Abstract
Background
Sarcopenia is an age-related, multifactorial syndrome. Previous studies have shown that air pollutants are associated with inflammation and oxidative stress. However, the association between long-term exposure to air pollution and sarcopenia is not completely understood.
Methods
The Taiwan National Health Research Database (NHIRD) contains medical records of almost all Taiwanese residents. Daily air pollution data collected by the Taiwan Environmental Protection Agency was used to analyze concentrations of sulfur oxide (SO2), carbon monoxide (CO), nitrogen monoxide (NO), nitrogen dioxide (NO2), and particulate matter (PM2.5, PM10). The databases were merged according to the insurants’ living area and the location of the air quality monitoring station. We categorized the pollutants into quartiles (Q1, Q2, Q3, and Q4).
Results
Our study population consisted of 286,044 patients, among whom 54.9% were female and 45.1% were male. Compared to Q1 levels of pollutants, Q4 levels of SO2 (adjusted hazard ratio [aHR] = 8.43; 95% confidence interval [CI] = 7.84, 9.07); CO (aHR = 3.03; 95%CI = 2.83, 3.25); NO (aHR = 3.47; 95%CI = 3.23, 3.73); NO2 (aHR = 3.72; 95%CI = 3.48, 3.98); PM2.5 (aHR = 21.9; 95% CI = 19.7, 24.5) and PM10 (aHR = 15.6; 95%CI = 14.1, 17.4) increased risk of sarcopenia.
Conclusions
Our findings indicated a significantly increased risk of sarcopenia in both male and female residents exposed to high levels of air pollutants.
Similar content being viewed by others
Introduction
Over the past decade, air pollution has become an important public health issue in industrialized countries [1, 2]. Long-term exposure triggers tissue-specific inflammation and increases oxidative stress, resulting in an increased risk of carcinoma, cardiovascular diseases, cerebrovascular diseases, neurodegenerative diseases, and osteoporosis, among others [3,4,5,6,7]. Sarcopenia is an age-related, multifactorial syndrome caused by low physical activity, poor nutritional consumption, changes in sex hormones, and oxidative stress, [8,9,10,11,12] primarily presenting as a loss of skeletal muscle mass and strength [13]. Thus, it often causes fractures, functional decline, frailty, and mortality, especially in patients with solid tumors, kidney dysfunction, diabetes, and cirrhosis [14,15,16,17]. Nevertheless, its etiology and pathogenesis are yet to be elucidated [18]. Inflammatory markers and oxidative stress have been proven to be associated with the onset of sarcopenia [19,20,21]. Past sarcopenia-related research was most frequently conducted on middle-aged or elderly people [22,23,24]. In a cross-sectional study, for each unit Increase in air pollutants, the sarcopenia risks were increased by 11.1%, 4.3%, 22.6%, and 9.3% in PM2.5, PM10, SO2, and O3, respectively [22]. Zhihan et al. observed an increased risk of probable sarcopenia with each interquartile range increment in the following air pollutants: PM2.5 (Odds Ratio (OR): 1.06; 95% Confidence Interval (CI): 1.04, 1.07); PM10 (OR: 1.15; 95% CI: 1.13, 1.17); PM coarse (O: 1.02; 95% CI: 1.01, 1.03); PM2.5 (OR: 1.08; 95% CI: 1.07, 1.10); NO2 (OR: 1.12; 95% CI: 1.10, 1.14); NOx (OR: 1.06; 95% CI: 1.05, 1.08) in older people [25].
However, > 10% of young adults of most ethnicities have been diagnosed with sarcopenia [26]. However, air is an indispensable necessity for life. It is not like hormones, nutritional status, or activity that changes due to differences in age and gender. Thus, we conducted this nationwide retrospective cohort study of Taiwanese residents aged > 18 years to evaluate the risk of sarcopenia associated with long-term exposure to air pollution.
Methods
Data source
The Taiwan National Health Research Database (NHIRD) contains the medical records of almost all Taiwanese residents. Patient demographic information, except for private data, time of diagnosis, and use of medicine, was extracted from the NHIRD. Disease coding followed the International Classification of Diseases, Ninth Revision, Tenth Revision, and Clinical Modification (ICD-9-CM and ICD-10-CM). In this study, we used the Longitudinal Generation Tracking Database, a subset of data from the NHIRD with a population of 2 million. For air pollution data, we used daily data from the Taiwan Environmental Protection Agency (EPA). The Taiwan EPA, Executive Yuan has established 74 ambient air quality monitoring stations (AQMS) on Taiwan’s main island, with locations based on population density. In this study, we obtained air pollutant levels from 31, 15, 23 and 5 AQMS located in northern, central, southern, and eastern Taiwan respectively. We analyzed temperature, humidity, sulfur oxide (SO2), carbon monoxide (CO), nitrogen monoxide (NO), nitrogen dioxide (NO2), and particulate matter (PM2.5, PM10). The databases were merged according to insurants’ living area and location of the air quality monitoring station.
This study was approved by the Institutional Review Board of the China Medical University Hospital Research Ethics Committee (CMUH109-REC2-031).
Study population, outcome, and comorbidities
We observed that the population aged > 18 years in 2003 lived in an area with air quality monitoring stations and had no history of sarcopenia before 2003. Sarcopenia (ICD-9-CM: 728.2; ICD-10-CM: M62.84) was defined as two or more outpatient diagnoses or one admission record. The study period began in 2003 and ended in 2016. Residents with missing data on living areas, medical records, and pollutant levels were excluded, as were those previously diagnosed with sarcopenia before the baseline.
The annual average amount of air pollutants to which each subject was exposed during the follow-up period was calculated: the sum of the pollutant concentrations for the days of exposure is divided by the number of days of exposure. The quartile of each air pollutant was as follows: SO2 (Q1: < 2.63 ppb; Q2: 2.63–3.21 ppb; Q3: 3.21–3.54 ppb; Q4: > 3.54 ppb); CO (Q1: < 0.37 ppm; Q2: 0.37–0.45 ppm; Q3: 0.45–0.55 ppm, Q4: > 0.55 ppm); NO (Q1: < 2.56 ppm; Q2: 2.56–4.05 ppm; Q3: 4.05–6.12 ppm; Q4: > 6.12 ppm); NO2 (Q1: < 16.1 ppm; Q2: 16.1–21.2 ppm; Q3: 21.2–26.9 ppm; Q4: > 26.9 ppm); PM2.5 (Q1: < 17.0 µg/m3; Q2: 17.0–18.4 µg/m3; Q3: 18.4–26.3 µg/m3; Q4: > 26.3 µg/m3); and PM10 (Q1: < 34.8 µg/m3; Q2: 34.8–39.9 µg/m3; Q3: 39.9-–49.7 µg/m3; Q4: > 49.7 µg/m3). Related comorbidities, such as alcohol abuse/dependence, tobacco abuse/dependence, chronic obstructive pulmonary disease (COPD), asthma, obesity, osteoporosis, and estrogen supplementation, were considered covariates.
Statistical analysis
The variables of sex, urbanization level, comorbidities, medication, and sarcopenia were presented as counts and percentages. Urbanization was defined using criteria that included population density (people per square kilometer), the proportion of individuals with a college-level education or higher, the proportion of people aged 65 years and older, the proportion of agricultural workers, and the density of physicians per 100,000 residents [27]. Variables related to environmental factors were expressed as means and standard deviations. The incidence rate ratio (IRR) was computed using Poisson regression.
Alcohol abuse, tobacco abuse, COPD, and asthma were related to smoking status and alcohol drinking patterns [28,29,30,31,32,33,34]. Obesity, osteoporosis and estrogen were also associated with the development of sarcopenia [8,9,10,11,12, 35]. To control for these confounders, the multivariable Cox proportional models were used to estimate the adjusted hazard ratio (aHR) to reveal the risk of sarcopenia with air pollution exposure in Taiwan residents. We also performed multivariable Cox proportional models with stratification to clarify the association between air pollution and the risk of sarcopenia development. Analyses were performed using SAS (version 9.4; SAS Institute Inc., Cary, NC, USA). Statistical significance was set at p < 0.05.
Results
Our study population comprised 286,044 patients, of whom 54.9% were female and 45.1% were male. The mean age was 41.0 (± 16.2) years, and the high-level urbanization area was the dominant group. A total of 12.6% of patients had comorbidities such as COPD, and 11.6% had previously developed asthma. Approximately 29.4% of participants took estrogen supplements. As shown in Table 1, the yearly average values of SO2, CO, NO, NO2, PM2.5, and PM10 that participants were exposed to within the follow-up period were 3.29 ppb (± 1.08), 0.49 ppm (± 0.19), 6.47 ppm (± 6.94), 23.4 ppm (± 11.7), 21.0 µg/m3 (± 6.19) and 43.20 µg/m3 (± 11.9), respectively. A total of 8,904 participants developed sarcopenia, and the mean follow-up time was 12.4 ± 2.20 years.
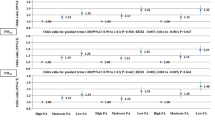
Table 2 shows the relationship between air pollutants and sarcopenia using the IRR. We divided the concentration of pollutants into four quartiles. Considering Q1 levels of each pollutant as the reference, the IRR of sarcopenia in Q2 levels of SO2, CO, NO, NO2, and PM2.5 was 1.37 (95% confidence interval [CI] = 1.25, 1.50), 1.29 (95%CI = 1.19, 1.40), 1.53 (95%CI = 1.42, 1.66), 1.50 (95%CI = 1.39, 1.61), and 0.18 (95%CI = 0.13, 0.25), respectively. Patients exposed to Q3 levels of CO (IRR = 2.00; 95%CI = 1.86, 2.16), NO (IRR = 1.86; 95%CI = 1.72, 2.01), PM2.5 (IRR = 4.88; 95%CI = 4.35, 5.49), and PM10 (IRR = 5.93; 95%CI = 5.32, 6.61) had an increased rate of sarcopenia. Q4 levels of all pollutants were related to sarcopenia (SO2: IRR = 8.74, 95%CI = 8.13, 9.40; CO: IRR = 2.88, 95%CI = 2.69, 3.08; NO: IRR = 3.33, 95%CI = 3.10, 3.57; NO2: IRR = 3.63, 95%CI = 3.40, 3.88; PM2.5: IRR = 23.0; 95%CI = 20.6, 25.7; PM10: IRR = 16.2, 95%CI = 14.6, 18.0). In women, the association between air pollutants and sarcopenia was similar to that observed in the entire population. However, the IRR of sarcopenia in Q3 levels of NO2 relative to Q1 levels was 1.23 (95%CI = 1.09, 1.38) in men.
Based on the Cox proportional hazards model (Table 3), the level of pollutants was directly proportional to the risk of sarcopenia. Similarly, compared to Q1 levels of pollutants, Q2 levels of SO2 (aHR = 1.40; 95% CI = 1.28, 1.54); CO (aHR = 1.33; 95%CI = 1.23, 1.44); NO (aHR = 1.53; 95%CI = 1.41, 1.66); and NO2 (aHR = 1.51; 95%CI = 1.40, 1.63) increased the risk of sarcopenia, but Q2 levels of PM2.5 (aHR = 0.18; 95%CI = 0.13, 0.26) did not. In addition to SO2, Q3 levels of pollutants increased the incidence of sarcopenia by 2.08 times (95%CI = 1.93, 2.24) for CO, 1.92 times (95%CI = 1.77, 2.08) for NO, 1.12 times (95% CI = 1.04, 1.21) for NO2, 4.82 times (95%CI = 4.29, 5.42) for PM2.5, and 5.94 times (95% CI = 5.33, 6.63) for PM10. Regarding Q4 levels of pollutants, PM2.5 showed the highest aHR of sarcopenia (21.9; 95% CI = 19.7, 24.5), followed by PM10 (aHR = 15.6; 95%CI = 14.1, 17.4), and SO2 (aHR = 8.43; 95%CI = 7.84, 9.07). Pollutants in Q4 levels of CO (aHR = 3.03; 95%CI = 2.83, 3.25); NO (aHR = 3.47; 95%CI = 3.23, 3.73); and NO2 (aHR = 3.72; 95%CI = 3.48, 3.98) increased the risk of sarcopenia more than threefold. The relationship between pollutant levels and sarcopenia remained the same in both men and women.
Discussion
We conducted a nationwide retrospective study with approximately 12-year follow-up data from 286,044 Taiwanese residents to evaluate the association between sarcopenia and air pollutant exposure, and found a significantly increased risk of sarcopenia in both male and female residents exposed to high levels of air pollutants.
There is growing evidence that air pollution exposure increases inflammatory markers, such as C-reactive protein (CRP); interleukins (ILs)-1, 6, 8, 10; tumor necrosis factor (TNF)-α; vascular cell adhesion molecule-1 (VCAM-1); intercellular adhesion molecule-1 (ICAM-1); reactive oxygen species (ROS); and reactive nitrogen species (RNS) [36,37,38,39,40,41,42,43]. CRP was independently associated with muscle strength impairment [44]. Furthermore, elevated concentrations of TNF-α, IL-6 and CRP are frequently identified in sarcopenia patients [45, 46] These cytokines probably trigger the activation of the ubiquitin-protease system and develop sarcopenia [47, 48].
Aging has been shown to decrease skeletal muscle mass and strength via oxidative stress and molecular inflammation in both human and animal studies [20, 21, 49]. During the aging process, the balance between degradation and resynthesis of skeletal muscle proteins is disrupted [50]. The results of a community-based study suggested that increased oxidative stress is significantly associated with grip strength in older women [20]. In addition, patients with sarcopenia have a significantly higher erythrocyte sedimentation rate (ESR) and CRP levels than controls, [19] and ROS/RNS plays an important role in the development of sarcopenia [51].
Air pollution sources include traffic emissions, fossil fuel combustion, and emissions from industries, agriculture, and power plants [52, 53]. The degree of air pollution is highly associated with urbanization [54]. We obtained air pollutant levels from 74 AQMS across all over Taiwan. Previous findings suggest that air quality in northern Taiwan has seen improvement over the last decade. Nonetheless, central and southern Taiwan have not experienced significant advancements in air quality. In these regions, PM concentrations have consistently exceeded the EPA’s established exposure standards, necessitating measures to mitigate their adverse effects on public health [55]. The study results show that the PM has the greatest impact on sarcopenia risk in residents living in central and southern regions.
Ethanol exposure impairs skeletal muscle protein synthesis and induces muscle autophagy [13, 56, 57]. However, previous studies have not found a significant association between alcohol consumption and sarcopenia [28,29,30]. Cigarette smoking is a common risk factor for many diseases including sarcopenia, as it causes oxidative stress and chronic inflammation [31,32,33,34]. COPD is a chronic inflammatory disease that affects the airways, [58, 59] showing similar symptoms to asthma. Both immune responses and chronic inflammatory diseases affect the airways [60, 61]. Sarcopenia frequently co-occurs with COPD or asthma, [62,63,64,65] similar to its relationship with osteoporosis [66,67,68]. In addition, several studies have shown that visceral fat with abundant inflammatory cells is the primary source of inflammatory cytokines, such as IL-1β, IL-6, and TNF-β [69,70,71]. Considering the above, we performed a sex-stratified multivariate Cox regression, adjusting for urbanization, asthma, obesity, osteoporosis, and estrogen supplementation.
This was a well-designed cohort study with a long follow-up period adjusted for various confounding factors. However, this study had some limitations. First, a surveillance bias might have been present. Level of urbanization might have been associated with medical convenience, resulting in different prevalence of sarcopenia in urban and rural areas. The Taiwanese government has established a single-payer compulsory social insurance system covering > 99% of residents, which balances medical convenience between urban and rural areas by providing free medical care [72, 73]. Second, living area was defined by the location of the institute where residents sought medical care during the study period. However, residents who had no medical records during this period were not enrolled; further, they tended to live in areas with low levels of air pollutants, resulting in an underestimated risk of sarcopenia. Third, although indoor air pollutant levels are associated with building characteristics, [74] their concentration could not be evaluated in this nationwide study. However, there is no evidence that buildings differ in places with different levels of air pollution in Taiwan, or that they cannot block outdoor air pollutants [75, 76]. Nevertheless, additional studies are required to clarify the impact of indoor air quality. Fourth, the major limitations of the NHIRD study are lack of healthy behavior and dietary habits. Diagnostic criteria for alcohol abuse/dependence were based on drinking behaviors and patients’ attitudes. In previous NHIRD studies, COPD, asthma, and tobacco abuse/dependence were considered instead of smoking status [77,78,79]. Thus, we consider COPD, asthma, tobacco abuse/dependence and alcohol abuse/dependence as the proxy variables for smoking status and alcohol consumption. Fifth, air pollution levels in the residential areas of NHIRD insurants were assessed based on the nearest air quality monitoring stations to clinics or hospitals. This approach may introduce bias in the results, as the measured air quality and urbanization levels could deviate from the true values, particularly when participants have lengthy commutes between their residences and medical facilities. Therefore, additional personal air sampling is necessary to validate these observations.
In summary, this study discovered a significant positive relationship between air pollution exposure and development of sarcopenia in both men and women. According to this finding, less polluting residential areas are required in older societies to slow the progression of chronic diseases like sarcopenia. However, further clinical and experimental studies are required to confirm these findings.
Data availability
The data that support the findings of this study are available from Taiwan Ministry of Health and Welfare (MOHW), but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the Taiwan MOHW.
References
Cohen AJ, et al. Estimates and 25-year trends of the global burden of Disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet. 2017;389(10082):1907–18.
Lelieveld J, et al. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature. 2015;525(7569):367–71.
Chang KH et al. Long-term exposure to Air Pollution associates the risk of Benign Brain Tumor: a Nationwide, Population-Based, Cohort Study in Taiwan. Toxics, 2022. 10(4).
Chen SW et al. Exposure to Air pollutants increases the risk of Chronic Rhinosinusitis in Taiwan residents. Toxics, 2022. 10(4).
Chang KH, et al. Traffic-related air pollutants increase the risk for age-related macular degeneration. J Investig Med. 2019;67(7):1076–81.
Andersen ZJ, et al. Stroke and long-term exposure to outdoor air pollution from nitrogen dioxide: a cohort study. Stroke. 2012;43(2):320–5.
Chang KH, et al. Exposure to air pollution increases the risk of osteoporosis: a nationwide longitudinal study. Med (Baltim). 2015;94(17):e733.
Brioche T, Lemoine-Morel S. Oxidative stress, Sarcopenia, antioxidant strategies and Exercise: molecular aspects. Curr Pharm Des. 2016;22(18):2664–78.
Marzetti E, et al. Physical activity and exercise as countermeasures to physical frailty and sarcopenia. Aging Clin Exp Res. 2017;29(1):35–42.
Yanai H. Nutrition for Sarcopenia. J Clin Med Res. 2015;7(12):926–31.
Messier V, et al. Menopause and sarcopenia: a potential role for sex hormones. Maturitas. 2011;68(4):331–6.
Maggio M, Lauretani F, Ceda GP. Sex hormones and Sarcopenia in older persons. Curr Opin Clin Nutr Metabolic Care, 2013. 16(1).
Thomas DR. Loss of skeletal muscle mass in aging: examining the relationship of Starvation, sarcopenia and cachexia. Clin Nutr. 2007;26(4):389–99.
Cruz-Jentoft AJ, Sayer AA. Sarcopenia the Lancet. 2019;393(10191):2636–46.
Tantai X, et al. Effect of Sarcopenia on survival in patients with Cirrhosis: a meta-analysis. J Hepatol. 2022;76(3):588–99.
Shu X, et al. Diagnosis, prevalence, and mortality of Sarcopenia in dialysis patients: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2022;13(1):145–58.
Feng L, et al. Prevalence and risk factors of Sarcopenia in patients with Diabetes: a Meta-analysis. J Clin Endocrinol Metab. 2022;107(5):1470–83.
Boirie Y. Physiopathological mechanism of Sarcopenia. JNHA - The Journal of Nutrition Health and Aging. 2009;13(8):717–23.
Can B, et al. Serum markers of inflammation and oxidative stress in Sarcopenia. Aging Clin Exp Res. 2017;29(4):745–52.
Howard C, et al. Oxidative protein damage is associated with poor grip strength among older women living in the community. J Appl Physiol (1985). 2007;103(1):17–20.
Siu PM, Pistilli EE, Alway SE. Age-dependent increase in oxidative stress in gastrocnemius muscle with unloading J Appl Physiol (1985), 2008. 105(6): p. 1695 – 705.
Zhang F, et al. Air pollution weaken your muscle? Evidence from a cross-sectional study on Sarcopenia in central China. Ecotoxicol Environ Saf. 2023;258:114962.
Jiang M, et al. Association between Household Solid Fuel Use and Sarcopenia Risk among Middle-aged and older adults in China: a Nationwide Population-based Cohort Study. J Nutr Health Aging. 2023;27(6):472–8.
Zhang B, et al. Impact of household solid fuel use on Sarcopenia in China: a nationwide analysis. Sci Total Environ. 2023;877:162814.
Lai Z et al. Is ambient air pollution associated with Sarcopenia? Results from a nation-wide cross-sectional study. Age Ageing, 2022. 51(11).
Jung HN, Jung CH, Hwang YC. Sarcopenia in Youth Metabolism. 2023;144:155557.
Liu C-Y, et al. Incorporating Development Stratification of Taiwan townships into Sampling Design of large Scale Health interview survey. J Health Manage. 2006;4(1):1–22.
Akune T, et al. Exercise habits during middle age are associated with lower prevalence of Sarcopenia: the ROAD study. Osteoporos Int. 2014;25(3):1081–8.
Lin CC, et al. Sarcopenia prevalence and associated factors in an elderly Taiwanese metropolitan population. J Am Geriatr Soc. 2013;61(3):459–62.
Liu LK, et al. Sarcopenia, and its association with cardiometabolic and functional characteristics in Taiwan: results from I-Lan Longitudinal Aging Study. Geriatr Gerontol Int. 2014;14(Suppl 1):36–45.
Landi F, et al. Prevalence and risk factors of Sarcopenia among nursing home older residents. J Gerontol A Biol Sci Med Sci. 2012;67(1):48–55.
Lee JS, et al. Associated factors and health impact of Sarcopenia in older Chinese men and women: a cross-sectional study. Gerontology. 2007;53(6):404–10.
Burke A, Fitzgerald GA. Oxidative stress and smoking-induced vascular injury. Prog Cardiovasc Dis. 2003;46(1):79–90.
Zuo L, et al. Interrelated role of cigarette Smoking, oxidative stress, and immune response in COPD and corresponding treatments. Am J Physiol Lung Cell Mol Physiol. 2014;307(3):L205–18.
Urano T, Inoue S. Recent genetic discoveries in osteoporosis, Sarcopenia and obesity. Endocr J. 2015;62(6):475–84.
Panasevich S, et al. Associations of long- and short-term air pollution exposure with markers of inflammation and coagulation in a population sample. Occup Environ Med. 2009;66(11):747–53.
Zhang Y, et al. Inflammatory response and endothelial dysfunction in the hearts of mice co-exposed to SO(2), NO(2), and PM(2.5). Environ Toxicol. 2016;31(12):1996–2005.
Fashi M, Agha H, Alinejad, Asilian H, Mahabadi. The Effect of Aerobic Exercise in Ambient Particulate Matter on Lung tissue inflammation and Lung Cancer. Iran J Cancer Prev. 2015;8(3):e2333.
Block ML, Calderon-Garciduenas L. Air pollution: mechanisms of neuroinflammation and CNS Disease. Trends Neurosci. 2009;32(9):506–16.
Kunzli N, et al. Ambient air pollution and the progression of Atherosclerosis in adults. PLoS ONE. 2010;5(2):e9096.
Zhang R, et al. Reduced pulmonary function and increased pro-inflammatory cytokines in nanoscale carbon black-exposed workers. Part Fibre Toxicol. 2014;11:73.
Wang C, et al. Particulate air pollution and circulating biomarkers among type 2 diabetic mellitus patients: the roles of particle size and time windows of exposure. Environ Res. 2015;140:112–8.
Risom L, Moller P, Loft S. Oxidative stress-induced DNA damage by particulate air pollution. Mutat Res. 2005;592(1–2):119–37.
Shokri-mashhadi N, et al. Association of circulating C-reactive protein and high-sensitivity C-reactive protein with components of Sarcopenia: a systematic review and meta-analysis of observational studies. Exp Gerontol. 2021;150:111330.
Dalle S, Rossmeislova L, Koppo K. The role of inflammation in age-related Sarcopenia. Front Physiol. 2017;8:1045.
Bian A-L, et al. A study on relationship between elderly Sarcopenia and inflammatory factors IL-6 and TNF-α. Eur J Med Res. 2017;22(1):25.
Ferrucci L, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47(6):639–46.
Toth MJ, et al. Immune activation is associated with reduced skeletal muscle mass and physical function in chronic Heart Failure. Int J Cardiol. 2006;109(2):179–87.
Meng SJ, Yu LJ. Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci. 2010;11(4):1509–26.
Koopman R, van Loon LJ. Aging, exercise, and muscle protein metabolism. J Appl Physiol (1985). 2009;106(6):2040–8.
Bohnert KR, McMillan JD, Kumar A. Emerging roles of ER stress and unfolded protein response pathways in skeletal muscle health and Disease. J Cell Physiol. 2018;233(1):67–78.
Forsberg B, Stjernberg N, Wall S. People can detect poor air quality well below guideline concentrations: a prevalence study of annoyance reactions and air pollution from traffic. Occup Environ Med. 1997;54(1):44–8.
Colvile RN, et al. The transport sector as a source of air pollution. Atmos Environ. 2001;35(9):1537–65.
Diapouli E, et al. Evolution of air pollution source contributions over one decade, derived by PM10 and PM2.5 source apportionment in two metropolitan urban areas in Greece. Atmos Environ. 2017;164:416–30.
Hsieh H-H, et al. Taiwan Air Quality Variation and Trend. Min Metallurgy: Bull Chin Inst Min Metall Eng. 2008;52(4):46–54.
Domiciano DS, et al. Discriminating Sarcopenia in community-dwelling older women with high frequency of overweight/obesity: the Sao Paulo Ageing & Health Study (SPAH). Osteoporos Int. 2013;24(2):595–603.
Thapaliya S, et al. Alcohol-induced autophagy contributes to loss in skeletal muscle mass. Autophagy. 2014;10(4):677–90.
Nussbaumer-Ochsner Y, Rabe KF. Systemic Manifestations of COPD Chest. 2011;139(1):165–73.
Sethi S, et al. Inflammation in COPD: implications for management. Am J Med. 2012;125(12):1162–70.
Hamid Q, Tulic M. Immunobiology of Asthma. Annu Rev Physiol. 2009;71(1):489–507.
Rovina N, Koutsoukou A, Koulouris NG. Inflammation and Immune Response in COPD: Where Do We Stand? Mediators of Inflammation, 2013. 2013: p. 413735.
Sepulveda-Loyola W, et al. Diagnosis, prevalence, and clinical impact of Sarcopenia in COPD: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2020;11(5):1164–76.
Benz E et al. Sarcopenia in COPD: a systematic review and meta-analysis. Eur Respir Rev, 2019. 28(154).
Borda MG, et al. [Sarcopenia in the elderly with a history of COPD/asthma: results of the SABE-Bogotá study]. Rev Esp Geriatr Gerontol. 2017;52(6):313–6.
Won HK, et al. Relationship between Asthma and sarcopenia in the elderly: a nationwide study from the KNHANES. J Asthma. 2023;60(2):304–13.
Nielsen BR, et al. Sarcopenia and osteoporosis in older people: a systematic review and meta-analysis. Eur Geriatr Med. 2018;9(4):419–34.
Crepaldi G, Maggi S. Sarcopenia and osteoporosis: a hazardous duet. J Endocrinol Investig. 2005;28(10 Suppl):66–8.
Reiss J, et al. Sarcopenia and osteoporosis are interrelated in geriatric inpatients. Z Gerontol Geriatr. 2019;52(7):688–93.
Spoto B, et al. Pro- and anti-inflammatory cytokine gene expression in subcutaneous and visceral fat in severe obesity. Nutr Metabolism Cardiovasc Dis. 2014;24(10):1137–43.
Nam SY, et al. The effect of abdominal visceral fat, circulating inflammatory cytokines, and leptin levels on reflux esophagitis. J Neurogastroenterol Motil. 2015;21(2):247–54.
Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol, 2004. 15(11).
Huang N, et al. Trends in rural and urban differentials in incidence rates for ruptured Appendicitis under the National Health Insurance in Taiwan. Public Health. 2006;120(11):1055–63.
Siroux V, et al. Relationships of active Smoking to Asthma and Asthma severity in the EGEA study. Epidemiological study on the Genetics and Environment of Asthma. Eur Respir J. 2000;15(3):470–7.
Langer S, Bekö G. Indoor air quality in the Swedish housing stock and its dependence on building characteristics. Build Environ. 2013;69:44–54.
Blondeau P, et al. Relationship between outdoor and indoor air quality in eight French schools. Indoor Air. 2005;15(1):2–12.
Boomhower SR, et al. A review and analysis of personal and ambient PM2.5 measurements: implications for epidemiology studies. Environ Res. 2022;204:112019.
Chang KH, et al. Increased risk of Dementia in patients with osteoporosis: a population-based retrospective cohort analysis. Age (Dordr). 2014;36(2):967–75.
Chang KH, et al. A large-scale study indicates increase in the risk of Epilepsy in patients with different risk factors. Including Rheumatoid Arthritis Medicine (Baltimore). 2015;94(36):e1485.
Tsai SC, et al. Long-term exposure to air pollution and the risk of develo** sudden sensorineural hearing loss. J Transl Med. 2021;19(1):424.
Acknowledgements
We are grateful to Health Data Science Center, China Medical University Hospital for providing administrative, technical and funding support.
Funding
This study is supported in part by Tungs’ Taichung Metro Harbor hospital research grant (TTMHH-R1130010), Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW109-TDU-B-212-124004), China Medical University Hospital (DMR-110-105), Tseng-Lien Lin Foundation, Taichung, Taiwan.
Author information
Authors and Affiliations
Contributions
Conceptualization, K.-H.C. and S.-W. C.; methodology, K.-H.C. and C.-L. L.; software, C.-L. L.; validation, S.-W. C., C.-Y. L., C.-Y. C., C.-L. L.,T.-L.H., F.-J. T., and K.-H.C.; formal analysis, K.-H.C. and C.-L. L.; investigation, K.-H.C. and C.-L. L.; resources, S.-W. C. F.-J.T., and K.-H.C.; data curation, K.-H.C. and C.-L. L.; writing—original draft preparation, S.-W. C., C.-Y. L., and K.-H.C.; writing—review and editing, K.-H.C.; visualization, S.-W. C., C.-Y. L., and K.-H.C.; supervision, K.-H.C.; project administration, S.-W. C. F.-J.T., and K.-H.C.; funding acquisition, S.-W. C. F.-J.T., and K.-H.C. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Review Board of China Medical University and Hospital, Taiwan (CMUH109-REC2-031 (CR2)). The IRB waived the consent requirement.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, SW., Lin, CY., Chen, CY. et al. Long-term exposure to air pollution and risk of Sarcopenia in adult residents of Taiwan: a nationwide retrospective cohort study. BMC Public Health 23, 2172 (2023). https://doi.org/10.1186/s12889-023-17091-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-023-17091-8