized into 20–39, 40–59, and ≥ 60. Race/ethnicity data were collected via self-reporting and segmented into five classifications: Mexican American, non-Hispanic Black, non-Hispanic White, Other Hispanic, and Other (comprising participants of multiple races). Educational attainment may act as a protective factor or a risk factor. Those with higher levels of education may have greater access to health knowledge and self-management skills, but may also be more susceptible to chronic stress due to the nature of their work. Education level was stratified as below high school, high school or equivalent, and beyond high school. The marital status of an individual may influence the efficacy of chronic stress relief through the influence of social support networks. Marital status was categorized as married, never married, or other (including widowed, divorced, and separated individuals). PIR is a significant indicator of household poverty, and economic stress represents a significant source of chronic stress. PIR is calculated as the ratio of a family's income to the appropriate poverty threshold for that family size, as determined by the U.S. Department of Health and Human Services. PIR values were partitioned into < 1.5, 1.5–3.5, and ≥ 3.5, and a larger value indicates a higher per capita family income. Smoking is a recognized stressor in humans and has been linked to liver disease, meaning that being a covariate can help to control for the effect of smoking on study outcomes. The smoking variable gauged whether an individual had smoked a minimum of 100 cigarettes throughout their lifetime and was sorted as yes or no. The level of a healthy diet may have a direct impact on liver health, and as a covariate can help explain the potential impact of different dietary patterns on hepatic steatosis and liver fibrosis. Healthy diet level was self-reported and grouped as excellent and good, fair, and poor. Activity level was determined by whether or not an individual partook in any moderate-intensity exercise, fitness, or recreational activities during a typical week and classified as yes or no. Lastly, ALT, AST, ALP, and GGT were derived from the laboratory data of NHANES. These are liver function tests whose changes reflect liver health and as covariates can help control for the impact of the liver disease process on study outcomes.
Statistical analysis
This study employed a complex sampling design and utilized weighted methods to minimize significant fluctuations in the dataset, in line with NHANES analysis recommendations. The baseline characteristics of the study population were statistically described based on CAP and LSM subgroups. Data for continuous variables follow a normal distribution and are expressed as means and standard deviation (SD), while data for categorical variables are expressed as numbers and percentages(%). Pearsonχ2 test was used for categorical variables, while one-way analysis of variance was used for continuous variables. After adjusting for potential confounding variables, weighted multivariate linear regression models were employed to investigate the linear association between ALS and CAP and LSM, calculating beta values and a 95% confidence interval. To verify the correlation between ALS and hepatic steatosis and fibrosis, we further grouped the variables based on the CAP and LSM values to change continuous variables into categorical variables. Since the degree of hepatic steatosis and fibrosis was used as a multicategorical response variable, we further analyzed the relationship between ALS and the degree of hepatic steatosis and liver fibrosis using weighted multiple logistic regression analysis. Model 1 was the crude model without any adjustments, while model 2 adjusted for age, gender, race/ethnicity, marital status, PIR, and education level. Model 3 adjusted for all covariates.
Furthermore, subgroup-stratified models were established to assess potential interactions between ALS and CAP and LSM, considering stratification by gender, age, education level, PIR level, healthy diet level, and smoking status. In the sensitivity analysis, we further classified each biomarker based on clinical cutoff points, assigning a score of 1 if the biomarker fell within the high-risk range. The cutoff ranges were as follows: systolic blood pressure ≥ 130 mmHg, diastolic blood pressure ≥ 80 mmHg, Tc ≥ 240 mg/dL, HDL ≤ 40 mg/dL, HbA1c ≥ 6.5%, Alb ≤ 35 g/L, BMI ≥ 30 kg/m2, and CRP ≥ 3 mg/dL.
All analyses in this study were conducted using R, version 4.2.0 (R Project for Statistical Computing), the survey package (version 4.11), and EmpowerStats (version 4.1).
Statistical significance was established when P-value was less than 0.05.
Results
Baseline characteristics of participants
In this study, a total of 4307 adult participants were included based on rigorous inclusion and exclusion criteria. The subjects had an average age of 52.69 ± 17.08 years. Within these participants, males constituted 43.86% while females accounted for 56.14%. The average (SD) concentrations of CAP, LSM, and ALS were 265.96 (62.07) dB/m, 6.05 (5.69) kPa, and 2.15 (1.63), respectively.
Table 1 presents the weighted baseline characteristics of the participants stratified based on their CAP levels. Among different CAP levels, significant disparities (all p < 0.05) were observed in gender, age, race/ethnicity, education level, marital status, PIR, healthy dietary level, smoking status, activity level, ALT, AST, GGT, ALP, LSM, and ALS, and these disparities exhibit statistical significance (P < 0.05). In comparison to the non-SLD group, individuals experiencing severe hepatic steatosis exhibited a predisposition towards older age, male gender, Non-Hispanic Black ethnicity, married, lower levels of education, diminished PIR, higher smoking status, limited moderate activity, lower healthy dietary level, as well as higher levels of ASL, ALT, ALP, GGT, LSM, and ALS.
Table 2 depicts the weighted baseline characteristics of individuals stratified based on their LSM levels. Significant differences (all p < 0.05) were observed in several variables, including gender, age, education level, PIR, healthy dietary level, smoking status, activity level, ALT, AST, GGT, ALP, CAP, and ALS among different LSM levels. When compared to the non-fibrosis group, individuals afflicted with liver cirrhosis were more inclined towards advanced age, male, lower levels of education, decreased PIR, higher smoking status, limited moderate activity, lower healthy dietary level, as well as higher levels of ASL, ALT, ALP, GGT, CAP, and ALS.
The association between als and cap and lsm
The results of a sample-weighted multivariate linear regression analysis were employed to examine the association between ALS and CAP and LSM, as demonstrated in Table 3. In the fully adjusted model (model 3), we observed a significant positive association between ALS and CAP and LSM (CAP, Model 3: β = 15.56, 95% CI: 14.50–16.62; LSM, Model 3: β = 0.58, 95% CI: 0.48–0.67), and maintained relative stability across models. This implies that chronic stress may be significantly associated with an increased risk of hepatic steatosis and fibrosis. Furthermore, this relationship remains significant when analyzing ALS using clinical cutoff point modeling (all p < 0.001).
Table 4 displays the results of our further weighted multinomial logistic regression analysis, where CAP and LSM were converted from continuous variables to categorical variables. When stratifying by the degree of hepatic steatosis, strong positive associations were observed between ALS and both hepatic steatosis and severe hepatic steatosis in all three models (p < 0.001). In the fully adjusted model (Model 3), every one-unit increase in ALS was associated with a 0.3369-fold and 1.0182-fold increased risk of SLD and severe SLD, respectively. When stratifying by the degree of liver fibrosis, strong positive associations were found between ALS and significant fibrosis, severe fibrosis, and cirrhosis in all three models (p < 0.001). In the fully adjusted model (Model 3), every one-unit increase in ALS had a 0.3878-fold, 0.6910-fold, and 0.6567-fold increased risk of significant fibrosis, severe fibrosis, and cirrhosis, respectively, compared to non-fibrosis. Moreover, the significance (p < 0.001) of all the considerable associations persevered when ALS was delineated using clinical threshold values. Abbreviations: ALP, alkaline phosphatase; ALT, alanine transaminase; ALS, allostatic load score; AST, aspartate transaminase; CAP, controlled attenuation parameter; GGT, gamma glutamyl transferase; LSM, liver stiffness measurement; PIR, poverty income ratio. Model 1 was the crude model without adjustment for covariates. Model 2 was adjusted for age, sex, race, marital status, education level, PIR. Model 3 was adjusted as for model 2, additionally adjusted for ALT, AST, GGT, ALP, healthy diet level, smoking status, physical activity
Subgroup analyses
Figures 2 and 3 present subgroup analyses aimed at further evaluating the robustness of the correlation between ALS and CAP and LSM. The outcomes of our subgroup analysis demonstrate that this positive correlation remains consistent across diverse population settings (all p < 0.05). It was noted that in males, aged between 20–39, lower education levels, lower PIR values, poorer dietary habits, and higher smoking status, a significantly magnified correlation was evident between ALS and both CAP and LSM. Statistically, there was strong evidence of interaction effects between ALS and CAP across different age groups (p for interaction < 0.001), healthy diet levels (p for interaction = 0.003), and PIR (p for interaction = 0.048) categories. Additionally, a significant interaction effect was observed between ALS and LSM specifically within different healthy diet levels (p for interaction = 0.003). However, no significant interactions were observed within other predetermined subgroups (p for interaction > 0.05).
Discussion
In this nationally representative study, ALS levels were positively correlated with the degree of hepatic steatosis and liver fibrosis, especially among young individuals aged 20–39, those with poorer dietary habits, and individuals with lower PIR values. Our study findings suggest that ALS levels serve as independent risk factors for monitoring the risk of hepatic steatosis and liver fibrosis.
To the best of our knowledge, this is the first study to assess the association between ALS and the degree of hepatic steatosis and liver fibrosis. Previous studies have demonstrated a significant association between stress and the incidence and mortality of liver disease [24, 25], particularly the role of the stress response in metabolic dysfunction-associated fatty liver disease [13]. A review by György described the utility of allostasis in non-alcoholic fatty liver disease from a molecular point of view, which was in line with our findings [12]. Yunzi Liu et al. constructed an experimental animal model of chronic stress and discovered that notwithstanding the chronicity of stress induces diminished dietary consumption and visceral adiposity, there is pronounced elevation in hepatic triglycerides and cholesterol levels, accompanied by augmented serum transaminase and inflammatory cytokine concentrations [26]. Czech et al. ascertained that mice subjected to a chronic stress model (congested communal living environment) displayed notable hepatic oxidative stress and inflammatory reactions in comparison to mice raised in a single housing environment when nourished with a normal diet [27]. Although heterogenous stress models were applied in these experimental inquiries, they collectively indicate a plausible correlation between the allostatic burden and the pathogenesis of hepatic steatosis and chronic inflammation.
Previous research has established a correlation between hepatic steatosis and fibrosis with subcomponents included in the ALS. Cardiovascular factors, such as dyslipidemia and hypertension, exhibit intricate interconnections with hepatic steatosis and fibrosis [28,29,30]. Metabolic factors like diabetes and high body mass index are causally linked to the development of hepatic steatosis [31, 32]. Lastly, systemic biomarkers of inflammation, including C-reactive protein levels, positively correlate with the severity of hepatic steatosis and fibrosis [31].
In our study, we did not explore potential associations between individual factors and hepatic steatosis or fibrosis in the adjusted analysis. Instead, chronic diseases typically extend their influence beyond singular biological functions or organs, encompassing the development of co-morbidities that impact overall outcomes. These comorbidities may arise from shared disease processes or diverse etiologies [12]. The concept of allostatic load offers a conceptual framework to comprehend the cumulative physiologic alterations inherent in chronic illnesses, demonstrating greater utility in stress evaluation and the appraisal of associated biological burden when compared to isolated biomarkers [11, 33]. Heightened allostatic load correlates with the advanced stages of ailments [11, 34]. As allostatic load gradually accumulates, it exacerbates deviation from the initial homeostatic set point, thus culminating in late-stage disease where physiological functions progressively deteriorate and become irreparable. Elevated allostatic load poses constraints not only on the likelihood of spontaneous remission but also on the efficacy of medical interventions. Consequently, applying the models of allostasis and allostatic load in the degree of liver steatosis and liver fibrosis may foster an enriched comprehension of disease progression, as well as facilitate the formulation of strategies encompassing prediction, prevention, and management.
Currently, the existing body of research on the association between allostatic load and human fatty liver disease is relatively scant, particularly when considering disease subgroups and interactions. Our subgroup analysis reveals that allostatic load impacts the severity of hepatic steatosis and fibrosis more pronouncedly in younger individuals with lower socio-economic status and subpar dietary health. This group may lack the requisite resources to cope with environmental stresses, which can result in the livers of such individuals working harder to maintain health and repair damage. This can lead to hepatic steatosis and excessive fibrosis, which in turn can exacerbate the severity of liver disease. Importantly, the SLD significantly correlates with the high prevalence of obesity and metabolic syndrome among young individuals, leading to a substantial escalation in disease occurrence over recent decades and substantially heightening the risk of liver cirrhosis and the necessity for liver transplantation [35]. Consequently, recognizing the allostatic load experienced by adolescents and young adults is of utmost importance. A systematic review by Jenny Guidi et al., encompassing 267 original investigations, suggested that factors such as low socioeconomic status, residing in impoverished communities, and indulging in unhealthy lifestyle habits (e.g., sedentariness, poor dietary patterns, and disturbed sleep) contributed to an augmented allostatic load [10]. Moreover, individual psychological well-being and co** mechanisms may moderate the association between social demographic influences and allostatic load. Nevertheless, the interpretation of these findings must be approached with caution due to the restricted nature of the sample size in this study and the potential influence of confounding factors. Consequently, further meticulously devised prospective studies are essential to gain a deeper understanding of this subject matter.
The exact mechanisms underlying the positive correlation between allostatic load and liver steatosis and fibrosis remain unclear. From an adaptive perspective, during sustained calorie surplus, excess fat is increasingly stored in non-adipose organs such as pancreatic cells, muscles, and the liver to accommodate the increased fat storage demands. Higher adaptation load can alter dietary habits, increase cravings for high-calorie foods, and lead to further fat accumulation to meet the body's increased demand for glucose levels [36]. Additionally, when the sympathetic nervous system (SNS) and hypothalamic–pituitary–adrenal (HPA) axis are chronically activated, a large number of catecholamines are released, which can promote lipolysis and lead to fat accumulation in the liver [37]. Furthermore, emotional stressors under high adaptation load can activate the hypothalamus to secrete various neurohormones, promoting the synthesis and secretion of glucocorticoids (GCs). Excessive levels of GCs have been shown to cause abnormal fat breakdown, resulting in the production of large amounts of free fatty acids, elevated blood glucose levels, and insulin resistance (IR) [14]. The continued presence of IR can disrupt hepatic lipid and glucose metabolism as well as systemic low-grade inflammation (manifested by increased levels of tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, IL-6, and CRP) [38]. Moreover, stress and activation of the HPA axis can lead to gut dysbiosis, which is connected to liver steatosis, meaning the gut-liver axis [13]. Of considerable importance is the observation that elevated levels of oxidative stress in hepatic tissue stimulate additional adaptive responses that modulate the course of non-alcoholic fatty liver disease. Excessive lipid peroxidation can induce oxidative injury to genomic DNA, which is intricately linked to the pathogenesis of hepatocellular carcinoma. Lastly, it is imperative to underscore that allostatic load is intricately associated with an elevation of pro-inflammatory responses [39]. Potential mechanisms that contribute to this effect include dysbiosis and increased intestinal permeability in the gut-liver axis, as well as the intricate immunomodulatory effects of glucocorticoids and the development of resistance to corticosteroids [40]. However, this adaptive response is determined by various molecular pathways involved in regulating adipocyte differentiation, connective tissue growth, and angiogenesis, as well as their genetic driving factors.
Our study holds its strengths. Firstly, this study represents the first exploration of the association between ALS and the degree of liver steatosis and fibrosis in a large, representative sample of the US population. Secondly, the data employed in this study are derived from the NHANES, furnishing a sizable and representative sample with stringent quality control protocols and well-trained personnel meticulously collecting high-quality clinical variables, thus ensuring the reliability and validity of the findings. However, our study has some limitations. Firstly, due to its cross-sectional nature, this analysis lacks the ability to establish temporal order and ascertain causality. Secondly, the degree of hepatic steatosis and fibrosis in this study was assessed using transient elastography, while multiple studies have demonstrated its high diagnostic accuracy [41,42,43], liver tissue biopsy remains the gold standard. Thirdly, the calculation of allostatic load scores exhibits noteworthy variability, and despite our utilization of multiple approaches, the exclusion of certain biomarkers involved in the stress response may have potentially influenced the findings of the study. Future investigations should explore alternative algorithms for calculating ALS. However, by simultaneously incorporating clinical criteria and biological markers in evaluating ALS, our study will help elucidate the relationship between clinical and biological parameters and better identify states of excessive allostatic load than using either criterion alone. Fourth, the potential for bias inherent in the study design must be acknowledged. Given the limitations of the dataset, there is a possibility of selection bias and generalisability limitations in the results. Some of the covariates, such as dietary health level and physical activity data, were self-reported by participants through recall, which may have introduced a degree of memory bias. Lastly, despite rigorous adjustment for several pertinent confounding factors, the potential residual influence of other confounders cannot be entirely ruled out, necessitating cautious interpretation of the study findings. For instance, the long-term use of certain medications, such as antibiotics and non-steroidal anti-inflammatory drugs, is linked to an increased risk of hepatic steatosis and hepatic fibrosis. Conversely, the long-term use of glucose-lowering drugs is associated with a decreased risk of these conditions. Certain comorbidities, such as cardiovascular or other chronic diseases, and genetic factors can bias the results.
Conclusion
The present study suggests that ALS levels are associated with the extent of liver steatosis and fibrosis, particularly within specific individual behavioral contexts. To validate our findings, larger-scale prospective investigations are warranted.
Availability of data and materials
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://wwwn.cdc.gov/nchs/nhanes.
Abbreviations
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine transaminase
- ALS:
-
Allostatic load score
- AST:
-
Aspartate transaminase
- BMI:
-
Body mass index
- CRP:
-
C-reactive protein
- CAP:
-
Controlled attenuation parameter
- GGT:
-
Gammaglutamyl transferase
- HDL:
-
High-density lipoprotein
- HbA1c:
-
Glycated hemoglobin
- VCTE:
-
Vibration-controlled transient elastography
- LSM:
-
Liver stiffness measurement
- NHANES:
-
National health and nutrition examination survey
- PIR:
-
Poverty income ratio
- SLD:
-
Steatotic liver disease
- Tc:
-
Total serum cholesterol
References
**e R, **ao M, Li L, Ma N, Liu M, Huang X, et al. Association between SII and hepatic steatosis and liver fibrosis: A population-based study. Front Immunol. 2022;13: 925690.
Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397(10290):2212–24.
Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol. 2019;70(3):531–44.
Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156(6):1717–30.
Zhang X, Wong GL, Wong VW. Application of transient elastography in nonalcoholic fatty liver disease. Clin Mol Hepatol. 2020;26(2):128–41.
Sterling P. Allostasis: a model of predictive regulation. Physiol Behav. 2012;106(1):5–15.
McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med. 1993;153(18):2093–101.
Fava GA, Guidi J, Semprini F, Tomba E, Sonino N. Clinical assessment of allostatic load and clinimetric criteria. Psychother Psychosom. 2010;79(5):280–4.
Duong MT, Bingham BA, Aldana PC, Chung ST, Sumner AE. Variation in the Calculation of Allostatic Load Score: 21 Examples from NHANES. J Racial Ethn Health Disparities. 2017;4(3):455–61.
Guidi J, Lucente M, Sonino N, Fava GA. Allostatic Load and Its Impact on Health: A Systematic Review. Psychother Psychosom. 2021;90(1):11–27.
Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35(1):2–16.
Baffy G. Allostasis in nonalcoholic fatty liver disease: implications for risk assessment. Dig Dis Sci. 2013;58(2):302–8.
Demori I, Grasselli E. The Role of the Stress Response in Metabolic Dysfunction-Associated Fatty Liver Disease: A Psychoneuroendocrineimmunology-Based Perspective. Nutrients. 2023;15(3):795.
Woods CP, Hazlehurst JM, Tomlinson JW. Glucocorticoids and non-alcoholic fatty liver disease. J Steroid Biochem Mol Biol. 2015;154:94–103.
Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;78(6):1966–86.
**e R, Liu M. Relationship Between Non-Alcoholic Fatty Liver Disease and Degree of Hepatic Steatosis and Bone Mineral Density. Front Endocrinol (Lausanne). 2022;13: 857110.
Roulot D, Czernichow S, Le Clésiau H, Costes JL, Vergnaud AC, Beaugrand M. Liver stiffness values in apparently healthy subjects: influence of gender and metabolic syndrome. J Hepatol. 2008;48(4):606–13.
Du EY, Jiang K, Carlson MC, Reed NS, Deal JA. Hearing Impairment and Allostatic Load in Older Adults. JAMA Otolaryngol Head Neck Surg. 2023;149(7):597–606.
Frei R, Haile SR, Mutsch M, Rohrmann S. Relationship of Serum Vitamin D Concentrations and Allostatic Load as a Measure of Cumulative Biological Risk among the US Population: A Cross-Sectional Study. PLoS ONE. 2015;10(10): e0139217.
Petrova D, Ubago-Guisado E, Garcia-Retamero R, Redondo-Sánchez D, Pérez-Gómez B, Catena A, et al. Allostatic Load and Depression Symptoms in Cancer Survivors: A National Health and Nutrition Examination Survey Study. Cancer Nurs. 2023. Published online ahead of print.
Tian T, Zhang J, **e W, Ni Y, Fang X, Liu M, et al. Dietary Quality and Relationships with Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) among United States Adults, Results from NHANES 2017–2018. Nutrients. 2022;14(21):4505.
Wan H, Jiang Y, Yang J, Ma Q, Liu L, Peng L, et al. Sex-specific associations of the urinary fourteen-metal mixture with NAFLD and liver fibrosis among US adults: A nationally representative study. Ecotoxicol Environ Saf. 2022;248: 114306.
Aldwin CM, Sutton KJ, Chiara G, Spiro A 3rd. Age differences in stress, co**, and appraisal: findings from the Normative Aging Study. J Gerontol B Psychol Sci Soc Sci. 1996;51(4):P179–88.
Lebeaupin C, Vallée D, Hazari Y, Hetz C, Chevet E, Bailly-Maitre B. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J Hepatol. 2018;69(4):927–47.
Russ TC, Kivimäki M, Morling JR, Starr JM, Stamatakis E, Batty GD. Association Between Psychological Distress and Liver Disease Mortality: A Meta-analysis of Individual Study Participants. Gastroenterology. 2015;148(5):958-66.e4.
Liu YZ, Chen JK, Zhang Y, Wang X, Qu S, Jiang CL. Chronic stress induces steatohepatitis while decreases visceral fat mass in mice. BMC Gastroenterol. 2014;14:106.
Czech B, Neumann ID, Müller M, Reber SO, Hellerbrand C. Effect of chronic psychosocial stress on nonalcoholic steatohepatitis in mice. Int J Clin Exp Pathol. 2013;6(8):1585–93.
Yuan S, Chen J, Li X, Fan R, Arsenault B, Gill D, et al. Lifestyle and metabolic factors for nonalcoholic fatty liver disease: Mendelian randomization study. Eur J Epidemiol. 2022;37(7):723–33.
Ciardullo S, Monti T, Sala I, Grassi G, Mancia G, Perseghin G. Nonalcoholic Fatty Liver Disease and Advanced Fibrosis in US Adults Across Blood Pressure Categories. Hypertension. 2020;76(2):562–8.
Katsiki N, Mikhailidis DP, Mantzoros CS. Non-alcoholic fatty liver disease and dyslipidemia: An update. Metabolism. 2016;65(8):1109–23.
Zhu C, Huang D, Ma H, Qian C, You H, Bu L, et al. High-Sensitive CRP Correlates With the Severity of Liver Steatosis and Fibrosis in Obese Patients With Metabolic Dysfunction Associated Fatty Liver Disease. Front Endocrinol (Lausanne). 2022;13: 848937.
Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol. 2017;14(1):32–42.
Carbone JT, Clift J, Alexander N. Measuring allostatic load: Approaches and limitations to algorithm creation. J Psychosom Res. 2022;163: 111050.
Parker HW, Abreu AM, Sullivan MC, Vadiveloo MK. Allostatic Load and Mortality: A Systematic Review and Meta-Analysis. Am J Prev Med. 2022;63(1):131–40.
Doycheva I, Watt KD, Alkhouri N. Nonalcoholic fatty liver disease in adolescents and young adults: The next frontier in the epidemic. Hepatology. 2017;65(6):2100–9.
Spencer SJ, Tilbrook A. The glucocorticoid contribution to obesity. Stress. 2011;14(3):233–46.
Grynberg A, Ziegler D, Rupp H. Sympathoadrenergic overactivity and lipid metabolism. Cardiovasc Drugs Ther. 1996;10(Suppl 1):223–30.
Gasmi A, Noor S, Menzel A, Doşa A, Pivina L, Bjørklund G. Obesity and Insulin Resistance: Associations with Chronic Inflammation, Genetic and Epigenetic Factors. Curr Med Chem. 2021;28(4):800–26.
Cruz-Topete D, Cidlowski JA. One hormone, two actions: anti- and pro-inflammatory effects of glucocorticoids. NeuroImmunoModulation. 2015;22(1–2):20–32.
Silverman MN, Sternberg EM. Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann N Y Acad Sci. 2012;1261:55–63.
Shen M, Lee A, Lefkowitch JH, Worman HJ. Vibration-controlled Transient Elastography for Assessment of Liver Fibrosis at a USA Academic Medical Center. J Clin Transl Hepatol. 2022;10(2):197–206.
Pavlov CS, Casazza G, Nikolova D, Tsochatzis E, Burroughs AK, Ivashkin VT, et al. Transient elastography for diagnosis of stages of hepatic fibrosis and cirrhosis in people with alcoholic liver disease. Cochrane Database Syst Rev. 2015;1(1):Cd010542.
Tapper EB, Afdhal NH. Vibration-controlled transient elastography: a practical approach to the noninvasive assessment of liver fibrosis. Curr Opin Gastroenterol. 2015;31(3):192–8.
Acknowledgements
We appreciate the contribution of all staff and participants in the U.S. National Health and Nutrition Examination Survey (NHANES).
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
**aohui Zhou has full access to all of the data in this study and assumes responsibility for study supervision. **aohui Zhou and Zhikun Dai conceptualized and designed the study, collected and analyzed data, carried out the initial analyses, and reviewed and revised the manuscript. Zhikun Dai acquired, analyzed, and interpreted the data, and drafted the initial manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocols of the NHANES were approved by the National Center for Health Statistics institutional review board, with all individuals providing informed written consent. Human study considerations were exempted due to the anonymity of the data.
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dai, Z., Zhou, X. Associations between allostatic load and hepatic steatosis and liver fibrosis: evidence from NHANES 2017–2020. BMC Public Health 24, 1602 (2024). https://doi.org/10.1186/s12889-024-19111-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-024-19111-7